Introduction
Urinary-derived sepsis is closely associated with
the systemic inflammatory response syndrome (SIRS). It is caused by
direct damage from bacterial toxins and the subsequent excessive
body defense following bacterial infection in the urinary system.
Bacterial infection in the urinary tract is most commonly caused by
Escherichia coli (1). It
leads to urinary-derived sepsis, which is a severe condition among
urinary infections. The kidneys are one of the critical organs that
are prone to multiple organ dysfunction syndrome (MODS) (2). Previous studies demonstrated
(3,4) that during sepsis, repeated
stimulation of the kidneys led to the production of a large number
of pro-inflammatory cytokines [including tumor necrosis factor
(TNF)-α, interleukin (IL)-6 and nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB)], followed by
the rapid release of large amounts of anti-inflammatory cytokines
[including IL-10 and transforming growth factor β (TGF-β)].
Following this, peak concentrations of proinflammatory cytokines
and anti-inflammatory cytokines were observed in the blood
circulation. Early and effective regulation of the inflammatory
response and the timely intervention during SIRS and sepsis is
important for the prevention and treatment of MODS (5).
Hydrogen sulfide (H2S) may be generated
endogenously. Following processing by cystathionine-β-synthase
(CBS) and cystathionine-γ-lyase (CSE), sulfur-containing amino
acids are able to generate H2S (6). It has been revealed that endogenous
H2S is extensively involved in many physiological and
pathological processes (7,8). CBS and CSE are expressed in kidney
tissues and, thus, endogenous H2S is also produced,
which plays a significant role in regulating renal functions
(9). H2S is also
important in regulating immune responses. A study by Pan et
al revealed that NaHS (a H2S donor) inhibited the
inflammatory response of endothelial cells to lipopolysaccharides
(10). Tokuda et al
observed that the inhalation of H2S was able to reduce
endotoxin-induced systemic inflammation (11). Furthermore, a study by Ang et
al demonstrated that H2S was able to reduce
sepsis-induced acute lung injury and inflammation (12).
NF-κB is an important intracellular signaling
molecule in signal transduction. NF-κB plays a significant role in
sepsis and organ failure (4). It
regulates a variety of genes involved in infection-related immune
responses, plays an important role in inflammation and leads to
organ dysfunction and mortality in patients with sepsis. Further
evidence has demonstrated that H2S is able to inhibit
the NF-κB signaling pathway (13)
and regulate the expression and activity of NF-κB (14).
In the present study, an upper urinary tract
infection that caused sepsis was established in rabbits by inducing
acute upper urinary tract obstruction and injecting Escherichia
coli. The renal CSE activity and endogenous H2S
concentration in the renal tissue of the septic rabbits was
observed in order to determine the association between kidney
injury induced by urinary-derived sepsis and endogenous
H2S. NaHS was used as a donor for H2S in
order to observe the effect of exogenous H2S on kidney
injury and NF-κB expression in renal tissue. The possible mechanism
underlying the role of H2S in the prevention of kidney
injury induced by urinary-derived sepsis was further analyzed.
Materials and methods
Animals and reagents
Thirty healthy male rabbits, weighing between
1.80–2.20 kg, were obtained from the Experimental Animal Center of
the University of South China (Henyang, China). They were kept in
standard conditions with free access to food and water. All animal
experiments were conducted according to the ethical guidelines of
the University of South China.
Escherichia coli (ATCC 25922) was provided by
the Department of Microbiology of the Second Affiliated Hospital of
University of South China (Henyang, China). NaHS was purchased from
Sigma (St. Louis, MO, USA). TNF-α, IL-10 and NF-κB antibodies were
obtained from Beijing Biosynthesis Biotechnology, Co., Ltd.
(Beijing, China). Rabbit SP-HRP (streptavidin-biotin-peroxidase)
kit and 3,3′-diaminobenzidine (DAB) chromogenic kit were provided
by Beijing Kangwei Century Biotech Co., Ltd. (Beijing, China).
Sepsis model establishment
Rabbits were randomly divided into five groups:
control, sham, sepsis, NaHS 2.8 μmol/kg and NaHS 8.4 μmol/kg
groups, with six rabbits in each group. In the control group, the
rabbits were kept in standard conditions without any treatment. In
the sham group, the rabbits were anesthetized through
intraperitoneal injection with 10% chloral hydrate (3 ml/kg) and an
incision was made through the left rectus abdominis. The middle
section of the left ureter was separated and the incision was
sutured. In the sepsis group, the middle section of the left ureter
was separated and ligated to form an acute upper urinary tract
obstruction. A suspension of Escherichia coli (1 ×
108/ml; 0.5 ml/kg) was injected into the ureter with
distal ligation. The surgical procedure in the NaHS 2.8 μmol/kg and
NaHS 8.4 μmol/kg groups was the same as that in the sepsis group.
NaHS (50 mmol/l) was administered postoperatively through an
injection into the ear vein at doses of 2.8 and 8.4 μmol/kg in the
NaHS 2.8 μmol/kg and NaHS 8.4 μmol/kg groups, respectively. Rabbits
were kept in standard conditions following the surgery. Left kidney
tissue samples were collected at 72 h following surgery.
Blood routine examination and renal
function test
At 24 h prior to surgery and 24, 48 and 72 h
following surgery, the white blood cell (WBC) count was determined
by automatic cell counting. Renal function was analyzed by
measuring the levels of creatinine (Cr) and blood urea nitrogen
(BUN). To do this, 5 ml venous blood was collected and centrifuged
at 1,629 × g for 10 min. The supernatant was analyzed using an
Olympus AU800 automated biochemical analyzer (Olympus, Tokyo,
Japan).
Hematoxylin and eosin (H&E)
staining
Kidney tissues were cut into 0.5-mm3
sections, fixed, embedded in paraffin and further cut into smaller
tissue sections. The tissue sections were dewaxed in xylene and
rehydrated in graded alcohols. Following washing, the sections were
stained with hematoxylin and following a second wash, the sections
were differentiated. The sections were subsequently stained with
eosin after washing. Following dehydration and differentiation in
alcohol, the sections were mounted and observed by microscopy
(Eclipse E200; Nikon, Tokyo, Japan).
Transmission electron microscopy
observation
Pathological changes in the kidney tissues were
observed by transmission electron microscopy. The kidney tissue was
fixed in 2.5% glutaric dialdehyde for 24 h. Following rinsing three
times with phosphate-buffered saline (PBS), the kidney tissue was
treated with 2% osmium tetroxide for 2 h. It was subsequently
dehydrated in a graded series of acetones after washing with PBS.
Following dehydration, the kidney tissue was saturated in
acetone/resin (1:1) at 37°C for 24 h, embedded in Epon, polymerized
in an oven at 60°C for 24 h and cut into semi-thin sections (1 μm).
The semi-thin sections were stained with toluidine blue for viewing
with a light microscope (Nikon). They were subsequently cut into
ultra-thin sections (500 Å) using an LKB III ultramicrotome (LKB
Bromma, Sollentuna, Sweden). The ultra-thin sections were stained
with lead nitrate and uranyl acetate, and examined with a
transmission electron microscope H7500 (Hitachi, Tokyo, Japan).
Immunohistochemical staining
Expression of TNF-α, IL-10 and NF-κB in renal tissue
was detected by immunohistochemical staining. Paraffin-embedded
renal tissue sections were dewaxed and hydrated. The tissue was
subjected to antigen retrieval and 3% hydrogen peroxide was used to
reduce endogenous peroxidase activity. Following blocking with
normal serum, tissue sections were incubated with the primary
antibodies anti-TNF-α, anti-IL-10 and anti-NF-κB (1:300 dilution)
overnight at 4°C. Following rinsing with PBS, biotinylated goat
anti-rabbit secondary antibody (Beijing Kangwei Century Biotech
Co., Ltd.) was added and the sections were incubated at room
temperature. The tissue sections were subsequently incubated with
horseradish peroxidase (HRP)-labeled streptavidin at room
temperature following rinsing with PBS. A DAB chromogenic reagent
was subsequently added for color development. Finally, the sections
were counterstained with hematoxylin, dehydrated, rendered
transparent with xylene, mounted and observed under a light
microscope.
Measurement of serum H2S
concentration
Experimental rabbits were anesthetized and 5–8 ml
venous blood was collected. Following centrifugation, 0.1 ml serum
sample was collected and combined with 0.5 ml 1% zinc acetate, 0.5
ml 20 mmol/l N,N-dimethyl-p-phenylenediamine sulfate and 0.4
ml 30 mmol/l FeCl3. Following incubation for 20 min at
room temperature, 1 ml 10% trichloroacetic acid was added to
precipitate the proteins. The absorbance of the supernatant
following precipitation was measured at a wavelength of 670 nm. A
standard curve was generated by serial dilution of NaHS. The
H2S concentration was calculated based on the standard
curve and was expressed in μmol/l.
Measurement of CSE activity
Kidney tissues were ground to form a 10% (w/v)
homogenate in potassium phosphate buffer (50 mmol/l, pH 6.8) at
4°C. The homogenate was centrifuged and the supernatant was
collected in a conical flask. Subsequently, a 5-pyridoxal
phosphate/potassium phosphate buffer solution (0.5%, pH 7.4, 100
mmol/l) and 0.5 mol/l L-cysteine was added to the reaction flask. A
1% zinc acetate solution (0.5 ml) and filter paper were added
through the central absorbent hole. The conical flask was filled
with nitrogen, sealed and incubated at 37°C in a water bath
oscillator for 90 min. To terminate the reaction, 0.5 ml 50%
trichloroacetic acid was added and the mixture was incubated at
37°C for 20 min. Upon termination of the reaction, the absorbance
was measured at a wavelength of 670 nm. A standard curve was
generated by serial dilution of NaHS. The H2S content
was measured according to the standard curve. CSE activity was
expressed as the amount of H2S generated per mg of
kidney tissue in one minute, with a unit of nmol/min/mg.
Statistical analysis
Data were processed using SPSS statistical software,
version 18.0 (SPSS, Inc., Chicago, IL, USA). All parameters are
presented as means ± standard deviations. One-way analysis of
variance (ANOVA) was performed to compare the differences among the
different groups. A paired t-test was carried out to analyze two
samples taken at the same time point. Measured data were analyzed
by a chi-square test. P<0.05 was considered to indicate a
statistically significant difference.
Results
NaHS treatment reduces the WBC level in
rabbits with sepsis
To determine the effect of NaHS on the WBC level in
rabbits with urinary-derived sepsis, a sepsis model was created in
the present study. The sepsis model was successfully established in
the rabbits. In the sepsis group, the rectal temperature,
respiratory rate and heart rate increased significantly (data not
shown), and no animals died during the observation period. At 24 h
prior to surgery, and 24, 48 and 72 h following surgery, the WBC
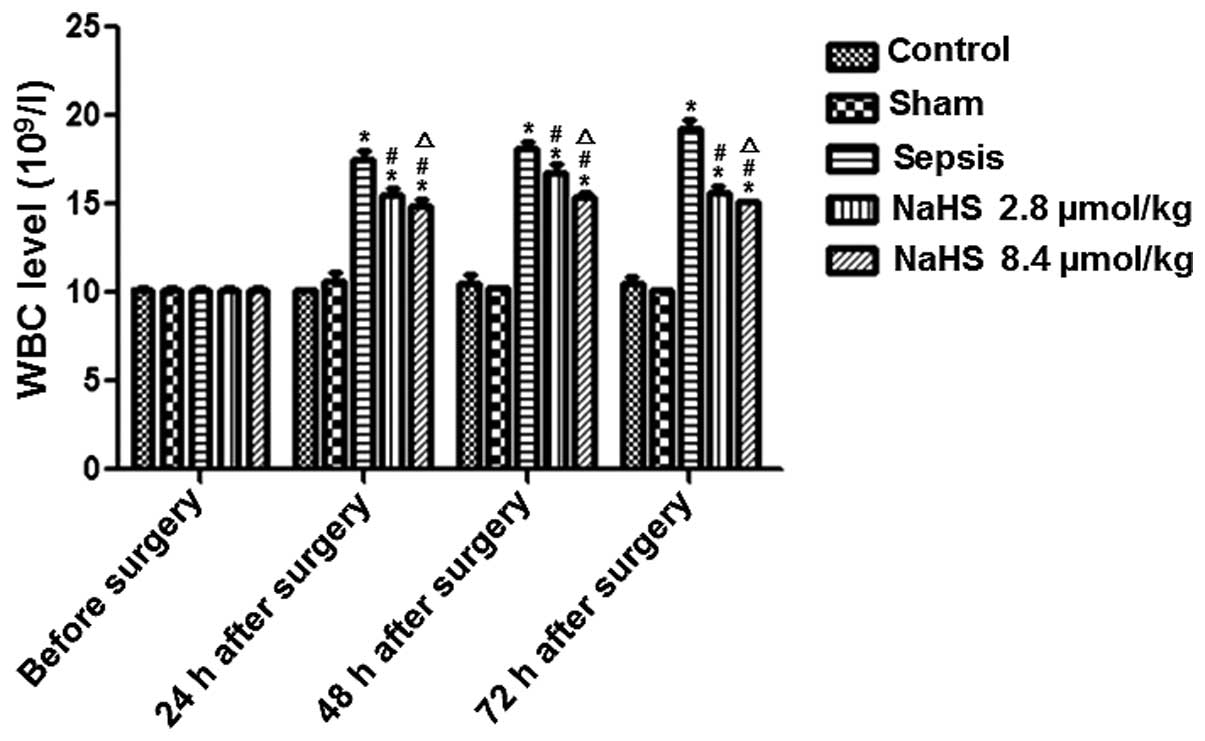
level was examined and compared. The results are shown in Fig. 1. The WBC level gradually increased
in the sepsis group compared with the levels in the control and
sham groups, and was significantly higher at 24, 48 and 72 h
following surgery (P<0.05). This result indicates that sepsis
increased the WBC level in rabbits. The WBC level in the NaHS 2.8
μmol/kg and NaHS 8.4 μmol/kg groups was significantly higher than
that in the control group and significantly lower than that in the
sepsis group at 24, 48 and 72 h following surgery (P<0.01).
Additionally, the WBC level in the NaHS 8.4 μmol/kg group was
significantly lower than that in the NaHS 2.8 μmol/kg group
(P<0.05). These results indicate that NaHS attenuated the
sepsis-induced increase in the WBC level.
NaHS treatment decreases Cr and BUN
levels in rabbits with sepsis
To determine the effect of NaHS treatment on kidney
injury induced by sepsis, the levels of Cr and BUN were examined at
24 h prior to surgery, and at 24, 48 and 72 h following surgery.
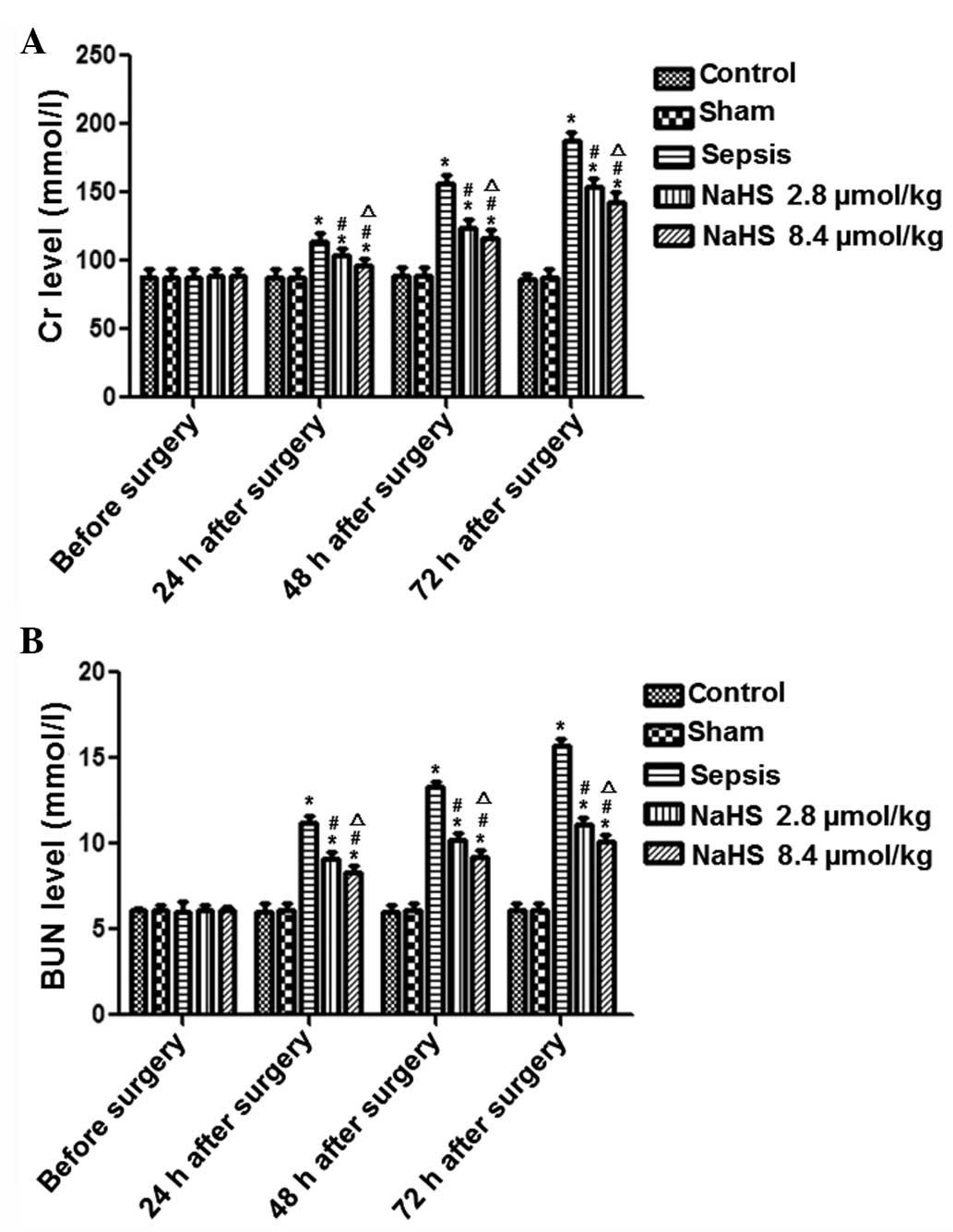
The results for Cr and BUN are shown in Fig. 2. In the sepsis group, the Cr and
BUN levels gradually increased with time. The Cr and BUN levels in
the sepsis group were significantly higher at 24, 48 and 72 h
following surgery compared with those in the control group
(P<0.05). In the NaHS groups, the Cr and BUN levels gradually
decreased in comparison with the levels in the sepsis group. This
difference between the NaHS groups and the sepsis group was
significant (P<0.01). Furthermore, there were significantly
higher levels of Cr and BUN in the NaHS 8.4 μmol/kg group than in
the NaHS 2.8 μmol/kg group (P<0.05). These results demonstrate
that sepsis induced elevated levels of Cr and BUN in rabbits and
that this elevation was inhibited by NaHS treatment.
NaHS treatment increases the plasma
H2S content in sepsis rabbits
To analyze the effect of NaHS on endogenous
H2S, serum samples were collected at 72 h following
surgery and the plasma H2S content was determined. As
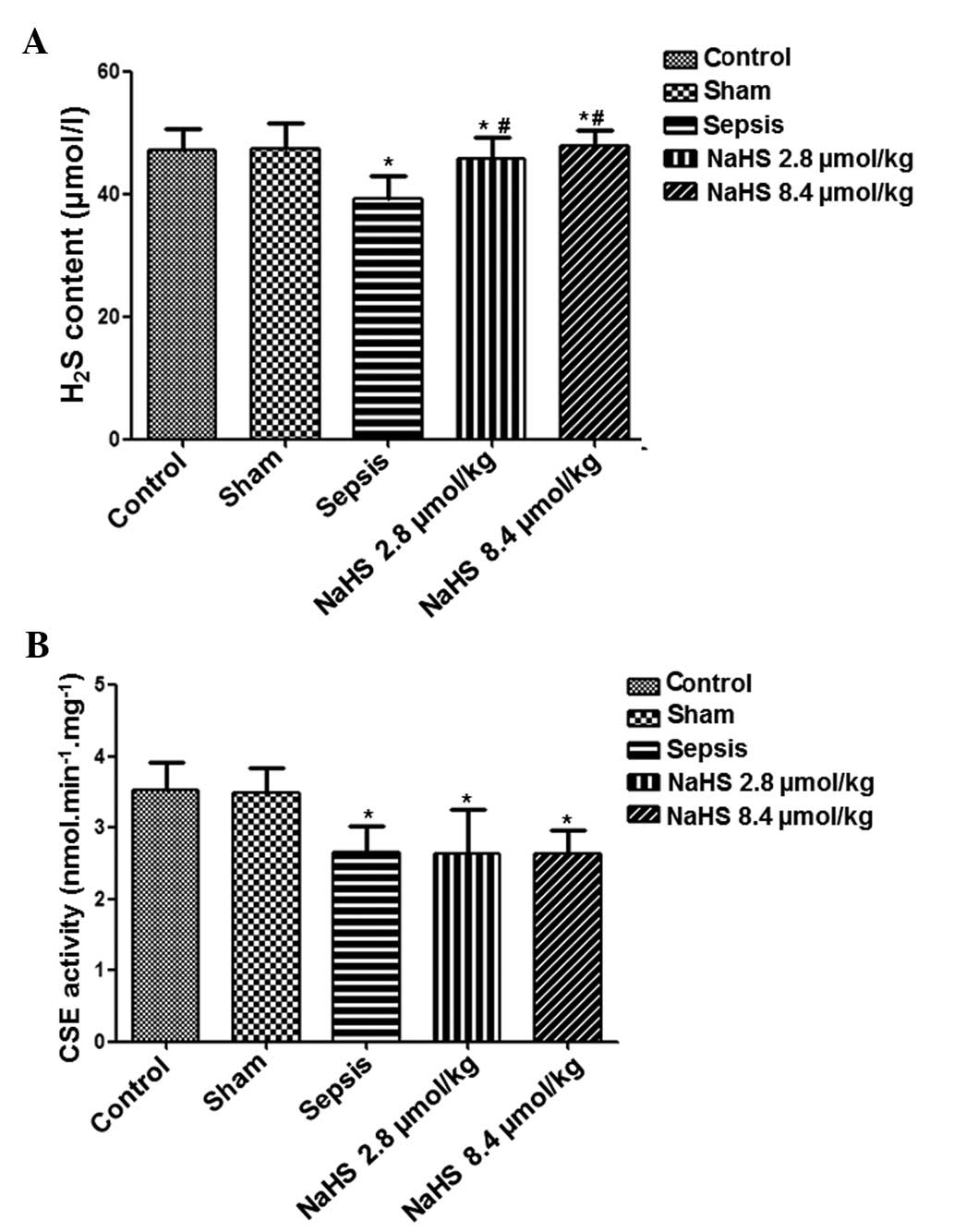
shown in Fig. 3, plasma
H2S content was significantly reduced in the sepsis
group compared with that in the control group (P<0.05).
Following treatment with NaHS, the plasma H2S content
increased in the NaHS 2.8 μmol/kg and NaHS 8.4 μmol/kg groups. The
plasma H2S contents in the NaHS 2.8 μmol/kg and NaHS 8.4
μmol/kg groups were significantly higher compared with that in the
sepsis group (P<0.05). These data indicate that NaHS treatment
increased the endogenous H2S levels of septic
rabbits.
The CSE activity in the renal tissue was analyzed.
Left renal tissue was collected at 72 h following surgery and the
CSE activity was detected in each group. As shown in Fig. 3, the CSE activity in the sepsis,
NaHS 2.8 μmol/kg and NaHS 8.4 μmol/kg groups was significantly
lower than that in the control group (P<0.05). However, no
significant difference was identified between the sepsis and NaHS
2.8 μmol/kg, sepsis and NaHS 8.4 μmol/kg, nor the NaHS 2.8 μmol/kg
and NaHS 8.4 μmol/kg groups. These results suggest that in
urinary-derived sepsis, the endogenous H2S content and
CSE activity are reduced, and that NaHS treatment may increase
H2S content but not CSE activity.
NaHS treatment alleviates the
pathological features of renal tissue in septic rabbits
To investigate the effect of NaHS on renal injury
induced by sepsis, the pathological features of renal tissue were
observed by H&E staining and transmission electron microscopy
at 72 h following surgery. The representative H&E staining and
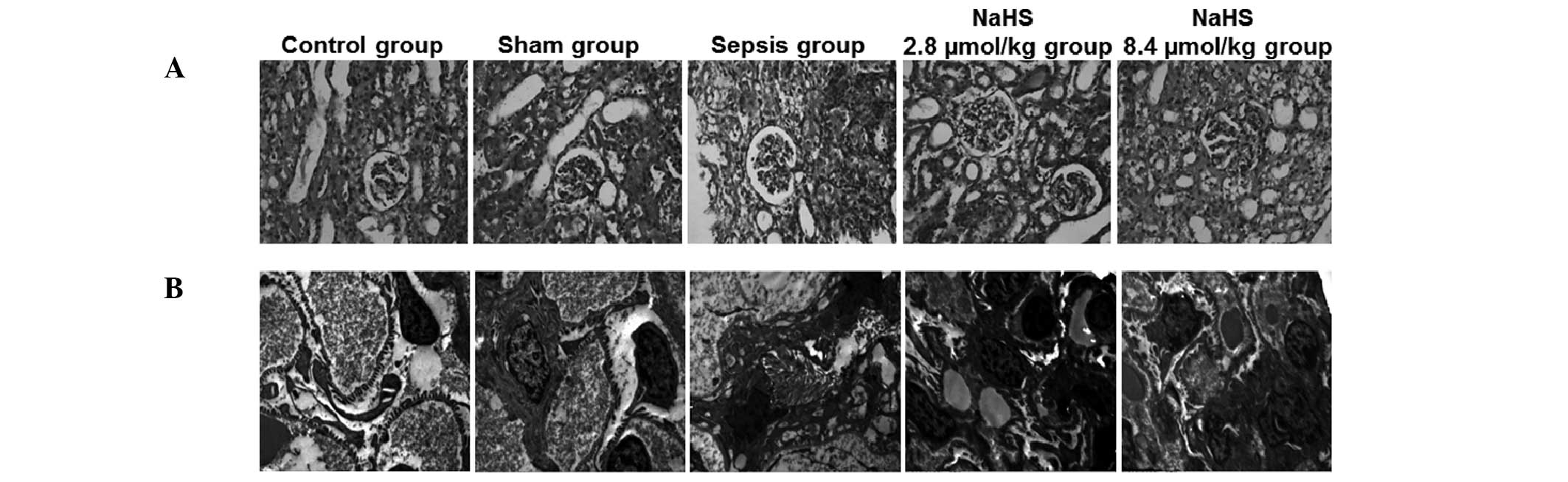
transmission electron microscopy results are shown in Fig. 4. The renal tissue revealed normal
morphology in the control and sham groups. There was no congestion,
edema or inflammatory cell infiltration. In the sepsis group,
mucosal necrosis and extensive neutrophil infiltration was present
in the pelvis and calyces as well as glomerular deformation and
renal capsule expansion. Swelling and necrosis was observed in the
renal tubular epithelial cells. The renal tubules were also
enlarged and infiltrated with neutrophils. In the kidney
interstitium, congestion, edema, inflammatory cell infiltration and
abscess formation was observed. Following treatment with NaHS,
these pathological changes were reduced in the NaHS 2.8 μmol/kg and
NaHS 8.4 μmol/kg groups.
The pathological changes in renal tissue were
further observed by transmission electron microscopy. As shown in
Fig. 4, no abnormal structures
were visible in the control and sham groups. There was no edema or
vacuolation in the epithelial cells of the renal tubules and the
mitochondria and endoplasmic reticulum were regularly arranged.
Podocytes and mesangial cells revealed a normal morphology and the
basement membrane also remained intact. In the sepsis group, edema
was present in the kidney interstitium, there was extensive atrophy
in the tubular epithelium and interstitial fibroblast
proliferation. Mitochondria in the renal tubular epithelial cells
were swollen and disorganized; their number was also reduced. Focal
fusion of glomerular podocytes was observed. Furthermore, there was
proliferation of the mesangial and endothelial cells. In the NaHS
2.8 μmol/kg and NaHS 8.4 μmol/kg groups, however, these
pathological changes were alleviated.
Collectively, these results suggest that the
pathological features of renal tissue induced by sepsis were
decreased by NaHS treatment.
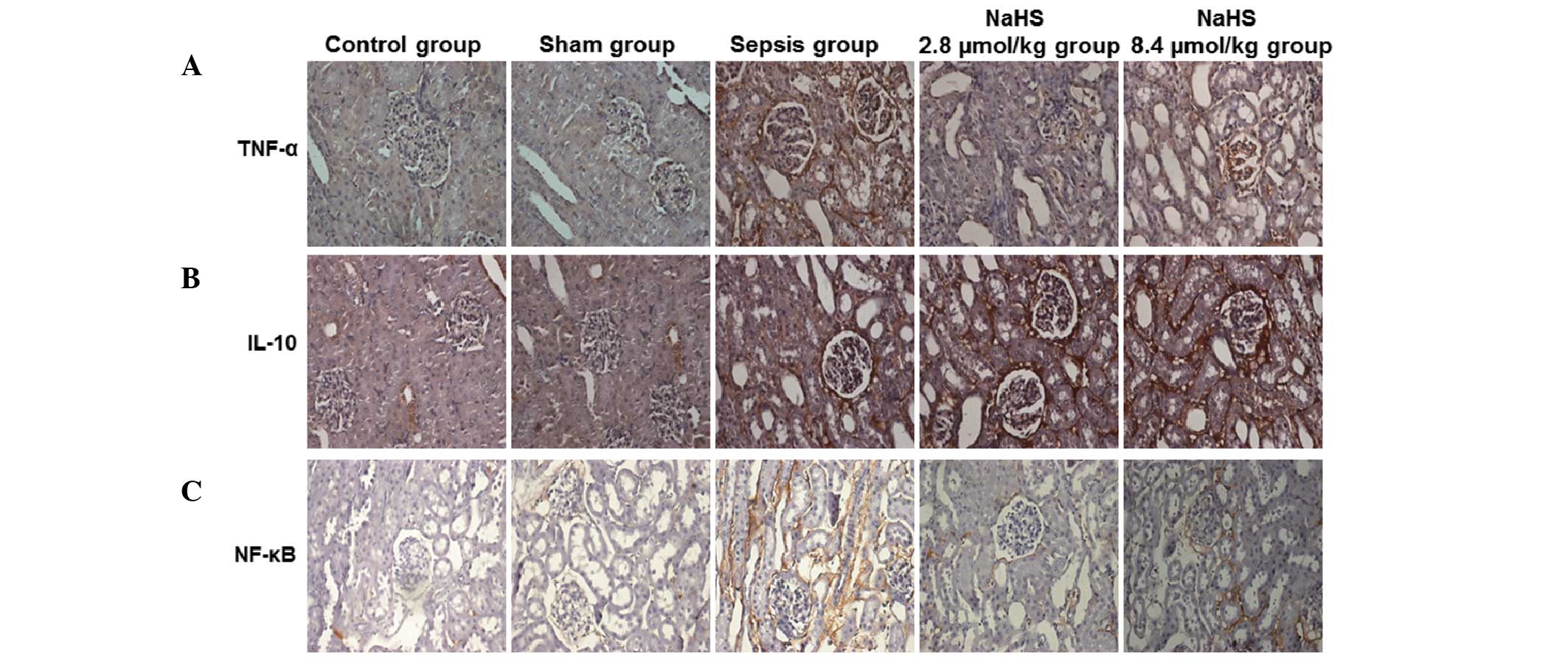
NaHS increases the expression level of
IL-10 and decreases the expression levels of TNF-α and NF-κB in the
renal tissue of septic rabbits
To analyze the possible mechanism by which
H2S prevents kidney injury induced by urinary-derived
sepsis, TNF-α, IL-10 and NF-κB protein expression in left kidney
tissue was determined by immunohistochemistry at 72 h following
surgery. The representative immunohistochemical results are shown
in Fig. 5 and the quantitative
results are shown in Table I.
Cells with yellow or brown particles were positively stained cells.
As shown in Fig. 5 and Table I, TNF-α, IL-10 and NF-κB proteins
were weakly expressed in each group. The expression levels of
TNF-α, IL-10 and NF-κB increased in the sepsis group compared with
those in the control group. In the NaHS 2.8 μmol/kg and NaHS 8.4
μmol/kg groups, the expression levels of TNF-α and NF-κB were lower
than those in the sepsis group. However, the expression levels of
IL-10 were higher in the NaHS groups than in the sepsis group.
Statistically, compared with those in the control group, the
expression levels of TNF-α, IL-10 and NF-κB were significantly
higher in the sepsis group (P<0.05). The expression levels of
TNF-α and NF-κB were significantly lower in the NaHS groups
compared with those in the sepsis group (P<0.05). Furthermore,
the NaHS 8.4 μmol/kg group had significantly higher levels of TNF-α
and NF-κB than the NaHS 2.8 μmol/kg group (P<0.05). By contrast,
the NaHS groups had a significantly higher level of IL-10
(P<0.05). The IL-10 level in the NaHS 8.4 μmol/kg group was
significantly higher than in the NaHS 2.8 μmol/kg group. These data
indicate that NaHS regulated the expression of TNF-α, IL-10 and
NF-κB in septic rabbits.
 | Table IIL-10, TNF-α and NF-κB expression
comparison in renal tissue at 72 h following surgery (OD, means ±
standard deviations, n=6). |
Table I
IL-10, TNF-α and NF-κB expression
comparison in renal tissue at 72 h following surgery (OD, means ±
standard deviations, n=6).
| Groups | TNF-α | IL-10 | NF-κB |
|---|
| Control | 0.141±0.023 | 0.114±0.014 | 0.110±0.016 |
| Sham | 0.139±0.013 | 0.114±0.014 | 0.111±0.017 |
| Sepsis | 0.264±0.017a | 0.225±0.015a | 0.277±0.017a |
| NaHS 2.8 μmol/kg | 0.222±0.015a,b | 0.267±0.015a,b | 0.265±0.017a,b |
| NaHS 8.4 μmol/kg | 0.198±0.009a–c | 0.275±0.016a–c | 0.221±0.017a–c |
Discussion
A previous study reported that ulinastatin
effectively prevented urinary-derived sepsis and reduced
inflammation by decreasing the level of TNF-α and increasing IL-10
(15). In patients with early
sepsis-induced acute kidney injury, renal replacement therapy is
effective in restoring organ function and increasing survival rate
through the removal of inflammatory mediators IL-10 and IL-6
(16). In a study by Souza et
al (4), sepsis was induced in
rats and it was identified that erythropoietin prevented the acute
kidney injury induced by sepsis through the inhibition of NF-κB and
the upregulation of endothelial nitric oxide synthase.
Studies have demonstrated that H2S plays
an important regulatory role in sepsis-related inflammation by
inhibiting the NF-κB signaling pathway and the expression of
inflammatory cytokines. A study by Li et al reported that by
downregulating NF-κB expression, H2S was able to promote
neutrophil aggregation to the site of inflammation, increase
neutrophil migration and adhesion, reduce plasma levels of TNF,
IL-1 and IL-6, and increase the level of IL-10 (17). H2S may also reduce
inflammation by inhibiting the NF-κB/cyclooxygenase-2 (COX-2)
pathways and decreasing the production of IL-1β, IL-6 and IL-8
(18). A study by Pan et al
(19) revealed that H2S
was able to inhibit NF-κB activation, increase the level of heme
oxygenase 1 (HO-1) and reduce TNF-α-induced inflammation.
The present study revealed that endogenous
H2S levels decreased in the renal tissue of rabbits with
urinary-derived sepsis. Throughout treatment with NaHS, the levels
of NF-κB and TNF-α decreased, the level of IL-10 increased, and
kidney function improved. These results suggest that NaHS protected
the kidneys during urinary-derived sepsis by inhibiting NF-κB,
reducing the level of TNF-α and increasing the level of IL-10,
thereby reducing inflammation in urinary-derived sepsis. The
protective effect of 8.4 μmol/kg NaHS was more significant than
that of 2.8 μmol/kg NaHS, indicating that NaHS protected the
kidneys from injury in a dose-dependent manner.
Acknowledgements
This study was supported by Joint Funds from the
Natural Science Foundation of Hunan Province (No. 13JJ9009).
References
|
1
|
Farshad S, Ranjbar R, Japoni A, et al:
Microbial susceptibility, virulence factors, and plasmid profiles
of uropathogenic Escherichia coli strains isolated from
children in Jahrom, Iran. Arch Iran Med. 15:312–316.
2012.PubMed/NCBI
|
|
2
|
Bagshaw SM, George C and Bellomo R: ANZICS
Database Management Committee: Early acute kidney injury and
sepsis: a multicentre evaluation. Crit Care. 12:R472008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadik NA, Mohamed WA and Ahmed MI: The
association of receptor of advanced glycated end products and
inflammatory mediators contributes to endothelial dysfunction in a
prospective study of acute kidney injury patients with sepsis. Mol
Cell Biochem. 359:73–81. 2012. View Article : Google Scholar
|
|
4
|
Souza AC, Volpini RA, Shimizu MH, et al:
Erythropoietin prevents sepsis-related acute kidney injury in rats
by inhibiting NF-κB and upregulating endothelial nitric oxide
synthase. Am J Physiol Renal Physiol. 302:F1045–1054.
2012.PubMed/NCBI
|
|
5
|
Makkonen J, Pietiläinen KH, Rissanen A, et
al: Genetic factors contribute to variation in serum alanine
aminotransferase activity independent of obesity and alcohol: a
study in monozygotic and dizygotic twins. J Hepatol. 50:1035–1042.
2009. View Article : Google Scholar
|
|
6
|
Kimura H: Hydrogen sulfide: its
production, release and functions. Amino Acids. 41:113–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calvert JW, Coetzee WA and Lefer DJ: Novel
insights into hydrogen sulfide-mediated cytoprotection. Antioxid
Redox Signal. 12:1203–1217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou CF and Tang XQ: Hydrogen sulfide and
nervous system regulation. Chin Med J (Engl). 124:3576–3582.
2011.PubMed/NCBI
|
|
9
|
Bełtowski J: Hypoxia in the renal medulla:
implications for hydrogen sulfide signaling. J Pharmacol Exp Ther.
334:358–363. 2010.PubMed/NCBI
|
|
10
|
Pan LL, Liu XH, Gong QH, et al: Hydrogen
sulfide attenuated tumor necrosis factor-α-induced inflammatory
signaling and dysfunction in vascular endothelial cells. PLoS One.
6:e197662011.
|
|
11
|
Tokuda K, Kida K, Marutani E, et al:
Inhaled hydrogen sulfide prevents endotoxin-induced systemic
inflammation and improves survival by altering sulfide metabolism
in mice. Antioxid Redox Signal. 17:11–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ang SF, Sio SW, Moochhala SM, et al:
Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E
metabolite in sepsis-evoked acute lung injury via transient
receptor potential vanilloid type 1 channel activation. J Immunol.
187:4778–4787. 2011. View Article : Google Scholar
|
|
13
|
Chattopadhyay M, Kodela R, Nath N, et al:
Hydrogen sulfide-releasing aspirin suppresses NF-κB signaling in
estrogen receptor negative breast cancer cells in vitro and
in vivo. Biochem Pharmacol. 83:723–732. 2012.PubMed/NCBI
|
|
14
|
Sen N, Paul BD, Gadalla MM, et al:
Hydrogen sulfide-linked sulfhydration of NF-κB mediates its
antiapoptotic actions. Mol Cell. 45:13–24. 2012.PubMed/NCBI
|
|
15
|
Chen X, Wang Y, Luo H, et al: Ulinastatin
reduces urinary sepsis-related inflammation by upregulating IL-10
and downregulating TNF α levels. Mol Med Rep. 8:29–34.
2013.PubMed/NCBI
|
|
16
|
Payen D, Lukaszewicz AC, Legrand M, et al:
A multicenter study of acute kidney injury in severe sepsis and
septic shock: association with inflammatory phenotype and HLA
genotype. PLoS One. 7:e358382012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Salto-Tellez M, Tan CH, et al:
GYY4137, a novel hydrogen sulfide-releasing molecule, protects
against endotoxic shock in the rat. Free Radic Biol Med.
47:103–113. 2009. View Article : Google Scholar
|
|
18
|
Yang C, Yang Z, Zhang M, et al: Hydrogen
sulfide protects against chemical hypoxia-induced cytotoxicity and
inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2
pathway. PLoS One. 6:e219712011.PubMed/NCBI
|
|
19
|
Pan LL, Liu XH, Gong QH, et al: Hydrogen
sulfide attenuated tumor necrosis factor-α-induced inflammatory
signaling and dysfunction in vascular endothelial cells. PLoS One.
6:e197662011.
|