Introduction
The preservation of vascular endothelial integrity
is a dynamic process involving the injury and repair of endothelial
cells. Vascular endothelial injury and dysfunction is considered to
be the first and crucial step in the development of cardiovascular
disease (1–3). Restoring the injured endothelium
quickly and effectively may prevent or reverse disease progression.
Previous studies have indicated that endothelial progenitor cells
(EPCs), which are the precursors of endothelial cells, play a
pivotal role in vascular homeostasis and endothelial repair, and
have been implicated in vasculogenesis or neovascularization
associated with cardiovascular disease (4–7).
Under conditions of tissue ischemia or injury, EPCs may be
mobilized from the bone marrow into the peripheral blood (5), where they migrate to sites of injured
endothelium and differentiate into endothelial cells (6). This stimulates angiogenesis and
endothelial cell repair (7). A
number of studies have indicated that patients with cardiovascular
disease, including pulmonary hypertension (PH) and coronary artery
disease, exhibit a reduced EPC number and function (8,9).
Thus, the upregulation of EPCs may improve the outcome of disease
(9), and the EPC number and
function may be used as predictive factors for the severity and
outcome of cardiovascular disease.
EPCs were first isolated from human peripheral blood
using magnetic bead selection (10). Succeeding studies revealed that
blood from the umbilical cord or bone marrow contain a more
abundant source of EPCs (11,12).
To obtain and expand EPCs in vitro for functional
assessment, mononuclear cells are isolated from the peripheral
blood or bone marrow by Ficoll density gradient centrifugation for
further culture. However, in order to avoid cell loss and the
influence of the procedure on EPC function, the manipulation of
this technique is elaborate and difficult in small experimental
animals. Thus, a more convenient and effective method may be
preferable. A previous study indicated that whole bone marrow cell
(WBMC) culture may expand the quantity of mesenchymal stem cells
with normal functions (13). Thus,
it was hypothesized that a similar technique may also be suitable
for EPCs.
To assess the feasibility of WBMC cultures for
expanding EPCs in small experimental animals, C57BL/6 mice (age,
3–4 weeks) were used as the experimental animals in the present
study. WBMCs were isolated from the femora and tibiae and cultured
in endothelial cell growth medium-2 (EGM-2; Lonza Systems, Basel,
Switzerland). The growth, phenotype and function of the EPCs were
assessed in vitro and in vivo.
Materials and methods
Animals
C57BL/6 male mice, either aged 3–4 weeks with a body
weight of 9.47±0.76 g or aged 6–8 weeks with a body weight of
23.35±2.74 g, were purchased from the Capital Medical University
(SCXK2005-0006; Beijing, China) and housed under specific
pathogen-free conditions in the Department of Laboratory Animals at
the Capital Medical University. All animal studies and procedures
were approved by the Institutional Animal Care and Use Committee of
the Capital Medical University.
WBMC culture for EPCs
A total of 12 mice (age, 3–4 weeks) were randomly
divided into two groups (n=6 per group) for the WBMC and bone
marrow mononuclear cell (BMMC) cultures. The mice were sacrificed
by decapitation and the femora and tibiae of the mice were
harvested aseptically. WBMCs were flushed using phosphate-buffered
saline (PBS) in a 1-ml syringe with a 25-gauge needle. Following
collection of the cells by centrifugation at 300 × g for 5 min, the
WBMCs were resuspended at a density of 107 cells/ml in
EGM-2. The medium included endothelial cell basal medium-2 (EBM-2),
supplemented with 5% fetal bovine serum (FBS), basic fibroblast
growth factor, vascular endothelial growth factor, epidermal growth
factor, recombinant insulin-like growth factor, ascorbic acid and
gentamicin/amphotericin-B. The cell suspension was plated into
24-well culture plates (1 ml/well), pre-coated with fibronectin
(Sigma-Aldrich, St. Louis, MO, USA), and incubated at 37°C in a
humidified environment with 5% carbon dioxide (CO2).
Half of the medium was renewed every day for the first three days,
and then replaced every two days thereafter.
Following seven days of culture, the cells were
incubated for 4 h with 10 μg/ml
1,10-dioctadecyl-3,3,30,30-tetramethylindocarbocyanine
perchlorate-labeled acetylated low-density lipoprotein (DiI-acLDL;
Molecular Probes®; Invitrogen Life Technologies,
Carlsbad, CA, USA). Next, the cells were fixed with 4%
paraformaldehyde and incubated for 1 h with 10 μg/ml fluorescein
isothiocyanate (FITC)-conjugated lectin (Sigma-Aldrich). After
washing extensively with PBS, the cells were examined by confocal
laser scanning microscopy to identify the EPCs, as described
previously (12). Furthermore, the
cell-surface markers, cluster of differentiation (CD)34 and fetal
liver kinase 1 (Flk-1), were analyzed by flow cytometry (BD
Biosciences, Franklin Lanes, NJ, USA). Briefly, the cells were
detached using 0.25% trypsin and 0.04% ethylenediaminetetraacetate
acid (EDTA), and resuspended in 200 μl PBS. The cells were
incubated for 20 min in the dark at 4°C with 2 μl FITC-conjugated
rat monoclonal antibody against mouse Flk-1 (BD Biosciences) and 5
μl phycoerythrin (PE)-conjugated rat monoclonal antibody against
mouse CD34 (BD Biosciences). The samples were centrifuged at room
temperature for 5 min at 300 × g, resuspended in 500 μl PBS and
evaluated using flow cytometry. Furthermore, a BMMC culture for the
EPCs was performed as described previously (12), to be used as the control.
RNA isolation, reverse transcription and
quantitative polymerase chain reaction (qPCR)
Total RNA of the EPCs was extracted using an
E.Z.N.A.® Total RNA kit I (Omega Bio-Tek, Norcross, GA,
USA) and reverse transcribed to cDNA using an M-MLV Reverse
Transcriptase kit (Invitrogen Life Technologies). The qPCR analysis
for endothelial nitric oxide synthase (eNOS) was performed with
Platinum® SYBR® Green qPCR SuperMix-UDG w/ROX
(Invitrogen Life Technologies) with an Applied
Biosystems® 7300 Real-Time PCR system (Invitrogen Life
Technologies). The PCR primer sequences were as follows: GAPDH
forward, 5′-AGCCTCGTCCCGTAGACAAAA-3′ and reverse,
5′-TGGCAACAATCTCCACTTTGC-3′; eNOS forward,
5′-TGTCACTATGGCAACCAGCGT-3′ and reverse,
5′-GCGCAATGTGAGTCCGAAAA-3′.
EPC functions in vitro
To assess the functions of EPCs in vitro,
EPCs obtained from the bone marrow following seven days of culture
were separated using 0.25% trypsin and 0.04% EDTA. Functions,
including vascular network formation, proliferation, adhesion and
migration, were assessed as described previously (12,14).
With regard to the vascular network formation ability in
vitro, the EPCs were suspended in EGM-2 at a density of
105 cells/ml (100 μl/well), cultured in 96-well
round-bottomed plates that had been pre-coated with
Matrigel™ (BD Biosciences) and incubated at 37°C in a
humidified environment with 5% CO2. Images were captured
using a light microscope (14). To
determine the proliferation ability, 200 μl EPC suspension at a
density of 105 cells/ml in EGM-2 was cultured in 96-well
round-bottomed plates for 24 h. Subsequently, 20 μl thiazolyl blue
(5 g/l) was supplemented for the 4-h culture. At the end of
culture, the supernatant was discarded and 100 μl dimethyl
sulfoxide was added for a 10-min incubation. Absorbance was
measured at a wavelength of 490 nm. To measure the adhesion
ability, 500 μl EPC suspension, at a density of 105
cells/ml in EGM-2, was plated in 24-well plates that had been
pre-coated with fibronectin and cultured for 30 min. Following
washing three times with PBS, the attached EPCs were counted. To
determine the migration ability, 100 μl EPC suspension, at a
density of 5×105 cells/ml in EBM-2 + 0.5% FBS, were
plated in the upper chamber of a modified Boyden chamber (8 μm pore
size; Corning, Tewksbury, MA, USA), and placed in 24-well plates
containing 600 μl EGM-2/well. Following incubation for 24 h, the
cells on the lower membrane were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet. The migrated EPCs were
counted.
EPC function in vivo
To identify the functions of the EPCs derived from
the WBMC culture, a mouse model of PH was established to assess the
therapeutic effects of EPC transplantation. A total of 24 mice
(age, 6–8 weeks) were randomly divided into four groups (n=6 per
group): PH model, WBMCs-EPCs transplantation, BMMCs-EPCs
transplantation and the control. PH was induced by a single
subcutaneous injection of monocrotaline (MCT; 60 mg/kg;
Sigma-Aldrich) (9), while PBS was
administered to the mice in the control group. At day five
following the injection of MCT, the EPCs were transplanted into the
mice. For transplantation, the EPCs were detached using 0.25%
trypsin and 0.04% EDTA, and resuspended in 1 ml PBS at a density of
106 cells/ml. The cells were subsequently transplanted
into the mice via the caudal vein. To further observe the
distribution of transplanted EPCs in the lungs, two additional mice
received transplantion of EPCs pre-labeled with
CellTracker™ CM-DiI (Invitrogen Life Technologies) using
the same procedure. At day 16 following the transplantation of
EPCs, the mice were sacrificed by decapitation and the lung tissue
was removed. The tissue was fixed in 10% paraformaldehyde for 24 h
at room temperature and embedded in paraffin. Serial 5-μm sections
were stained with hematoxylin and eosin, and observed under a
microscope. The medial wall thickness (WT) of the pulmonary
arterioles was assessed according to a method described in a
previous study (9).
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used to
conduct the statistical analyses. Differences were compared using
the independent t-test or one-way analysis of variance, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
Growth of EPCs in vitro
Following two days of culture, a small ‘blood
island’ surrounded by spindle-like cells was observed in the WBMC
culture system (Fig. 1A). After
seven days of culture, the cells adhered to the base of the culture
plate and exhibited a spindle-like appearance (Fig. 1B). Using immunofluorescence
labeling with FITC-lectin and DiI-acLDL, the majority of cells were
shown to be positive for FITC-lectin and DiI-acLDL using confocal
laser scanning microscopy (Fig.
1C–E); thus, were defined as EPCs undergoing differentiation
(12). There was no difference in
the number of double fluorescence positive cells between the WBMC
and BMMC culture systems (92.8±8.7 vs. 93.2±9.3%; P>0.05;
Fig. 1I). Furthermore, the
expression levels of CD34 and Flk-1, as analyzed by flow cytometry,
revealed no statistically significant differences between the WBMC
and BMMC culture systems (CD34, 78.3±6.7 vs. 79.2±8.6%; Flk-1,
87.6±7.3 vs. 89.5±8.5%; double expression of CD34 and Flk-1,
75.8±5.4 vs. 76.3±6.2%; P>0.05; Fig. 1G, H and J–L). In the subsequent
culture of approximately one week, endothelial colony-forming
cells, which originated from the EPCs (15), gradually appeared (Fig. 1F). These cells exhibited a typical
cobblestone morphology and proliferated rapidly. The first colony
was detected following 15.2±3.8 days of culture in the WBMC culture
system and no statistically significant differences were identified
when compared with the BMMC culture system (14.7±4.5 days;
P>0.05; Fig. 1M).
 | Figure 1Whole bone marrow cell (WBMC) culture
for endothelial progenitor cells (EPCs) and their identification
in vitro. (A) Following two days of culture, a small ‘blood
island’ surrounded by spindle-like cells was observed. (B)
Following seven days of culture, cells exhibited a spindle-like
appearance. The majority of the cells were immunopositive for (C)
1,10-dioctadecyl-3,3,30,30-tetramethylindocarbocyanine
perchlorate-labeled acetylated low-density lipoprotein (DiI-acLDL)
and (D) FITC-conjugated Flk-1. (E) Cells positive for FITC-lectin
and DiI-acLDL were recognized as EPCs undergoing differentiation.
(F) Following cell culture for ~2 weeks, endothelial colony-forming
cells, which originated from the EPCs, appeared and exhibited a
typical cobblestone morphology. Cells expressed CD34 and Flk-1 in
the (G) WBMC and (H) bone marrow mononuclear cell culture systems.
FITC, fluorescein isothiocyanate; Flk-1, fetal liver kinase 1; CD,
cluster of differentiation. WBMC culture for EPCs and their
identification in vitro. Comparison between WMBC-EPCs and
BMMC-EPCs with regard to the expression levels of (I) double
fluorescence positive cells for DiI-acLDL and FITC-Flk-1, (J) CD34,
(K) Flk-1 and (L) double fluorescence positive cells for CD34 and
Flk-1, and (M) the time that the first endothelial colony-forming
cells appeared. Data (n=6) are presented as the mean ± standard
deviation.*P>0.05. WBMC, whole bone marrow cell;
BBMC, bone marrow mononuclear cell; EPCs, endothelial progenitor
cells; DiI-acLDL,
1,10-dioctadecyl-3,3,30,30-tetramethylindocarbocyanine
perchlorate-labeled acetylated low-density lipoprotein; FITC,
fluorescein isothiocyanate; Flk-1, fetal liver kinase 1; CD,
cluster of differentiation. |
Expression levels of eNOS
qPCR analysis demonstrated that the EPCs derived
from the WBMC culture system expressed similar levels of eNOS to
those from the BMMC culture system (ΔΔCt 2.68±0.43 vs.
2.52±0.47; P>0.05; Fig.
2C).
EPC functions in vitro
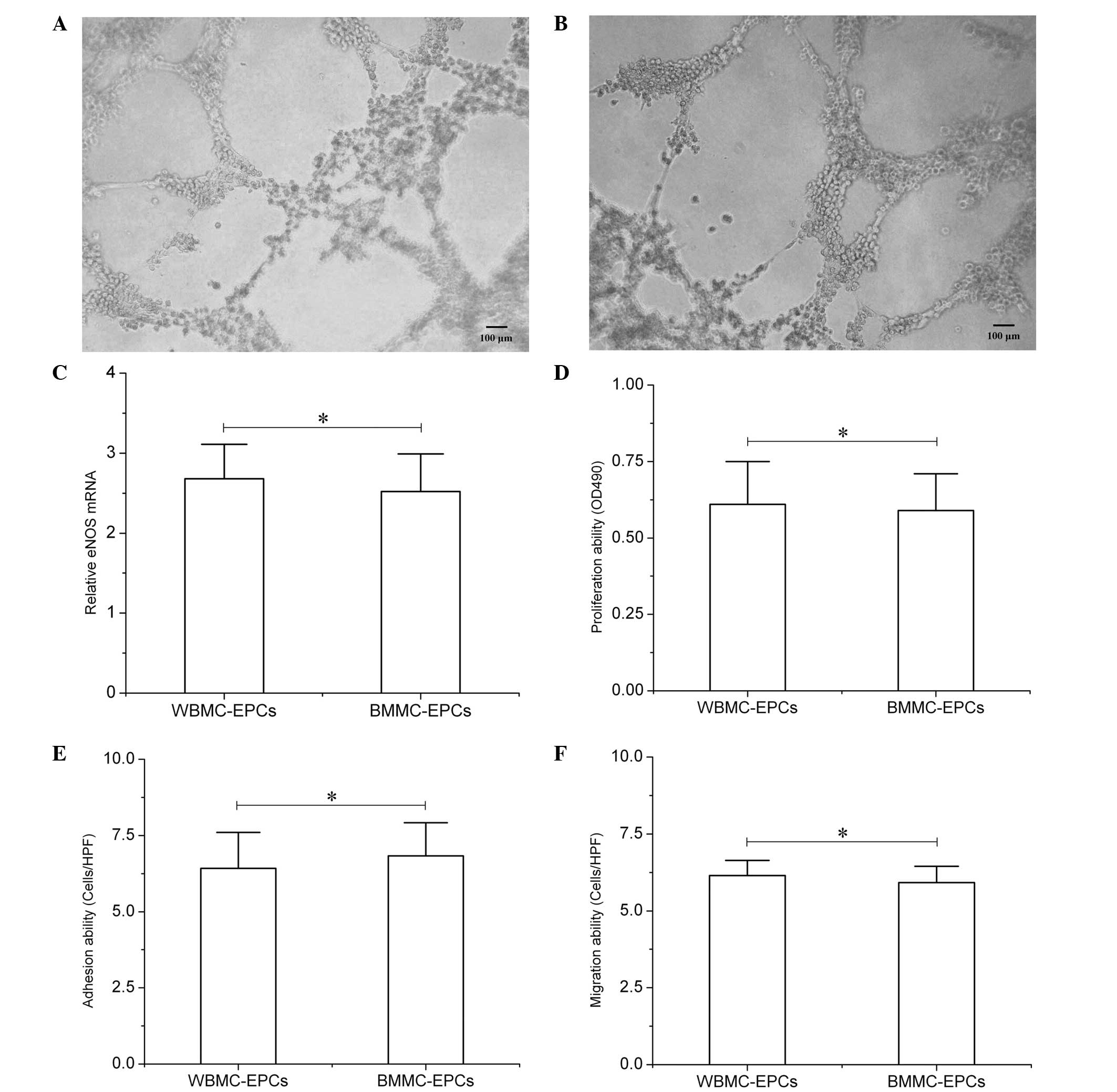
To assess the vascular network formation ability,
the EPCs derived from the BMMC culture system were plated in
96-well round-bottomed plates that had been pre-coated with
Matrigel. Following ~5 h of culture, the EPCs revealed a marked
morphological change, with the cells connecting to each other to
form two-dimensional networks (Fig.
2A). The same vascular network formation was also observed in
the EPCs derived from the WBMC culture system (Fig. 2B). Furthermore, analyses of EPC
proliferation, adhesion and migration ability in vitro were
performed to assess the functional status of the EPCs. The results
did not reveal any statistically significant differences between
the two culture systems [WBMC culture system: Proliferation optical
density (OD)490, 0.61±0.14; adhesion, 6.42±1.18
cells/high power field (HPF); migration, 6.15±0.49 cells/HPF; BMMC
culture system: Proliferation OD490, 0.59±0.12;
adhesion, 6.83±1.09 cells/HPF; migration, 5.92±0.53 cells/HPF;
P>0.05; Fig. 2D–F).
EPC functions in vivo
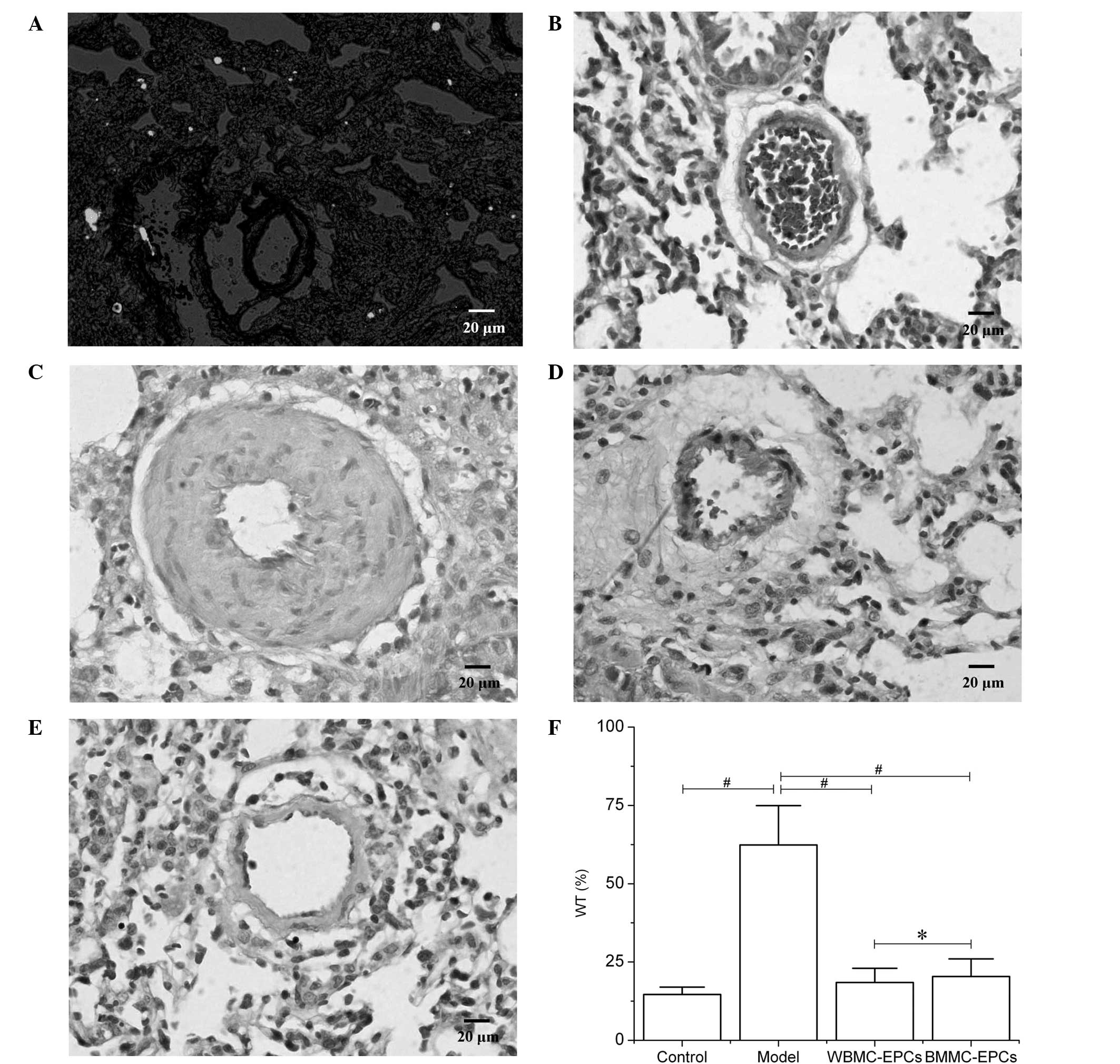
A mouse model of PH was used to further assess the
therapeutic effects of EPCs in vivo. The results revealed
that two days following the transplantation of CM-DiI-labeled EPCs,
an abundance of cells positive for CM-DiI were observed in the
lungs (Fig. 3A). At day 21
following the subcutaneous injection of MCT, histological
examination of the lungs indicated that medial hypertrophy of the
pulmonary muscular arterioles was evident in the PH model group
(Fig. 3C). In addition, the WT
increased in the model group when compared with the control group
(62.37±12.58 vs. 14.62±2.35%; P<0.01; Fig. 3F). However, transplantation of EPCs
derived from the BMMC and WBMC culture systems improved the medial
hypertrophy of the pulmonary muscular arterioles (Fig. 3D and E). The WT in the two
transplantation groups decreased when compared with the model group
and no statistically significant differences were observed between
the two transplantation groups (18.46±4.52 vs. 20.37±5.63%;
P>0.05; Fig. 3F).
Discussion
The present study demonstrated that EPCs were easily
obtained from WBMCs cultured in vitro. The cells exhibited
similar growth and biological characteristics to EPCs derived from
a traditional BMMC culture system. Thus, the EPCs were able to
simultaneously bind to lectin and cause phagocytosis of acLDLs. In
addition, the cells exhibited high expression levels of CD34 and
Flk-1, possessed similar functional properties to BMMC-derived EPCs
in vitro and improved the outcome of pulmonary vascular
disease when transplanted into a mouse model. These characteristics
indicate that the WBMC culture system is a more convenient and
effective method of obtaining EPCs, with the advantage of a
simplified procedure. Thus, this strategy may allow an improved
understanding of the EPC status in a number of vascular diseases,
particularly in small experimental animal models, and an improved
evaluation of the severity and outcome of the disease.
Numerous studies have indicated that changes to the
EPC number and function participate in the pathogenesis of
cardiovascular disease. Analysis of the EPC number and function
during the early stages of disease may improve the evaluation of
disease severity and outcome (8,9). The
outcome of the disease may be improved via the upregulation of EPCs
(9,16,17).
Thus, studies investigating EPCs in cardiovascular disease have
attracted increasing attention. Directly isolating a sufficient
number of EPCs from the peripheral blood for functional analysis is
difficult due to the low abundance of EPCs. Thus, EPC expansion in
a specific culture medium is commonly required. Since the first
isolation of EPCs from the peripheral blood (10), EPCs have subsequently been obtained
from umbilical cord blood, bone marrow, liver, tunica externa and
adipose tissue (11,12,18–21).
Among the diverse sources of EPCs, peripheral blood remains the
most common option for further mononuclear cell isolation and
culture. However, this procedure is not easily performed in small
experimental animals, as the EPC content of the peripheral blood is
low. Thus, bone marrow, which is a reservoir of circulating EPCs,
is normally used as a substitute. However, the procedure remains
difficult in small experimental animals at a very young age since
the EPC content in their bone marrow cells is too low for adequate
collection. Furthermore, the procedure of density gradient
centrifugation further aggravates cell loss. Thus, a more
convenient and effective method is required.
The procedure of WBMC culture for mesenchymal stem
cells (13) indicates that a
similar technique may also be suitable for EPCs. Based on this
hypothesis, in the present study, WBMCs segregated from mice aged
3–4 weeks-old and with a low body weight, were cultured in
endothelial cell growth medium to observe the growth of EPCs. The
results revealed that following seven days of culture, there was an
abundance of EPCs with a spindle-like appearance. The majority of
these EPCs were undergoing differentiation, with the
characteristics of simultaneous lectin binding and acLDL
phagocytosis, as well as high expression levels of specific
membrane molecules, including CD34 and Flk-1. Compared with the
traditional BMMC culture procedure, there were no differences with
regard to EPC characteristics, indicating the feasibility of WBMC
culture for expanding EPCs in small experimental animals.
Furthermore, in the subsequent culture period, endothelial
colony-forming cells gradually appeared and there was no difference
in the time point of the first colony appearance between the two
culture systems. Endothelial colony-forming cells are considered to
be important descendants of EPCs that play a crucial role in the
repair process of injured vascular endothelium (15). The similarity in growth
characteristics indicates that the EPCs derived from the WBMC
culture system may play a similar function in vascular endothelial
repair to those from the BMMC culture system. To further confirm
this hypothesis, EPC functions in vitro, including vascular
network formation, proliferation, adhesion and migration, were
assessed. The results did not reveal any differences between the
two culture systems. Furthermore, previous studies have indicated
that nitric oxide, an important regulatory molecule primarily
produced by EPCs or endothelial cells, may play a pivotal role in
cardioprotective effects (22,23).
Thus, the normal expression level of eNOS is a crucial index for
the EPC functional status. The present study demonstrated that
there was no statistically significant difference in the expression
levels of eNOS between EPCs derived from the WBMC and the
traditional BMMC culture systems. Therefore, the WBMC culture
system is a convenient and reliable procedure for the in
vitro study of EPCs.
To further analyze the in vivo function of
EPCs derived from the WBMC culture system, a mouse model of PH was
established to assess the therapeutic effect of EPC
transplantation. Previous studies have confirmed that EPC
transplantation is effective in preventing the progression of PH in
laboratory animal models (24,25).
Thus, the use of a PH model in the current study was a reliable
method for assessing EPC function in vivo. The results
revealed that the transplanted EPCs were able to successfully
migrate to the lungs, improving the medial hypertrophy of the
pulmonary muscular arterioles in the MCT-induced mouse model of PH.
There were no differences in the WT between the transplantation of
EPCs derived from the WBMC and the traditional BMMC culture
systems, indicating that the EPCs obtained by the WBMC culture
procedure possessed the same functional characteristics as the EPCs
obtained by the BMMC culture system. Thus, the WBMC culture system
is suitable for assessing the in vivo function of EPCs.
In conclusion, the results of the present study
demonstrated that the WBMC culture system is a convenient and
effective method of obtaining and expanding EPCs, with the
advantage of a simplified procedure and normal function. However,
the reliability of this technique remains controversial and
requires further investigation for the future treatment of
cardiovascular disease, particularly in small experimental animal
models.
References
|
1
|
Lin CP, Chen YH, Leu HB, Lin SJ, Chen YL,
Huang SL and Chen JW: Anti-inflammatory strategies for
homocysteine-related cardiovascular disease. Front Biosci (Landmark
Ed). 14:3836–3845. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaragoza C, Gomez-Guerrero C,
Martin-Ventura JL, Blanco-Colio L, et al: Animal models of
cardiovascular diseases. J Biomed Biotechnol. 2011:4978412011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu JT, Chen YL, Chen WC, Chen HY, Lin YW,
Wang SH, et al: Role of pigment epithelium-derived factor in
stem/progenitor cell-associated neovascularization. J Biomed
Biotechnol. 2012:8712722012.PubMed/NCBI
|
|
4
|
Umemura T and Higashi Y: Endothelial
progenitor cells: therapeutic target for cardiovascular diseases. J
Pharmacol Sci. 108:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aicher A, Zeiher AM and Dimmeler S:
Mobilizing endothelial progenitor cells. Hypertension. 45:321–325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirton JP and Xu Q: Endothelial precursors
in vascular repair. Microvasc Res. 79:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reinders ME, Rabelink TJ and Briscoe DM:
Angiogenesis and endothelial cell repair in renal disease and
allograft rejection. J Am Soc Nephrol. 17:932–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werner N, Kosiol S, Schiegl T, Ahlers P,
Walenta K, Link A, Böhm M and Nickenig G: Circulating endothelial
progenitor cells and cardiovascular outcomes. N Engl J Med.
353:999–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JF, DU ZD, Chen Z, Han ZC and He ZX:
Granulocyte colony-stimulating factor attenuates
monocrotaline-induced pulmonary hypertension by upregulating
endothelial progenitor cells via the nitric oxide system. Exp Ther
Med. 6:1402–1408. 2013.
|
|
10
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, et al: Isolation of putative progenitor
endothelial cells for angiogenesis. Science. 275:964–967. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Proctor SJ and Dickinson AM: The
effect of cryopreservation on umbilical cord blood endothelial
progenitor cell differentiation. Cell Transplant. 17:1423–1428.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JF, Du ZD, Chen Z, Lu DX, Li L, Guan
YQ and Wan SG: Endothelial progenitor cell down-regulation in a
mouse model of Kawasaki disease. Chin Med J (Engl). 125:496–501.
2012.PubMed/NCBI
|
|
13
|
Gunetti M, Tomasi S, Giammò A, Boido M,
Rustichelli D, Mareschi K, Errichiello E, et al: Myogenic potential
of whole bone marrow mesenchymal stem cells in vitro and in vivo
for usage in urinary incontinence. PLoS One. 7:e455382012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reinisch A, Hofmann NA, Obenauf AC,
Kashofer K, Rohde E, Schallmoser K, et al: Humanized large-scale
expanded endothelial colony-forming cells function in vitro and in
vivo. Blood. 113:6716–6725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ingram DA, Mead LE, Tanaka H, Meade V,
Fenoglio A, Mortell K, et al: Identification of a novel hierarchy
of endothelial progenitor cells using human peripheral and
umbilical cord blood. Blood. 104:2752–2760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu
Z, Hicklin DJ, Shimada K, et al: Granulocyte colony-stimulating
factor promotes neovascularization by releasing vascular
endothelial growth factor from neutrophils. FASEB J. 19:2005–2007.
2005.
|
|
17
|
Maruyama H, Watanabe S, Kimura T, Liang J,
Nagasawa T, Onodera M, Aonuma K and Yamaguchi I: Granulocyte
colony-stimulating factor prevents progression of
monocrotaline-induced pulmonary arterial hypertension in rats. Circ
J. 71:138–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: mobilization, differentiation, and homing.
Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar
|
|
20
|
Hu Y, Zhang Z, Torsney E, Afzal AR,
Davison F, Metzler B and Xu Q: Abundant progenitor cells in the
adventitia contribute to atherosclerosis of vein grafts in
ApoE-deficient mice. J Clin Invest. 113:1258–1265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Planat-Benard V, Silvestre JS, Cousin B,
André M, Nibbelink M, Tamarat R, Clergue M, et al: Plasticity of
human adipose lineage cells toward endothelial cells: physiological
and therapeutic perspectives. Circulation. 109:656–663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueda K, Takano H, Hasegawa H, Niitsuma Y,
Qin Y, Ohtsuka M and Komuro I: Granulocyte colony stimulating
factor directly inhibits myocardial ischemia-reperfusion injury
through Akt-endothelial NO synthase pathway. Arterioscler Thromb
Vasc Biol. 26:e108–e113. 2006. View Article : Google Scholar
|
|
23
|
Shimada K, Okabe TA, Mikami Y, Hattori M,
Fujita M and Kishimoto C: Therapy with granulocyte
colony-stimulating factor in the chronic stage, but not in the
acute stage, improves experimental autoimmune myocarditis in rats
via nitric oxide. J Mol Cell Cardiol. 49:469–481. 2010. View Article : Google Scholar
|
|
24
|
Zhao YD, Courtman DW, Deng Y, Kuqathasan
L, Zhang Q and Stewart DJ: Rescue of monocrotaline-induced
pulmonary arterial hypertension using bone marrow-derived
endothelial-like progenitor cells: efficacy of combined cell and
eNOS gene therapy in established disease. Circ Res. 96:442–450.
2005. View Article : Google Scholar
|
|
25
|
Nagaya N, Kangawa K, Kanda M, Uematsu M,
Horio T, Fukuyama N, Hino J, Harada-Shiba M, et al: Hybrid
cell-gene therapy for pulmonary hypertension based on phagocytosing
action of endothelial progenitor cells. Circulation. 108:889–895.
2003. View Article : Google Scholar : PubMed/NCBI
|