Introduction
Giant cell tumors of the bone (GCTB) are a rare
osteolytic primary bone neoplasm that occur in young adults,
characterized by the presence of numerous osteoclasts (1). The majority of GCTBs arise in the
metaphyseal-epiphyseal area and are most commonly found in the
distal femur, proximal tibia and distal radius (2,3).
GCTBs are rarely found in the vertebrae, and the majority of
vertebrae GCTBs are located in the sacrum, usually the upper sacrum
(4). The sacrum is the fourth most
common site, accounting for 1.7–8.2% of cases (5–7).
GCTBs also occur in the mobile spine; however, this location only
accounts for 2–4% of cases (8,9). In
all locations, the neoplasm most commonly occurs between the ages
of 20 and 45 years, affecting males and females with equal
frequency (9). The pathogenesis
and histogenesis remain unclear, since there is no predictable
value of histology for the clinical outcome. Although classified as
benign, GCTBs are aggressive and recur locally in ≤50% of cases. Up
to 5% of GCTBs metastasize to the lungs and spontaneous
transformation to a high-grade malignancy occurs in 1–3% of
patients (1,10). Recent advances have been made with
regard to the pathogenesis of GCTB. The osteoclast differentiation
factor, receptor activator of nuclear factor-κB ligand (RANKL), was
shown to be highly expressed in stromal cells within GCTBs
(11–13), leading to the prediction of the
neoplastic ‘driver’ role of stromal cells. In addition, RANKL
appears to be critical to the pathogenesis of GCTB. However, more
driving factors underlying the strong tumor growth capacity of GCTB
require investigation.
Hypoxia has become one of the key issues in the
study of tumor physiology. A group of transcription factors have
been reported to be involved in the regulation of genes responsible
for the metabolic changes under hypoxia (14,15).
A pivotal component of these factors is hypoxia-inducible factor
(HIF)-1, a heterodimer consisting of an oxygen-sensitive HIF-1α
subunit and a constitutively expressed HIF-1β subunit (16). HIF-1 binds to a conserved DNA
consensus on the promoter region of target genes, known as
hypoxia-responsive elements (17–19).
HIF induces a vast array of gene products, which control cellular
processes that are crucial for hypoxic adaptation (20). HIF-1 is a key regulator of vascular
endothelial growth factor (VEGF) and other angiogenic factors
(21,22), which play crucial roles in the
growth and progression of solid tumors (23–26).
When GCTBs are involved with the sacrum, patients
present with localized lower-back pain that may radiate to one or
both lower limbs. Neurological symptoms, if present, are often
subtle (27). Vague abdominal
discomfort, early satiety and a change in bowel/bladder habits are
possible. Due to the generally insidious onset of symptoms in
patients with sacral GCTBs, the tumor usually grows to a large size
prior to diagnosis; thus, may undergo hypoxia. However, little is
known with regard to the expression of HIF-1α and VEGF in GCTBs,
particularly in sacral GCTBs.
In the present study, the expression levels of
HIF-1α and VEGF were quantitatively determined in 22 sacral GCTB
samples using immunohistochemical methods. In addition, to provide
novel indicators for the degree of malignancy and the prognosis of
GCTBs, correlations between HIF-1α or VEGF expression with the
invasion and recurrence were assessed.
Materials and methods
Tissue samples and ethical approval
Use of the 22 sacral GCTB samples was approved by
the Internal Review Board of the Department of Orthopedics, First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China). The samples were surgical resections from patients with
sacral GCTBs registered in the aforementioned hospital between
January 1998 and December 2012. A total of 10 normal sacral samples
were used as a control. All the tissue samples used for
immunohistochemical staining were formalin-fixed and
paraffin-embedded following surgical resection, while the tissue
samples used for immunoblotting were frozen at −80°C immediately
after surgical resection. Hematoxylin-eosin slides, pathology
reports, other medical records and treatment procedures were
reviewed and standardized to ensure study homogeneity. The
specimens used in the study were human sacral GCTB specimens
removed by surgery as part of the cancer treatment. Prior to
surgery, the patients granted consent for the use of the excised
cancer tissue in medical or scientific research.
Immunohistochemical staining
GCTB sample slides were deparaffinized by heating at
55°C for 30 min, washed with xylene and rehydrated serially in 100,
90 and 70% ethanol and phosphate-buffered saline (PBS). Antigen
retrieval was performed by heating for 20 min at a constant
temperature of 98°C in 10 mM sodium citrate (pH 6.0; 250 ml), and
endogenous peroxidase activity was inhibited with 0.3% hydrogen
peroxide for 20 min. Rabbit polyclonal antibodies against HIF-1α
and VEGF (Abcam, Cambridge, UK), and a mouse monoclonal antibody
against CD34 (Abcam) were used to perform the immunohistochemical
assay. The antibodies were diluted 1:50 with goat serum separately.
Following incubation with the primary antibodies at room
temperature for 1 h, the sections were washed with PBS three times
for 5 min each, and incubated with a goat anti-rabbit/mouse
immunoglobulin G horseradish peroxidase (HRP)-conjugated secondary
antibody (Abcam). Following an additional three washes,
3,3′-diaminobenzidine HRP substrate (Abcam) was added for 1 min and
counterstained with Mayer’s hematoxylin. The samples were then
dehydrated and sealed with cover slips. Negative controls were
performed by omitting the primary antibodies. A semi-quantitative
system was used to analyze the level of antigen expression:
Immunoreactivity was scored as either negative (0), focal (1+;
<25% positive cells), moderate (2+; 25–50% positive cells) or
diffuse (3+; >50% positive cells). The intensity of
immunostaining was rated as follows: None (0), weak (+1), moderate
(+2) and intense (+3). The immunohistochemistry score was defined
as the sum of the aforementioned two scores. Specimens were
analyzed by two observers and scored following a consensus by the
observers (28).
Semi-quantitative immunoblotting
Tissue samples for immunoblotting were placed in 10
ml ice-cold isolation solution, containing 250 mM sucrose, 10 mM
triethanolamine (Sigma-Aldrich, St. Louis, MO, USA), 1 mg/ml
leupeptin (Sigma-Aldrich) and 0.1 mg/ml phenylmethylsulfonyl
fluoride (Sigma-Aldrich) titrated to pH 7.6, and the mixture was
homogenized at 13,600 × g with three strokes for 15 sec using a
tissue homogenizer (PowerGun 125; Thermo Fisher Scientific,
Pittsburgh, PA, USA). Following homogenization, the total protein
concentration was measured using a bicinchoninic acid protein assay
reagent kit (Thermo Fisher Scientific, Rockford, IL, USA), which
was adjusted to 2 mg/ml with isolation solution. Equal amounts of
protein and sample buffer were separated using 12% gradient
SDS-PAGE, stained with Coomassie Brilliant Blue and transferred to
a polyvinylidene fluoride membrane. The blotted membrane was
blocked with Tris-buffered saline containing 5% milk, and incubated
with HIF-1α, VEGF or CD34 rabbit polyclonal antibodies (1:500;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by
incubation with a HRP-coupled secondary antibody (1:1000; Cell
Signaling Technology, Inc., Danvers, MA, USA). The proteins were
detected using enhanced chemiluminescence (Thermo Fisher
Scientific). All immunoblots were representative of at least three
independent experiments.
Calculation of the tumor microvessel
density (MVD)
At a low-power field (magnification, ×200), the
tumor tissue sections were screened and five areas with the most
intense neovascularization (hot spots) were selected. Microvessel
counts of these areas were performed at a high-power field
(magnification, ×400). Any CD34 positive endothelial cell or
endothelial cell cluster clearly separated from adjacent
microvessels, tumor cells and connective tissue elements were
considered to be single countable microvessels. Branching
structures were counted as one, unless there was a break in the
continuity of the vessel, in which case the structure was counted
as two distinct vessels. Three fields per tumor section were
counted in the areas that appeared to contain the greatest number
of microvessels on scanning at low magnification. MVD was defined
as the mean score from the five fields.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). HIF-1α, VEGF and CD34
expression levels between the two groups were analyzed using the
Student’s t-test. Correlations between HIF-1α or VEGF expression
and the MVD value were analyzed using Spearman’s rank correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression levels of HIF-1α and VEGF
in the sacral GCTB samples
HIF-1α and VEGF expression levels were determined
using immunohistochemical staining in the sacral GCTB samples. The
results demonstrated that HIF-1α was primarily located in the
cytoplasm of the mononuclear stromal cells and rarely located in
the tumor cell nuclei, as shown in Fig. 1A and B. VEGF was also located in
the cytoplasm of mononuclear stromal cells or multinucleated giant
cells. Immunohistochemical staining revealed that more
HIF-1α-positive cells were observed in sacral GCTB samples compared
with normal sacral tissues (Fig. 1C
and D). In addition, more mononuclear stromal cells and
multinucleated giant cells were VEGF-positive in sacral GCTB
specimens compared with normal tissues.
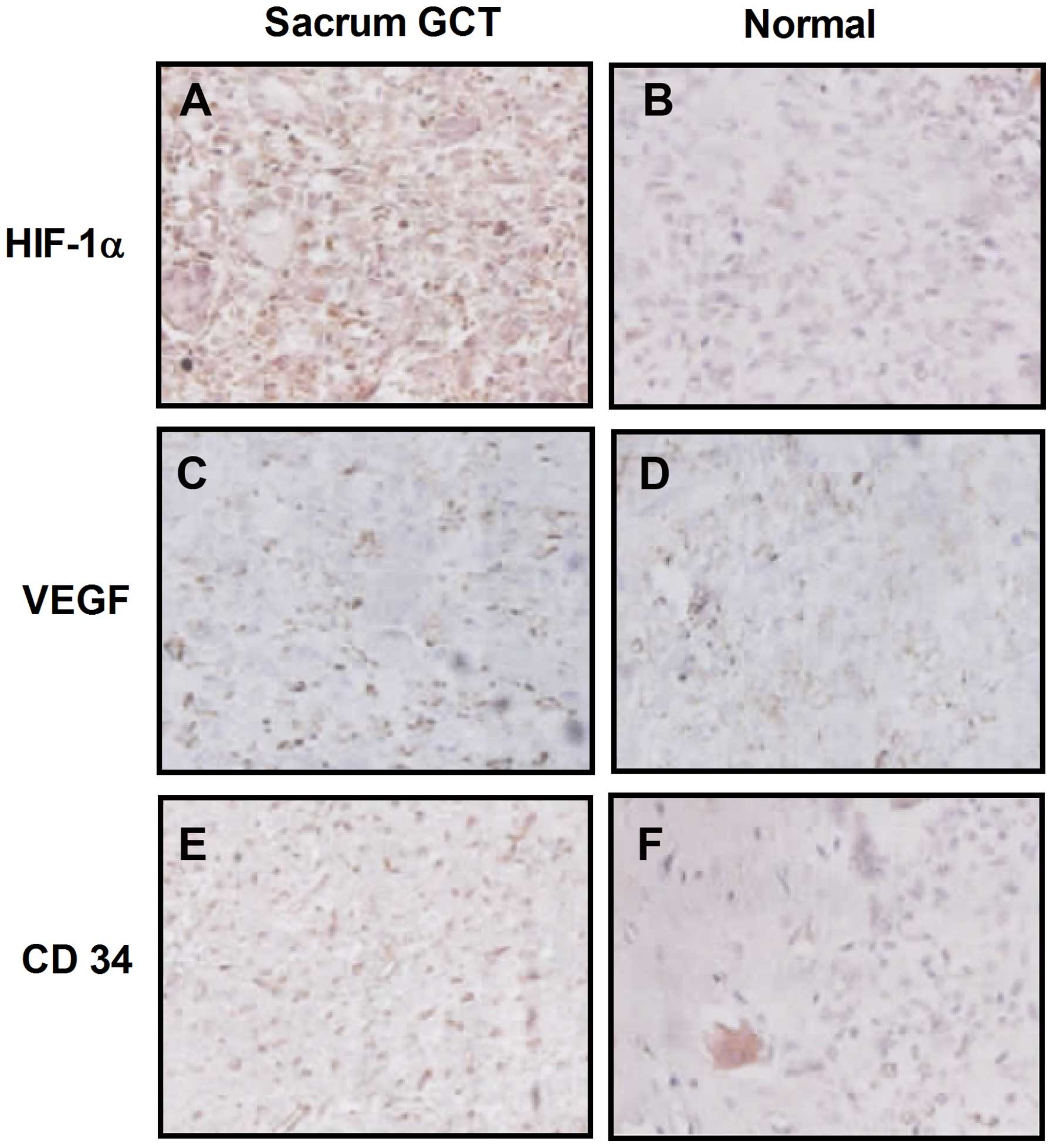 | Figure 1Representative immunohistochemical
staining for HIF-1α, VEGF and CD34 expression in (A, C and E)
sacral GCT samples, respectively, and (B, D and F) normal sacral
tissues, respectively (magnification, ×200; light microscopy).
HIF-1α, hypoxia-inducible factor 1α; VEGF, vascular endothelial
growth factor; GCT, giant cell tumor. |
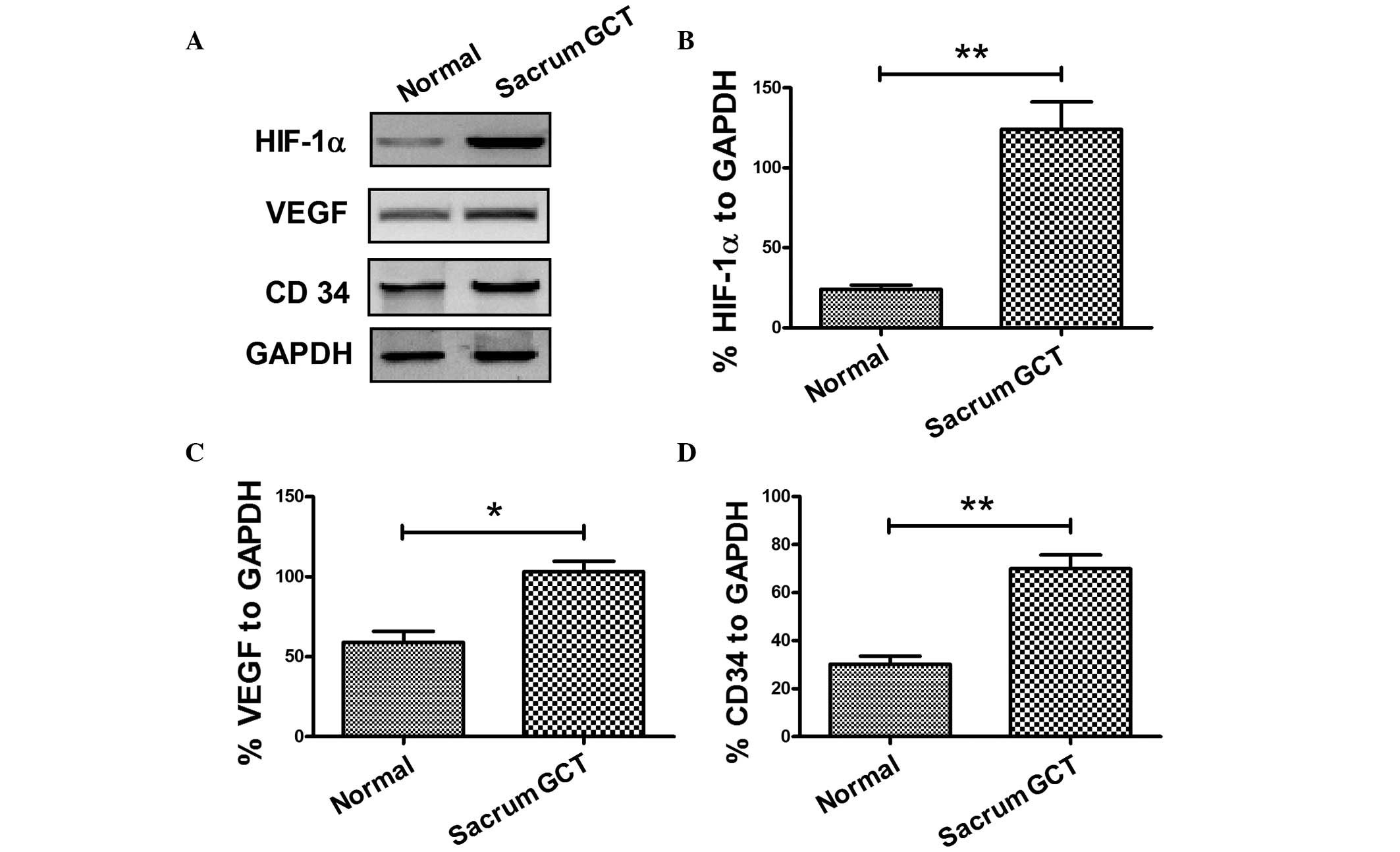
To confirm the expression of HIF-1α and VEGF in
sacral GCTB samples, the protein expression levels were analyzed
using western blot analysis. As shown in Fig. 2, HIF-1α and VEGF were overexpressed
in sacral GCTB specimens when compared with the normal sacral
tissues. The mean relative expression of HIF-1α against GAPDH in
the sacral GCTB specimens was 124.00±17.20%, while the mean value
in the normal sacral tissues was 24.20±2.60% (Fig. 2A and B), which produced a 95%
confidence interval (CI) of 59.68–139.9 and a statistically
significant difference (P<0.01). The mean relative expression of
VEGF in the sacral GCTB specimens was 103.00±6.63%, while the mean
value in the normal sacral tissues was 59.00±6.78% (Fig. 2A and C), which had a 95% CI of
22.12–65.88 and a statistically significant difference (P<0.05).
Therefore, HIF-1α and VEGF were overexpressed in the sacral GCTB
samples as compared with the normal sacral tissues.
Determination of the tumor MVD value in
the sacral GCTB samples
HIF-1 and VEGF are known to have key regulatory
roles in the vascular endothelial growth and angiogenesis of tumors
(21–26). To determine the effect of HIF-1α
and VEGF on sacral GCTB angiogenesis, the MVD of sacral GCTB
samples was determined using immunohistochemical staining and
western blot analysis, from which the correlations between HIF-1α
or VEGF expression with the intratumoral MVD value were analyzed.
Firstly, the expression of CD34, a molecular marker of vascular
endothelial cells, was determined and the MVD value in the sacral
GCTB samples was calculated. As shown in Fig. 1E, CD34 expression was primarily
located in the vascular endothelial cells surrounding the sacral
GCTB. Western blot analysis was also performed to determine CD34
expression in the sacral GCTB samples. The mean relative expression
of CD34 in the sacral GCTB specimens was 70.00±5.70%, while the
mean value in the normal sacral tissues was 30.20±3.26% (Fig. 2A and D), which produced a 95% CI of
24.65–54.95 and a statistically significant difference (P<0.01).
Thus, overexpression of CD34 was confirmed in the sacral GCTB
samples.
Overexpression of HIF-1α or VEGF is
correlated with a high MVD value in the sacral GCTB samples
To investigate the correlation between HIF-1α and
VEGF expression levels and the MVD of the sacral GCTB samples, the
immunohistochemical staining results of HIF-1α and VEGF expression
were semi-quantitatively interpreted by calculating the
immunohistochemistry score of each molecule in each sacral GCTB
sample. The mean immunohistochemistry score of HIF-1α in the sacral
GCTB samples was 4.53±0.40 compared with 2.58±0.39 in the control
samples (P=0.03); the mean immunohistochemistry score of VEGF in
the sacral GCTB specimens was 3.36±0.31 compared with 1.95±0.30 in
the control samples (P=0.049). The correlation between HIF-1α and
VEGF expression in the sacral GCTB samples with the MVD value was
then determined. As shown in Fig.
3, a positive correlation was observed between the HIF-1α or
VEGF expression and the MVD value in the GCTB samples (Fig. 3D and E). Therefore, overexpression
of HIF-1α or VEGF was confirmed to be correlated with a high MVD
value in the sacral GCTB samples.
Discussion
GCTB is a benign neoplasm characterized by the
presence of mononuclear cells, together with multinucleated giant
cells that resemble normal osteoclasts (29). GCTB may exhibit considerable local
aggressiveness, often associated with intense osteolytic activity.
In a small number of cases, GCTBs may develop lung metastases,
indicating that specific tumors may acquire an aggressive phenotype
(30). HIF mediates the
pathophysiological response to hypoxia in ischemic diseases,
including various types of cancer (31). Knowles et al (32) first described HIF expression in
GCTB and human osteoclasts in culture and in vivo. The
authors proposed a model whereby HIF-dependent VEGF secretion from
stromal cells mediates paracrine effects to stimulate osteoclast
differentiation (32).
In the present study, a total of 22 sacral GCTB
samples were collected, and HIF-1α and VEGF expression levels were
determined using immunohistochemical staining and western blot
analysis. Significantly high levels of HIF-1α and VEGF expression
were confirmed in the GCTB samples using the two methods. In
addition, the expression of CD34, an MVD marker, was determined
using immunohistochemical staining and western blot analysis. CD34
was also found to be significantly overexpressed in the sacral GCTB
samples. Furthermore, Spearman’s rank correlation analysis
demonstrated significant correlations between HIF-1α or VEGF
expression and the MVD value in the GCTB samples.
HIF-mediated induction of VEGF is known to have a
number of effects, including the recruitment of monocytes and
osteoclasts in GCTBs (33,
34) and supporting osteoclast
survival and activity (35). Local
hypoxia has been shown to correlate with HIF-1α expression in
osteoblasts, local VEGF production and increased numbers of
tartrate-resistant acid phosphatase-positive osteoclasts (36). However, hypoxia and growth factors
function indirectly on osteoclasts via the promotion of paracrine
secretion of osteoblast-derived VEGF. Osteoclasts in culture and
osteoclast-like giant cells in vivo were shown to express
HIF-1α and HIF-2α, which further induced the expression of VEGF and
other downstream genes.
The results of the present study indicated that
hypoxia and subsequent induced growth factors within the bone
microenvironment may contribute to the initiation and development
of GCTB. Local hypoxia may promote the production of HIF and VEGF
(37,38) and (pre)-osteoclast recruitment.
Within established tumors, hypoxia comprises chronic
diffusion-limited hypoxia due to inadequate tumor vasculature
(39), acute hypoxia due to
perfusion fluctuation (40) and
metabolic hypoxia due to fluctuations in the rate of oxygen
utilization (41). Despite GCTB
being highly vascular, it is likely that HIF expression within
these tumors is driven by hypoxia, as well as microenvironmental
growth factors.
In conclusion, the present study demonstrated that
HIF-1α, VEGF and CD34 are overexpressed in sacral GCTBs using
immunohistochemistry and western blot analysis. The MVD value,
calculated using CD34 expression, was also shown to be upregulated
in sacral GCTBs, and significantly correlate with HIF-1α or VEGF
expression in these GCTB samples. Therefore, the present study has
provided novel indicators for the tumor growth capacity of
GCTBs.
References
|
1
|
Anract P, De Pinieux G, Cottias P,
Pouillart P, Forest M and Tomeno B: Malignant giant-cell tumours of
bone. Clinico-pathological types and prognosis: a review of 29
cases. Int Orthop. 22:19–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gamberi G, Serra M, Ragazzini P, et al:
Identification of markers of possible prognostic value in 57 giant
cell tumors of bone. Oncol Rep. 10:351–356. 2003.PubMed/NCBI
|
|
3
|
Miszczyk L, Wydmański J and Spindel J:
Efficacy of radiotherapy for giant cell tumor of bone: given either
postoperatively or as sole treatment. Int J Radiat Oncol Biol Phys.
49:1239–1242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turcotte RE, Sim FH and Unni KK: Giant
cell tumor of the sacrum. Clin Orthop Relat Res. 291:215–221.
1993.PubMed/NCBI
|
|
5
|
Turcotte RE: Giant cell tumor of bone.
Orthop Clin North Am. 37:35–51. 2006. View Article : Google Scholar
|
|
6
|
Sung HW, Shu WP, Wang HM, Yuai SY and Tsai
YB: Surgical treatment of primary tumors of the sacrum. Clin Orthop
Relat Res. 215:91–98. 1987.PubMed/NCBI
|
|
7
|
Guo W, Ji T, Tang X and Yang Y: Outcome of
conservative surgery for giant cell tumor of the sacrum. Spine
(Phila Pa 1976). 34:1025–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonald DJ, Sim FH, McLeod RA and Dahlin
DC: Giant-cell tumor of bone. J Bone Joint Surg Am. 68:235–242.
1986.PubMed/NCBI
|
|
9
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.PubMed/NCBI
|
|
10
|
Olivera P, Perez E, Ortega A, et al:
Estrogen receptor expression in giant cell tumors of the bone. Hum
Pathol. 33:165–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan T, Atkins GJ, Trivett MK, et al:
Molecular profiling of giant cell tumor of bone and the
osteoclastic localization of ligand for receptor activator of
nuclear factor kappaB. Am J Pathol. 167:117–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skubitz KM, Cheng EY, Clohisy DR, Thompson
RC and Skubitz AP: Gene expression in giant-cell tumors. J Lab Clin
Med. 144:193–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atkins GJ, Haynes DR, Graves SE, et al:
Expression of osteoclast differentiation signals by stromal
elements of giant cell tumors. J Bone Miner Res. 15:640–649. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cummins EP and Taylor CT:
Hypoxia-responsive transcription factors. Pflugers Arch.
450:363–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Licausi F, Weits DA, Pant BD, Scheible WR,
Geigenberger P and van Dongen JT: Hypoxia responsive gene
expression is mediated by various subsets of transcription factors
and miRNAs that are determined by the actual oxygen availability.
New Phytol. 190:442–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
17
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006.PubMed/NCBI
|
|
20
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and hif-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.
|
|
22
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
23
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pralhad T, Madhusudan S and Rajendrakumar
K: Concept, mechanisms and therapeutics of angiogenesis in cancer
and other diseases. J Pharm Pharmacol. 55:1045–1053. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taylor RM, Kashima TG, Knowles HJ and
Athanasou NA: VEGF, FLT3 ligand, PIGF and HGF can substitute for
M-CSF to induce human osteoclast formation: Implications for giant
cell tumour pathobiology. Lab Invest. 92:1398–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saikia B, Goel A and Gupta SK: Fine-needle
aspiration cytologic diagnosis of giant-cell tumor of the sacrum
presenting as a rectal mass: A case report. Diagn Cytopathol.
24:39–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hara H, Akisue T, Fujimoto T, et al:
Expression of vegf and its receptors and angiogenesis in bone and
soft tissue tumors. Anticancer Res. 26:4307–4311. 2006.PubMed/NCBI
|
|
29
|
Athanasou NA, Bliss E, Gatter KC, Heryet
A, Woods CG and McGee JO: An immunohistological study of giant-cell
tumour of bone: evidence for an osteoclast origin of the giant
cells. J Pathol. 147:153–158. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bertoni F, Present D and Enneking WF:
Giant-cell tumor of bone with pulmonary metastases. J Bone Joint
Surg Am. 67:890–900. 1985.PubMed/NCBI
|
|
31
|
Brahimi-Horn MC and Pouysségur J:
Harnessing the hypoxia-inducible factor in cancer and ischemic
disease. Biochem Pharmacol. 73:450–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knowles HJ and Athanasou NA:
Hypoxia-inducible factor is expressed in giant cell tumour of bone
and mediates paracrine effects of hypoxia on monocyte-osteoclast
differentiation via induction of VEGF. J Pathol. 215:56–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barleon B, Sozzani S, Zhou D, Weich HA,
Mantovani A and Marmé D: Migration of human monocytes in response
to vascular endothelial growth factor (VEGF) is mediated via the
VEGF receptor flt-1. Blood. 87:3336–3343. 1996.PubMed/NCBI
|
|
34
|
Engsig MT, Chen QJ, Vu TH, et al: Matrix
metalloproteinase 9 and vascular endothelial growth factor are
essential for osteoclast recruitment into developing long bones. J
Cell Biol. 151:879–889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aldridge SE, Lennard TW, Williams JR and
Birch MA: Vascular endothelial growth factor receptors in
osteoclast differentiation and function. Biochem Biophys Res
Commun. 335:793–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mori S, Akagi M, Kikuyama A, Yasuda Y and
Hamanishi C: Axial shortening during distraction osteogenesis leads
to enhanced bone formation in a rabbit model through the
HIF-1alpha/vascular endothelial growth factor system. J Orthop Res.
24:653–663. 2006. View Article : Google Scholar
|
|
37
|
Zheng MH, Xu J, Robbins P, et al: Gene
expression of vascular endothelial growth factor in giant cell
tumors of bone. Hum Pathol. 31:804–812. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumta SM, Huang L, Cheng YY, Chow LT, Lee
KM and Zheng MH: Expression of VEGF and MMP-9 in giant cell tumor
of bone and other osteolytic lesions. Life Sci. 73:1427–1436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brizel DM, Rosner GL, Prosnitz LR and
Dewhirst MW: Patterns and variability of tumor oxygenation in human
soft tissue sarcomas, cervical carcinomas, and lymph node
metastases. Int J Radiat Oncol Biol Phys. 32:1121–1125. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hill SA, Pigott KH, Saunders MI, et al:
Microregional blood flow in murine and human tumours assessed using
laser Doppler microprobes. Br J Cancer Suppl. 27:S260–S263.
1996.PubMed/NCBI
|
|
41
|
Cohen-Jonathan E, Evans SM, Koch CJ, et
al: The farnesyltransferase inhibitor L744,832 reduces hypoxia in
tumors expressing activated H-ras. Cancer Res. 61:2289–2293.
2001.PubMed/NCBI
|