Introduction
Gastric cancer is the most common malignant tumor of
the digestive system and it is the second most lethal cancer
worldwide (1). The prevalence of
gastric cancer differs regionally, and ~70% of cases occur in
developing countries (2–4). The human epidermal growth factor
receptor 2 (Her-2) is a member of the epithelial growth factor
receptor (EGFR) family, the amplification of which may induce the
overexpression of EGFR. Once it is bound to its ligand, Her-2 is
phosphorylated and its function as a tyrosine kinase is activated,
thus promoting cell proliferation (5). Park et al (6) demonstrated that Her-2 is an
independent prognostic factor of gastric cancer. Her-2 is usually
detected using immunohistochemistry (IHC) and fluorescence in
situ hybridization (FISH), which each have advantages but also
disadvantages. Therefore, the investigation of novel methods is
necessary. In the present study, 426 cases of gastric cancer, all
pathogenically confirmed, were collected. The clinical data were
retrospectively reviewed. Her-2 expression in tumor tissue was
examined using IHC, and the Her-2/neu gene expression was examined
by quantitative polymerase chain reaction (qPCR). The aim of this
study was to provide a novel method for the detection of Her-2/neu
gene in gastric cancer tissues.
Materials and methods
Samples
Data was collected from patients admitted to the
General Military Hospital of Beijing PLA (124 cases), the 281st
Hospital of the PLA (107 cases), Zhejiang Cancer Hospital (99
cases) and Weifang People’s Hospital (96 cases) between 2011 and
2013. Written informed consent was obtained from all patients prior
to their participation in the study. All patients were
pathologically diagnosed with gastric cancer and Her-2 protein
expression was detected using IHC. None of the patients received
preoperative treatment with chemotherapy, radiotherapy or
immunotherapy. Samples consisted of 424 cases of adenocarcinoma
(including papillary adenocarcinoma, tubular adenocarcinoma, mucous
adenocarcinoma and signet ring cell carcinoma), one case of gland
scale cancer and one case of squamous carcinoma. The patients
included 149 cases of intestinal type, 244 cases of diffuse type
and 33 cases of mixed/unknown. The ages of the patients ranged
between 27 and 84 years (median age, 59.2 years) and included 322
males and 104 females. According to the World Health Organization
criteria (7), there were 192
poorly differentiated, 161 moderately differentiated and 73 highly
differentiated cases. A total of 310 cases were observed with lymph
node metastasis and 116 cases without lymph node metastasis. There
were 93 cases in the cardia, 180 cases in the antrum and 153 cases
in the stomach body. The cancer was classified as stage I in 40
cases, stage II in 108 cases, stage III in 248 cases and stage IV
in 30 cases, respectively, according to the TNM Cancer Staging
System of the American Joint Committee of Cancer (8).
Materials and reagents
The DNA extraction kit was purchased from Qiagen
(Hilden, Germany) and a Her-2/neu FISH testing kit was obtained
from Beijing ACCB Biotech Ltd.(Beijing, China). The Mx3000P qPCR
system was obtained from Stratagene (La Jolla, CA, USA).
DNA extraction
DNA extraction was performed using the QIAamp DNA
FFPE Tissue kit (Qiagen). The tissue was sectioned into slices (10
μm). The concentration and purity of the DNA was then measured in
accordance with the manufacturer’s instructions.
qPCR
The PCR reaction volume (20 μl) included 0.3 μl Taq
DNA polymerase, 0.4 μl substrate dNTP, 2.4 μl Mg2+, 2.0
μl buffer and 3.0 μl DNA. The primers were obtained from the
real-time PCR kit used and their catalogue numbers were Q/HDYKB007.
The cycling conditions were as follows: 95°C for 5 min, followed by
45 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 45 sec,
for a total of 40 cycles.
3 analysis
Her-2 gene was amplified with dual-color FISH (Her-2
gene real-time PCR kit, Guangzhou LBP Medical Science Technology
Co., Ltd., Guangzhou, China) in accordance with the manufacturer’s
instructions. Briefly, hybridization buffer, a DNA probe and
purified water were centrifuged and then heated to 65°C overnight
in a water bath. Tissue sections (4 μm) were placed on slides and
immersed in a denaturing bath (2× SSC) for 5 min at 73°C, followed
by dehydration in increasing ethanol concentrations and then dried.
The slides were incubated with the probe at 42°C for 30 min. The
slides were then washed with 0.4× SSC/0.3% NP-40 for 2 min,
air-dried in the dark, counterstained with
4′,6-diamidino-2-phenylindole (DAPI) and covered with a cover-slip.
The slides were observed under an Olympus BX51 fluorescence
microscope (Shanghai Pooher Photoelectric Technology Co., Ltd.,
Shanghai, China) equipped with a digital camera. A cell was
considered to be amplified when a definite cluster of >10
signals for Her-2 was found. Known positive and negative cells were
used as controls for each FISH assay. Gene amplification was scored
when ≥20 cancer cell nuclei exhibited a Her-2/CEP17 ratio ≥2, or
when a Her-2 signal cluster was observed (9).
IHC scoring
The 4 μm thick tissue sections of malignant tumor
cells on the slides were stained with brown staining (Zymed
Corporation, Inc., San Francisco, CA, USA). Briefly, sections were
deparaffinized in xylene and rehydrated in grade alcohols. The
antigen retrieval was performed using the wet autoclaving method in
the presence of citrate buffer pH 6.0. The sections were incubated
overnight in primary antibody at a dilution of 1:100 in blocking
buffer at 4°C. The sections were stained using a Polin-2 plus
Polymer HRP Detection System(ZSBIO, Beijing, China). Strong brown
staining in the cell membrane of malignant tumor cells indicates
positivity in this staining method. The HercepTest™ Interpretation
Guide (10) was used to grade the
membrane staining. The staining was scored as negative (0) when no
membrane staining was observed or when membranes were stained in
≤10% of tumor cells, weakly positive (+) if the focal membrane was
stained in ≥10% of tumor cells, intermediately positive (++) if
complete membranes were weakly-moderately stained in ≥10% of tumor
cells and strongly positive (+++) if complete membranes were
intensely stained in ≥10% of tumor cells.
Statistical analysis
Data were calculated using SPSS software, version
19.0 (SPSS, Inc., Chicago, IL, USA). χ2 and Fisher’s
exact tests were used to test for an association between Her-2
amplification or protein overexpression and clinicopathological
parameters. The kappa test was used to measure the consistency.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Correlation between Her-2 and
clinicopathological parameters in gastric cancer measured using
IHC
Using IHC analysis, the rate of overexpression of
Her-2 in cancerous tissues was found to be 13.38% (57/426). The
overexpression of Her-2 was significantly correlated with the depth
of invasion, lymph node metastasis and TNM stage (P<0.05), and
no significant correlation was identified between the
overexpression of Her-2 and age, gender, tumor location,
differentiation degree and Lauren classification (P>0.05;
Table I).
 | Table IExpression of Her-2 measured using
immunohistochemistry in cancerous tissue and clinicopathological
parameters. |
Table I
Expression of Her-2 measured using
immunohistochemistry in cancerous tissue and clinicopathological
parameters.
| | Expression level of
Her-2 | | |
|---|
| |
| | |
|---|
| Clinical
features | Number of cases | ++/+++ | −/+ | χ2 | P-value |
|---|
| Age | | | | 2.054 | 0.152 |
| ≤60 years | 202 | 22 | 180 | | |
| >60 years | 224 | 35 | 189 | | |
| Gender | | | | 0.933 | 0.334 |
| Male | 322 | 46 | 276 | | |
| Female | 104 | 11 | 93 | | |
| Tumor location | | | | 1.523 | 0.467 |
| Cardia | 93 | 9 | 84 | | |
| Antrum | 180 | 27 | 153 | | |
| Gastric body | 153 | 21 | 132 | | |
| Differentiation
degree | | | | 4.304 | 0.116 |
| Poor | 192 | 25 | 167 | | |
| Moderate | 161 | 27 | 134 | | |
| High | 73 | 5 | 68 | | |
| Lauren
classification | | | | 5.047 | 0.080 |
| Intestinal type | 149 | 27 | 122 | | |
| Diffuse type | 244 | 25 | 219 | | |
| Mixed
type/unknown | 33 | 5 | 28 | | |
| Depth of
invasion | | | | 5.732 | 0.017 |
| Serosal
invasion-negative | 55 | 13 | 42 | | |
| Serosal
invasion-positive | 371 | 44 | 327 | | |
| Lymphatic
metastasis | | | | 5.782 | 0.016 |
| Yes | 310 | 49 | 261 | | |
| No | 116 | 8 | 108 | | |
| TNM stage | | | | 44.761 | <0.001 |
| I | 40 | 0 | 40 | | |
| II | 108 | 4 | 104 | | |
| III | 248 | 39 | 209 | | |
| IV | 30 | 14 | 16 | | |
Correlation between Her-2/neu expression
and clinicopathological parameters in gastric cancer analyzed using
qPCR
Using PCR analysis, the positive expression rate of
Her-2/neu in cancerous tissue was found to be 11.17% (46/412). The
expression of Her-2/neu was significantly correlated with the depth
of invasion, lymphatic metastasis and TNM stage (P<0.05), and no
significant correlation was identified between the positive
expression of Her-2/neu and age, gender, tumor location,
differentiation degree and Lauren classification (P>0.05;
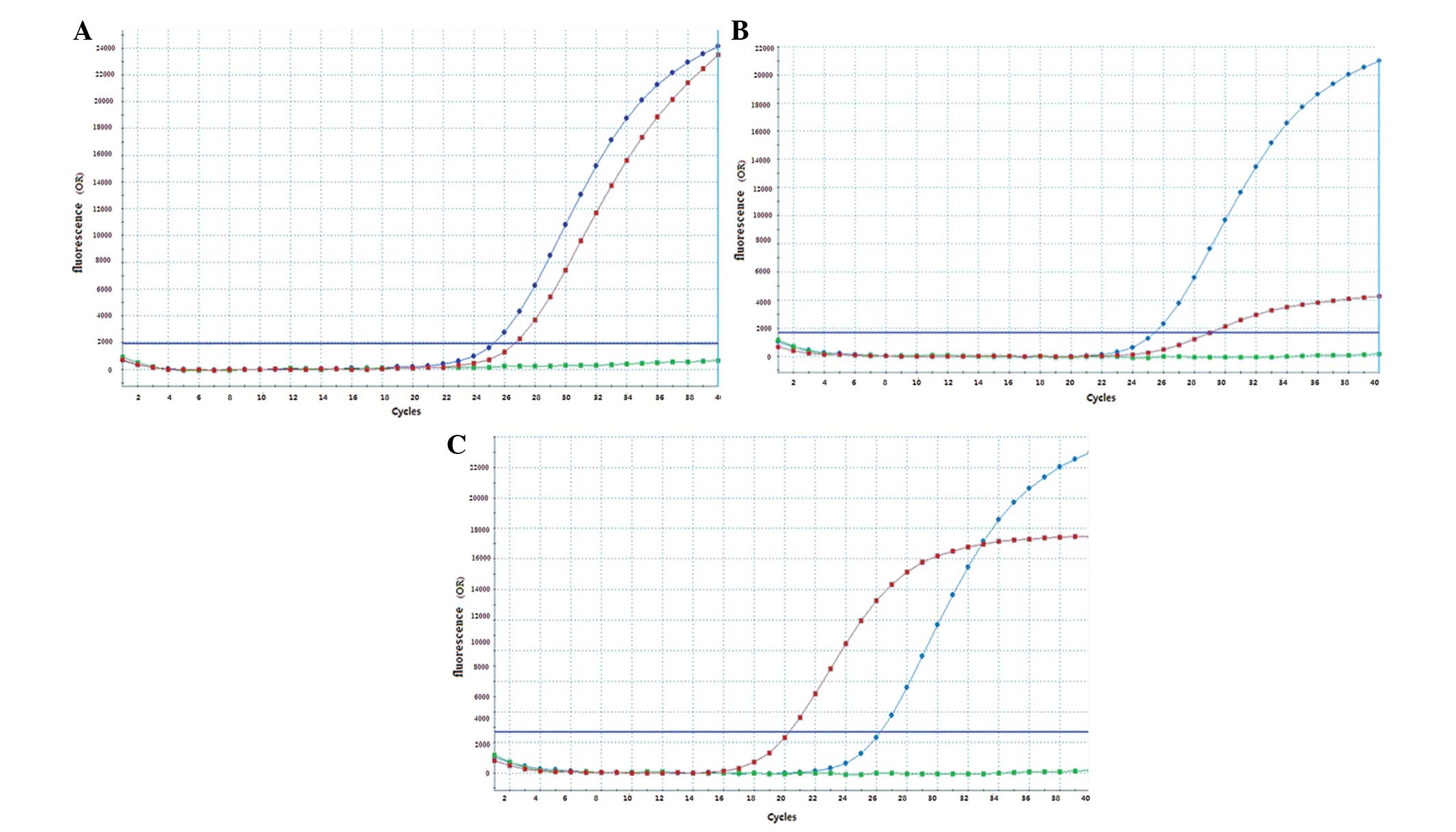
Fig. 1A–C; Table II).
 | Table IIExpression of Her-2/neu measured
using quantitative polymerase chain reaction in cancerous tissue
and clinicopathological parameters. |
Table II
Expression of Her-2/neu measured
using quantitative polymerase chain reaction in cancerous tissue
and clinicopathological parameters.
| | Expression of
Her-2/neu | | |
|---|
| |
| | |
|---|
| Clinical
features | Number of
cases | Positive
expression | Negative
expression | χ2 | P-value |
|---|
| Age | | | | 1.480 | 0.224 |
| ≤60 years | 196 | 18 | 178 | | |
| >60 years | 216 | 28 | 188 | | |
| Gender | | | | 1.794 | 0.180 |
| Male | 317 | 39 | 278 | | |
| Female | 95 | 7 | 88 | | |
| Tumor site | | | | 1.246 | 0.536 |
| Cardia | 89 | 7 | 82 | | |
| Antrum | 174 | 21 | 153 | | |
| Gastric body | 149 | 18 | 131 | | |
| Differentiation
degree | | | | 3.834 | 0.147 |
| Poor | 184 | 18 | 166 | | |
| Moderate | 155 | 23 | 132 | | |
| High | 73 | 5 | 68 | | |
| Lauren
classification | | | | 3.433 | 0.180 |
| Intestinal
type | 142 | 21 | 121 | | |
| Diffuse type | 240 | 21 | 219 | | |
| Mixed
type/unknown | 30 | 4 | 26 | | |
| Depth of
invasion | | | | 6.352 | 0.012 |
| Serosal
invasion-negative | 51 | 11 | 40 | | |
| Serosal
invasion-positive | 361 | 35 | 326 | | |
| Lymphatic
metastasis | | | | 4.285 | 0.038 |
| Yes | 296 | 39 | 257 | | |
| No | 116 | 7 | 109 | | |
| TNM stage | | | | 50.034 | <0.001 |
| I | 40 | 0 | 40 | | |
| II | 100 | 3 | 97 | | |
| III | 242 | 29 | 213 | | |
| IV | 30 | 14 | 16 | | |
Clinical pathological parameters of
gastric cancer cases with relative Her-2/neu gene copy number >2
and <4.5
There were 14 cases with a relative Her-2/neu gene
copy number >2 and <4.5, the definite judgment for which was
challenging. This range is used to determine whether the Her-2 gene
is amplification-positive or negative. A Her-2 gene copy number
>4.5 in the DNA sample that will be detected indicates an
amplification-positive Her-2 gene and a value <2 an
amplification-negative gene. Alternatively in situ
hybridization can be used to detect the Her-2 gene without the use
of the copy number. These comprised 9 females and 5 males. All
cases were TNM stage II–III, including 3 cases of IIa, 5 cases of
IIb, 4 cases of IIIa and 2 cases of IIIb. The expression of Her-2
was determined in 13 cases using IHC, and expression of Her-2/neu
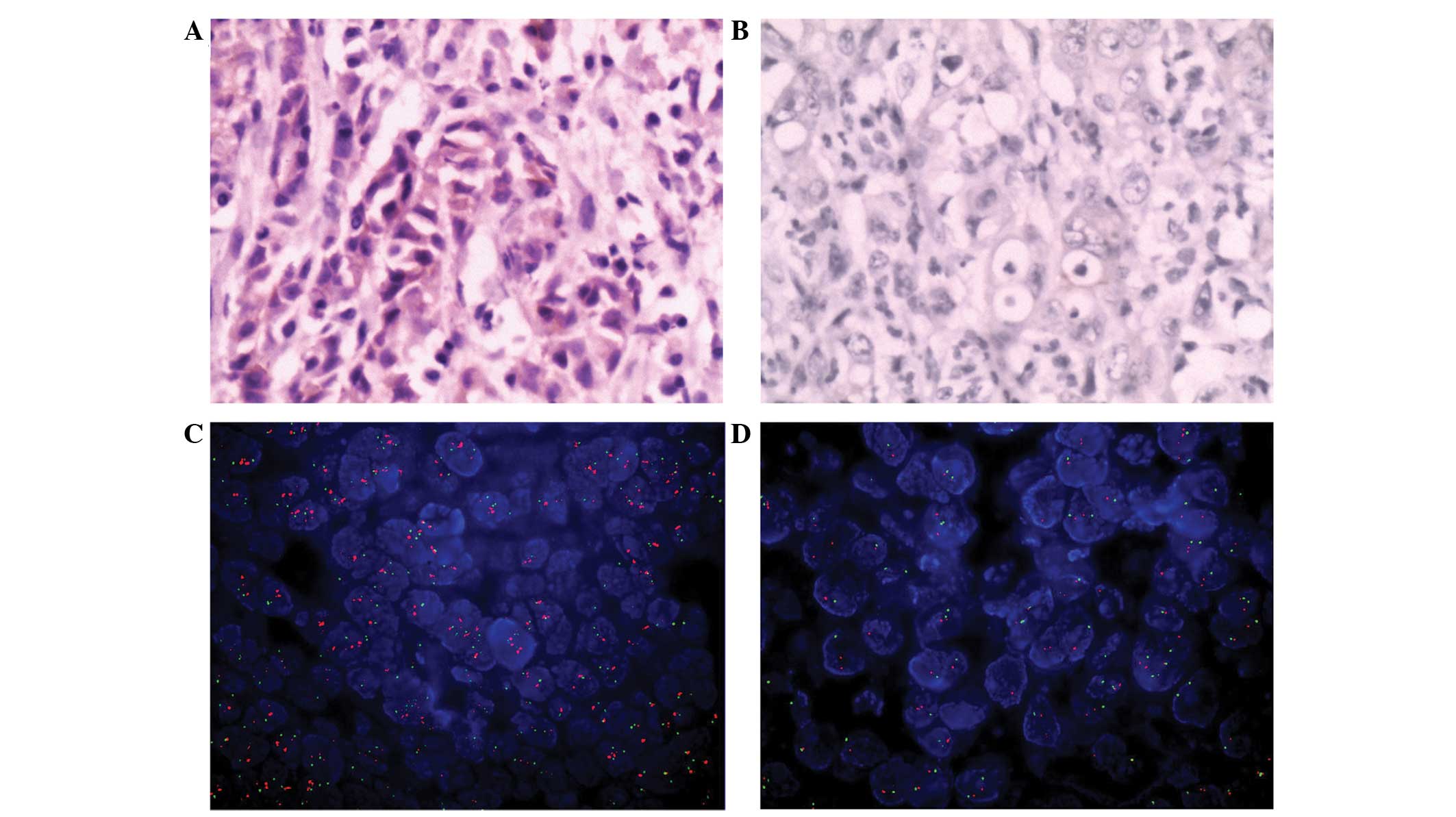
was determined in 8 cases using FISH (Table III; Fig 2A and B).
 | Table IIIClinicopathological parameters of
gastric cancer cases with relative Her-2/neu gene copy number >2
and <4.5. |
Table III
Clinicopathological parameters of
gastric cancer cases with relative Her-2/neu gene copy number >2
and <4.5.
| Case index | Gender | Lauren
classification | TNM stage | IHC | FISH |
|---|
| 1 | Female | Intestinal
type | IIIb | − | − |
| 2 | Female | Intestinal
type | IIIa | ++ | − |
| 3 | Male | Diffuse type | IIIb | +++ | + |
| 4 | Female | Intestinal
type | IIIa | ++ | − |
| 5 | Male | Diffuse type | IIb | + | − |
| 6 | Male | Intestinal
type | IIa | ++ | − |
| 7 | Female | Mixed type | IIa | ++ | + |
| 8 | Female | Intestinal
type | IIb | +++ | + |
| 9 | Female | Diffuse type | IIb | ++ | + |
| 10 | Male | Mixed type | IIb | ++ | − |
| 11 | Female | Mixed type | IIb | ++ | + |
| 12 | Female | Intestinal
type | IIIa | ++ | + |
| 13 | Male | Diffuse type | IIa | ++ | + |
| 14 | Female | Intestinal
type | IIIa | ++ | + |
Correlation between Her-2/neu gene
expression and the overexpression of Her-2 protein
Her-2 overexpression was observed in 53 cases
(12.86%) using IHC examination, Her-2/neu positive expression was
observed in 46 cases via qPCR. In the kappa test, good consistency
is indicated when 0.4<κ≤1 and poor consistency is indicated when
0≤κ≤0.4. For the studied population, there was a good consistency
of diagnosis between IHC and qPCR (κ=0.828; P<0.001; Table IV).
 | Table IVComparison between
immunohistochemistry and qPCR for diagnosis of gastic cancer. |
Table IV
Comparison between
immunohistochemistry and qPCR for diagnosis of gastic cancer.
| | qPCR | | |
|---|
| |
| | |
|---|
|
Immunohistochemistry | Number of
cases | Positive
expression | Negative
expression | P-value | κ-value |
|---|
| ++/+++ | 53 | 42 | 11 | <0.001 | 0.828 |
| −/+ | 359 | 4 | 355 | | |
Discussion
Her-2/neu is located on human chromosome 17q21 and
is a member of the epidermal growth factor receptor (EGFR) family.
Her-2/neu is a tyrosine kinase transmembrane glycoprotein with a
molecular weight of 185 kDa. It is involved in a variety of
biological activities of tumor cells, including cell proliferation,
adhesion, metastasis and differentiation (11).
The Her-2/neu gene has been demonstrated to be an
important prognostic indicator in patients with breast cancer, as a
prognostic factor for chemotherapy response and the target for
trastuzumab therapy (12). At the
annual meeting of the American Society of Clinical Oncology in
2009, Bang et al (13)
reported the results from the To-GA multi-center randomized
controlled clinical trial. This was the first clinical trial of
targeted therapy proven to prolong survival time of patients with
advanced gastric cancer, which opened a new chapter of targeted
therapy for advanced gastric cancer. It laid the foundation of
Her-2/neu gene detection in the diagnosis and treatment of gastric
cancer and supports the use of trastuzumab in gastric cancer
therapy. Based on the results from that trial, Herceptin
(trastuzumab, was approved for the treatment of advanced gastric
cancer in January 2010.
Previous studies have shown that >30% of human
tumors overexpress the Her-2/neu gene, including breast cancer,
ovarian cancer, endometrial cancer, fallopian tube cancer, stomach
cancer and prostate cancer. In breast cancer, its overexpression is
~20–40% (14), whilst gene or
protein expression of Her-2/neu in gastric cancer varies from 6 to
35% (15–18). Her-2/neu expression is affected by
numerous factors, including tumor location, histology and specimen
types.
The present study indicated that the rate of Her-2
protein expression analyzed using IHC was 13.38% (57/426) in
gastric cancer. In addition, Her-2 protein overexpression was found
to be significantly associated with the depth of tumor invasion
(P=0.017), lymph node metastasis (P=0.016) and TNM staging
(P<0.001), but not with age, gender, tumor location, the degree
of differentiation or Lauren classification (P>0.05). The
positive expression rate of Her-2/neu gene was 11.17% (46/412).
Similarly, its expression was significantly associated with the
depth of tumor invasion (P=0.012), lymph node metastasis (P=0.038)
and TNM staging (P<0.001), but was not found to be associated
with age, gender, tumor location, the degree of differentiation or
Lauren classification (P>0.05).
IHC is currently the most commonly used method for
Her-2 detection; however, FISH is also used. Although IHC is simple
and cheap, its results are influenced by tissue fixation, the
quality and origin of Her-2 antibody and the bias of the observer,
which results in poor sensitivity and specificity. FISH is
accurate, with a higher sensitivity and specificity; however, it is
complex, time-consuming and has a high failure rate. In the present
study, qPCR was used to detect Her-2 in tumor samples and the
results were compared with the IHC results. It was found that the
results from the qPCR were comparable with those from IHC
(κ=0.828).
Barberis et al (19) demonstrated that these two methods
have similar results for the detection of Her-2 in breast cancer,
and are cost-effective compared with other PCR methods approved by
the Food and Drug Administration. Bossard et al (20) obtained similar findings, indicating
that qPCR is an alternative method for Her-2/neu gene detection.
Tse et al (21)
demonstrated that PCR sensitivity and specificity for Her-2 was
87.5 and 100%, respectively, with reference to IHC, while the
sensitivity and specificity was 89.5 and 92%, respectively, with
reference to FISH. Nistor et al (22) reported that the concordance rate of
PCR and FISH was 92%. It has been demonstrated that Her-2/neu can
be amplified and accurately detected in the paraffin tissues from
breast cancer patients (23,24).
qPCR is able to detect 46 samples at a time by Mx3000P qPCR, which
meets the requirements of clinical laboratories. Inconsistencies
between the results of these two methods are likely to be due to
the process of sample collection and preparation. In terms of the
factors discussed above, however, PCR is better.
In conclusion, qPCR is simple, objective, efficient
and highly reproducible. The results of qPCR are consistent with
the results obtained using IHC and FISH, with significant cost
advantages. Therefore, it may be an alternative method for
Her-2/neu gene detection in gastric cancer in the future.
Acknowledgements
The authors would like to thank Guang-jun Zhu
prepared the manuscript and carrying out the literature search;
Guang-Jun Zhu for reviewing and editing the manuscript; Chun-Wei Xu
for correcting and revising the manuscript; Chun-Wei Xu for
performing the histopathological, immunohistochemical examinations;
and Mei-Yu Fang and Yu-Ping Zhang for reviewing the manuscript. All
authors read and approved the final manuscript.
Abbreviations:
|
Her-2
|
human epidermal growth fact or
receptor 2
|
|
EGFR
|
epithelial growth factor receptor
|
|
PCR
|
polymerase chain reaction
|
|
FISH
|
fluorescence in situ hybridization
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou XN, Duan JJ, Huangfu XM, Chen WQ and
Zhao P: Analysis of stomach cancer mortality in the national
retrospective sampling survey of death causes in China. Chin J Prev
Med. 44:390–397. 2010.(In Chinese).
|
|
5
|
Arteaga C: Targeting Her1/EGFR: a
molecular approach to cancer therapy. Semin Oncol. 30(Suppl 7):
3–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park DI, Yun JW, Park JH, et al: Her-2/neu
amplification is an independent prognostic factor in gastric
cancer. Dig Dis Sci. 51:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilton SR and Aaltonen LA: World Health
Organization classification of tumors. Pathology and Genetics of
Tumours of the Digestive System. IARC Press; Lyon, France: pp.
265–314. 2000
|
|
8
|
Ichikura T, Tomimatsu S, Uefuji K, et al:
Evaluation of the New American Joint Committee on
Cancer/International Union against cancer classification of lymph
node metastasis from gastric carcinoma in comparison with the
Japanese classification. Cancer. 86:553–558. 1999. View Article : Google Scholar
|
|
9
|
Moelans CB, van Diest PJ, Milne AN and
Offerhaus GJ: Her-2/neu testing and therapy in gastroesophageal
adenocarcinoma. Patholog Res Int. 2011:6741822010.PubMed/NCBI
|
|
10
|
Casalini P, Iorio MV, Galmozzi E and
Ménard S: Role of HER receptors family in development and
differentiation. J Cell Physiol. 200:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. Arch Pathol Lab
Med. 131:18–43. 2007.
|
|
13
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
14
|
Hayes DF, Yamauchi H, Broadwater G, et al:
Cancer and Leukemia Group B: Circulating Her-2/erbB-2/c-neu (Her-2)
extracellular domain as a prognostic factor in patients with
metastatic breast cancer: Cancer and Leukemia Group B Study 8662.
Clin Cancer Res. 7:2703–2711. 2001.PubMed/NCBI
|
|
15
|
Tsugawa K, Yonemura Y, Hirono Y, et al:
Amplification of the c-met, c-erbB-2 and epidermal growth factor
receptor gene in human gastric cancers: correlation to clinical
features. Oncology. 55:475–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yonemura Y, Ninomiya I, Tsugawa K, et al:
Prognostic significance of c-erbB-2 gene expression in the poorly
differentiated type of adenocarcinoma of the stomach. Cancer Detect
Prev. 22:139–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yonemura Y, Ninomiya I, Yamaguchi A, et
al: Evaluation of immunoreactivity for erbB-2 protein as a marker
of poor short term prognosis in gastric cancer. Cancer Res.
51:1034–1038. 1991.PubMed/NCBI
|
|
18
|
Uchino S, Tsuda H, Maruyama K, et al:
Overexpression of c-erbB-2 protein in gastric cancer. Its
correlation with long-term survival of patients. Cancer.
72:3179–3184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barberis M, Pellegrini C, Cannone M, et
al: Quantitative PCR and Her2 testing in breast cancer: a technical
and cost-effectiveness analysis. Am J Clin Pathol. 129:563–570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bossard C, Bieche I, Le Doussal V,
Lidereau R and Sabourin JC: Real-time RT-PCR: a complementary
method to detect Her-2 status in breast carcinoma. Anticancer Res.
25:4679–4683. 2005.PubMed/NCBI
|
|
21
|
Tse C, Brault D, Gligorov J, et al:
Evaluation of the quantitative analytical methods real-time PCR for
Her-2 gene quantification and ELISA of serum Her-2 protein and
comparison with fluorescence in situ hybridization and
immunohistochemistry for determining Her-2 status in breast cancer
patients. Clin Chem. 51:1093–1101. 2005.
|
|
22
|
Nistor A, Watson PH, Pettigrew N, et al:
Real-time PCR complements immunohistochemistry in the determination
of Her-2/neu status in breast cancer. BMC Clin Pathol. 6:22006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chariyalertsak S, Purisa W and Vinyuvat S:
Her-2/neu amplification determined by real-time quantitative PCR
and its association with clinical outcome of breast cancer in
Thailand. Asian Pac J Cancer Prev. 12:1703–1706. 2011.PubMed/NCBI
|
|
24
|
Page K, Hava N, Ward B, et al: Detection
of Her2 amplification in circulating free DNA in patients with
breast cancer. Br J Cancer. 104:1342–1348. 2011. View Article : Google Scholar : PubMed/NCBI
|