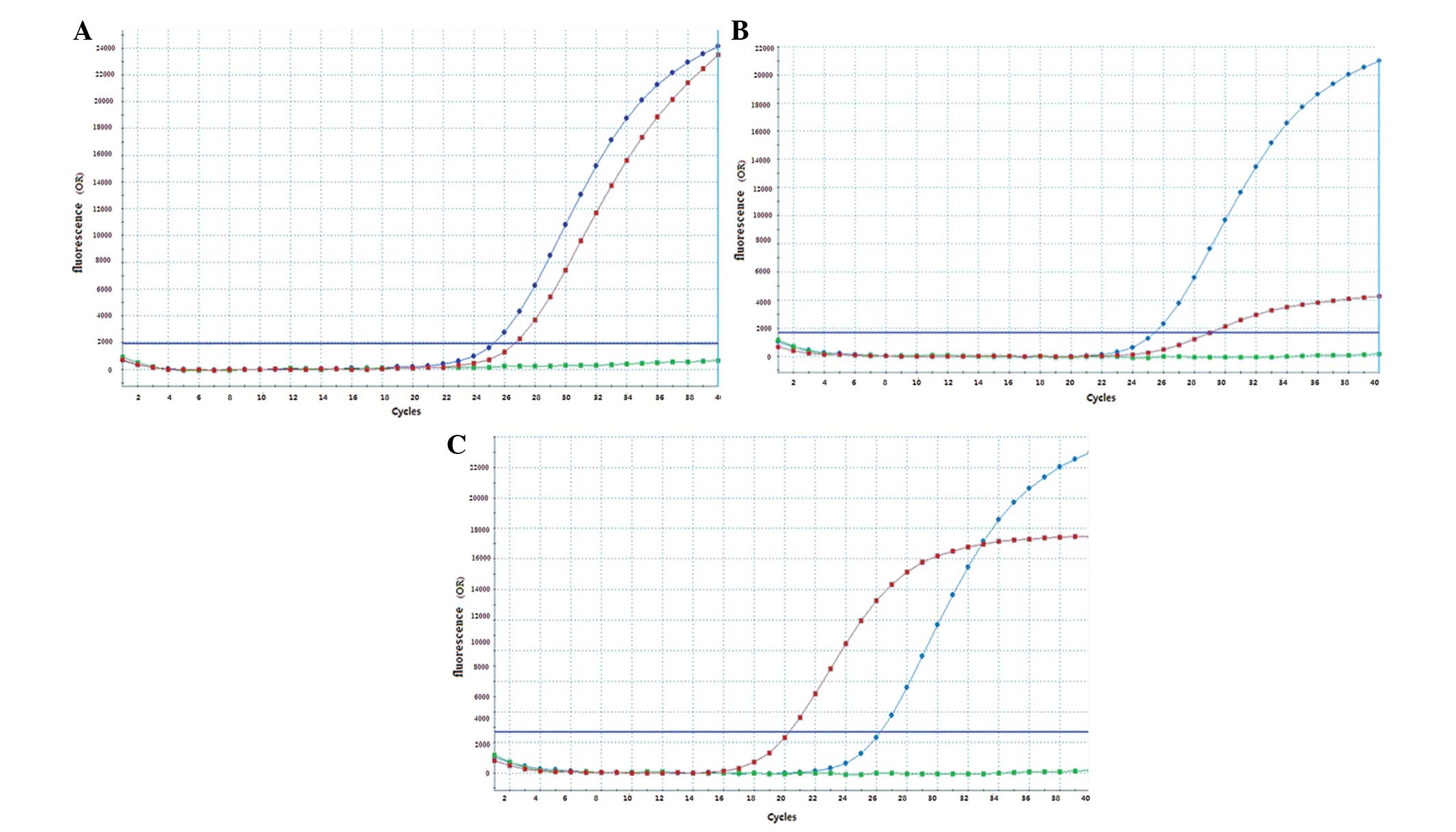
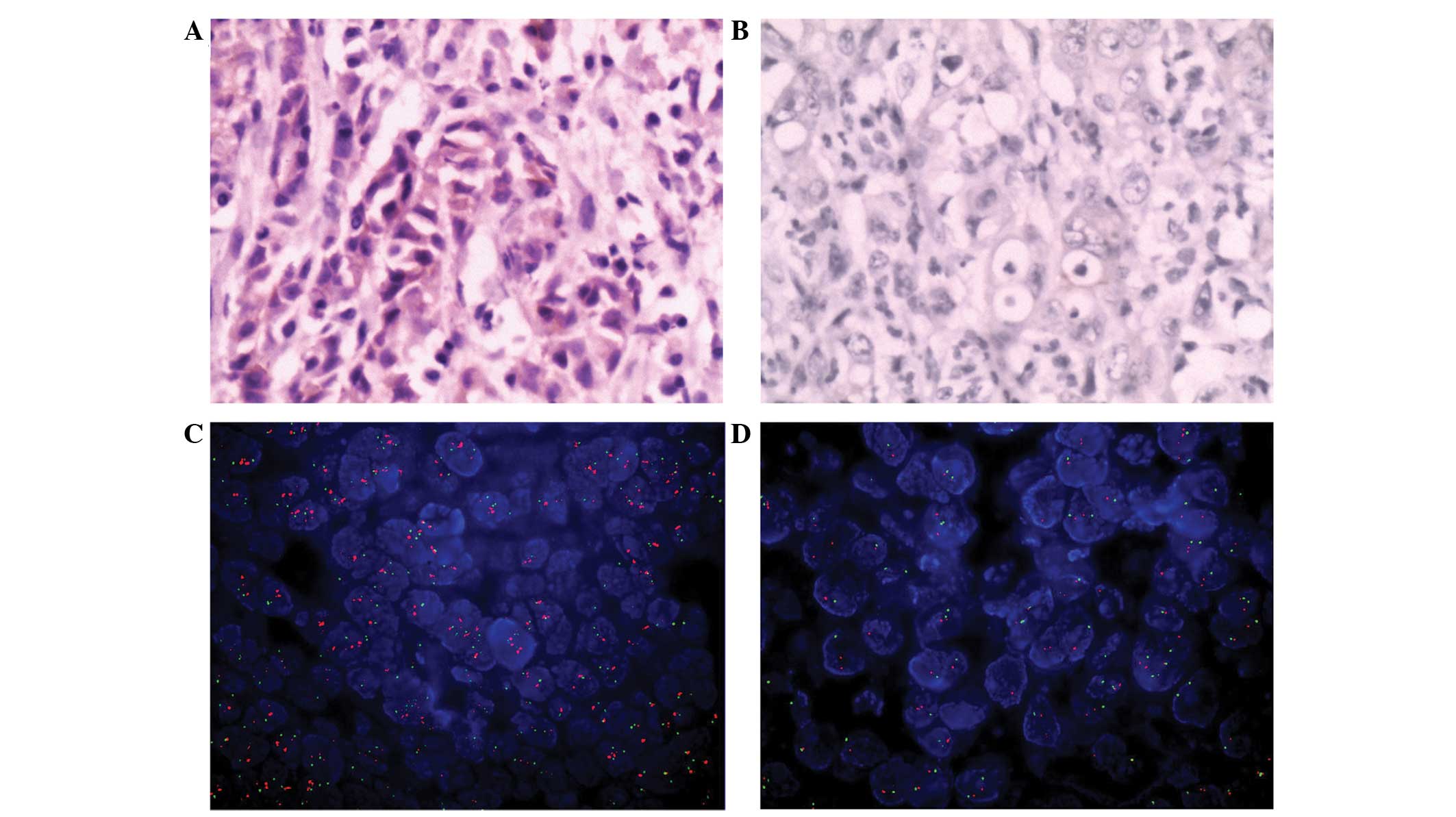
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou XN, Duan JJ, Huangfu XM, Chen WQ and
Zhao P: Analysis of stomach cancer mortality in the national
retrospective sampling survey of death causes in China. Chin J Prev
Med. 44:390–397. 2010.(In Chinese).
|
|
5
|
Arteaga C: Targeting Her1/EGFR: a
molecular approach to cancer therapy. Semin Oncol. 30(Suppl 7):
3–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park DI, Yun JW, Park JH, et al: Her-2/neu
amplification is an independent prognostic factor in gastric
cancer. Dig Dis Sci. 51:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilton SR and Aaltonen LA: World Health
Organization classification of tumors. Pathology and Genetics of
Tumours of the Digestive System. IARC Press; Lyon, France: pp.
265–314. 2000
|
|
8
|
Ichikura T, Tomimatsu S, Uefuji K, et al:
Evaluation of the New American Joint Committee on
Cancer/International Union against cancer classification of lymph
node metastasis from gastric carcinoma in comparison with the
Japanese classification. Cancer. 86:553–558. 1999. View Article : Google Scholar
|
|
9
|
Moelans CB, van Diest PJ, Milne AN and
Offerhaus GJ: Her-2/neu testing and therapy in gastroesophageal
adenocarcinoma. Patholog Res Int. 2011:6741822010.PubMed/NCBI
|
|
10
|
Casalini P, Iorio MV, Galmozzi E and
Ménard S: Role of HER receptors family in development and
differentiation. J Cell Physiol. 200:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. Arch Pathol Lab
Med. 131:18–43. 2007.
|
|
13
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
14
|
Hayes DF, Yamauchi H, Broadwater G, et al:
Cancer and Leukemia Group B: Circulating Her-2/erbB-2/c-neu (Her-2)
extracellular domain as a prognostic factor in patients with
metastatic breast cancer: Cancer and Leukemia Group B Study 8662.
Clin Cancer Res. 7:2703–2711. 2001.PubMed/NCBI
|
|
15
|
Tsugawa K, Yonemura Y, Hirono Y, et al:
Amplification of the c-met, c-erbB-2 and epidermal growth factor
receptor gene in human gastric cancers: correlation to clinical
features. Oncology. 55:475–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yonemura Y, Ninomiya I, Tsugawa K, et al:
Prognostic significance of c-erbB-2 gene expression in the poorly
differentiated type of adenocarcinoma of the stomach. Cancer Detect
Prev. 22:139–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yonemura Y, Ninomiya I, Yamaguchi A, et
al: Evaluation of immunoreactivity for erbB-2 protein as a marker
of poor short term prognosis in gastric cancer. Cancer Res.
51:1034–1038. 1991.PubMed/NCBI
|
|
18
|
Uchino S, Tsuda H, Maruyama K, et al:
Overexpression of c-erbB-2 protein in gastric cancer. Its
correlation with long-term survival of patients. Cancer.
72:3179–3184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barberis M, Pellegrini C, Cannone M, et
al: Quantitative PCR and Her2 testing in breast cancer: a technical
and cost-effectiveness analysis. Am J Clin Pathol. 129:563–570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bossard C, Bieche I, Le Doussal V,
Lidereau R and Sabourin JC: Real-time RT-PCR: a complementary
method to detect Her-2 status in breast carcinoma. Anticancer Res.
25:4679–4683. 2005.PubMed/NCBI
|
|
21
|
Tse C, Brault D, Gligorov J, et al:
Evaluation of the quantitative analytical methods real-time PCR for
Her-2 gene quantification and ELISA of serum Her-2 protein and
comparison with fluorescence in situ hybridization and
immunohistochemistry for determining Her-2 status in breast cancer
patients. Clin Chem. 51:1093–1101. 2005.
|
|
22
|
Nistor A, Watson PH, Pettigrew N, et al:
Real-time PCR complements immunohistochemistry in the determination
of Her-2/neu status in breast cancer. BMC Clin Pathol. 6:22006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chariyalertsak S, Purisa W and Vinyuvat S:
Her-2/neu amplification determined by real-time quantitative PCR
and its association with clinical outcome of breast cancer in
Thailand. Asian Pac J Cancer Prev. 12:1703–1706. 2011.PubMed/NCBI
|
|
24
|
Page K, Hava N, Ward B, et al: Detection
of Her2 amplification in circulating free DNA in patients with
breast cancer. Br J Cancer. 104:1342–1348. 2011. View Article : Google Scholar : PubMed/NCBI
|