Introduction
Recent studies have indicated that atherosclerosis
is closely associated with adipokines (1,2).
Acylation stimulating protein (ASP) is a source of adipokine
derived from complement component 3 (C3) (3). As a core factor of the complement
system, C3 functions as a key molecule, initiating an alternative
pathway for complement activation and participating in cascades. In
addition, C3 is considered to be a link between atherosclerosis and
high blood pressure, high cholesterol, insulin resistance or
obesity, functioning as an independent index for atherosclerosis
risk factors (4–6). Endogenous C3 is mainly derived from
fat tissues and the liver (7). In
a physiological state, chylomicrons stimulate the secretion of C3
from adipose cells. ASP is a cleaved fragment of C3 that functions
as a strong stimulating factor, promoting fatty acid esterification
and triglyceride synthesis. In addition, ASP is a bioactive
substance, closely associated with human fat metabolism (8).
ASP metabolic pathway dysfunction causes a number of
carbohydrate and lipid metabolic disorders, leading to obesity,
diabetes and cardiovascular diseases (9). However, studies investigating the
correlation of ASP and C3 with atherosclerosis have been rarely
reported.
In the present study, the role of ASP in the
occurrence and development of atherosclerosis was investigated by
analyzing the levels of ASP in healthy controls and patients with
coronary heart disease (CHD) or metabolic syndrome (MS). In
addition, the brachial-ankle pulse wave velocity (baPWV) and
ankle-brachial index (ABI) of patients suffering from MS were
determined, and the correlation with the ASP and C3 concentration
was determined. Furthermore, the correlation between the ASP and C3
concentration with the severity of coronary artery disease in CHD
patients was analyzed to determine whether ASP is a risk factor in
the occurrence and development of CHD. The CHD patients were
divided into mild, moderate and severe CHD groups, according to
coronary artery stenosis grading. The protein and mRNA expression
levels of C3 in the mild, moderate and severe CHD subgroups were
determined by western blotting and quantitative reverse
transcription-polymerase chain reaction (RT-PCR), respectively.
Materials and methods
Subjects
A total of 118 patients diagnosed with CHD in the
Department of Cardiology of Provincial Hospital of Shandong (Jinan,
China) were enrolled in the study and divided into the MS (n=56)
and CHD (n=62) groups. MS patients (male, 27; female, 29) were
diagnosed according to the MS diagnostic criteria (10). Patients suffering from heart
failure, acute coronary syndrome, an infectious disease,
rheumatism, cancer and secondary MS, or patients administered
anticoagulants, were excluded from the MS group. In the CHD
patients (male, 30; female, 32), the degree of vascular stenosis
was determined by coronary angiography. Patients suffering from
severe infections or connective tissue disease were excluded from
the CHD group. In addition, 42 healthy volunteers (male, 20;
female, 22) were recruited for the control group. The individuals
in the three groups were aged between 45 and 81 years. No
statistically significant differences existed in the age and gender
compositions between the groups. The study was approved by the
Ethics Committee of the Provincial Hospital of Shandong University
(Jinan, China) and written informed consent was obtained from the
patient.
Arteriosclerosis detection
An arteriosclerosis detection device BP-203RPEIII
(Omron Healthcare Co., Ltd, Tokyo, Japan) was used to determine the
baPWV and ABI values of the patients, measured after resting in the
supine position for >5 min. Basic information of the
individuals, including height, weight and age, were inserted in the
device. In addition, heart sounds were recorded from the fourth
intercostal space of the left sternal border, while
electrocardiogram electrodes were fixed around the wrists, and four
cuffs, connected with a plethysmograph and oscillometric pressure
sensor, were placed on the ankle brachial artery. Following
stabilization of the phonocardiogram signal, the average baPWV and
ABI values were determined by recording three consecutive
measurements. The highest bilateral limb value of baPWV and the
lowest bilateral limb value of ABI were selected for analysis.
baPWV values of <1,400 cm/sec were considered as normal
reference values, while values of ≥1,400 cm/sec were regarded as
abnormal. With regard to ABI, values between 0.9 and 1.4 were
considered to be normal, values of ≤0.9 indicated that patients may
suffer from arteriosclerotic occlusion in the lower limbs and
values of ≥1.4 indicated the possibility of blood vessel
calcification.
Coronary angiography
Selective coronary angiography was performed using
Judkins method. Quantitative analysis of the vascular stenosis
degree was performed according to the Gensini scoring system
(11), as follows: 1 point, ≤25%
stenosis; 2 points, 26–50% stenosis; 4 points, 51–75% stenosis; 8
points, 76–90% stenosis; 16 points, 91–99% stenosis; and 32 points,
100% stenosis. Different segments of the coronary artery were
multiplied with appropriate factors. The final score of coronary
artery stenosis for each patient was the sum of all the branch
points. According to the final scores, the individuals were divided
into the four groups as follows: Healthy control (0 points), mild
CHD (≤20 points), moderate CHD (21–40 points) and severe CHD
(>40 points) groups.
Enzyme-linked immunosorbent assay (ELISA)
and immunoturbidimetry
Fasting venous blood samples were collected from the
subjects, and the serum was separated by immediate centrifugation
at 1,411 × g at 4°C for 10 min. Serum aliquots were stored at −20°C
for further use. The serum ASP concentration was measured using an
OmniKine™ Human ACE ELISA kit (Assay Biotech Co., Inc., Sunnyvale,
CA, USA), according to the manufacturer’s instructions.
Immunoturbidimetry was performed to measure the concentration of C3
using an Immunoturbidimetry kit (Assay Biotech Co., Inc.).
Immunoblot assays
Total proteins were isolated from the serum of each
participant, separated by 10% sodium dodecyl sulfate/polyacrylamide
gel electrophoresis and subjected to immunoblot analyses. Primary
antibodies against C3 and actin were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA; anti-C3, cat no. sc-28294,
1:200; anti-actin, cat no. sc-130301, 1:10,000). A secondary goat
anti-mouse immunoglobulin G-horseradish peroxidase-conjugated
antibody was also used (Santa Cruz Biotechnology, Inc.; cat no.
sc-2005, 1:10,000). The signals were detected using an enhanced
chemiluminescence system (Pierce Biotechnology, Inc., Rockford, IL,
USA), and immunoblotting experiments were performed a minimum of
three times. The mean optical density (OD) of the C3 protein bands
was normalized against the OD of the actin band of each
individual.
Quantitative RT-PCR
Quantitative RT-PCR analysis was performed to
determine the mRNA expression levels of C3 and GAPDH. The total RNA
was harvested from the serum of all individuals using an RNeasy kit
(Qiagen, Valencia, CA, USA), according to the manufacturer’s
instructions. Briefly, the PCR procedures were as follows:
Pre-denaturation at 95°C for 30 sec, followed by 35 cycles of
denaturation at 95°C for 15 sec and annealing at 56°-60°C for 30
sec. RT-PCR experiments were performed a minimum of three
times.
RNA (1 μl) was reverse transcribed into cDNA using
random primers in a Reverse Transcription II system (Promega
Corporation, Madison, WI, USA), according to the manufacturer’s
instructions. The mRNA expression levels of C3 and GAPDH were
determined using quantitative PCR with an ABI Prism Sequence
Detection system (Applied Biosystems Life Technologies, Foster
City, CA, USA). The primers used are listed in Table I. An assay reagent containing
premixed primers and a VIC-labeled probe (Applied Biosystems Life
Technologies; cat no. 4310884E) was used to quantify the mRNA
expression level of endogenous GAPDH. The relative levels of C3
transcripts were normalized against the amount of GAPDH mRNA for
each sample.
 | Table IPrimers used in the study. |
Table I
Primers used in the study.
| Primers | Primer sequences |
|---|
| C3_F |
5′-AGAATCGAACATAGACAGATAGT-3′ |
| C3_R |
5′-CTTCGTCATGGTCATGTAGAAC-3′ |
| GAPDH_F |
5′-TCGCTCTTACAAGTCGATCCA-3′ |
| GAPDH_R |
5′-GACCAAGCTGACTCGTAGCT-3′ |
Statistical analysis
Statistical analyses were performed using the
Statistical Package for Social Sciences software (SPSS version
13.0; SPSS, Inc., Chicago, IL, USA). The data are expressed as the
mean ± standard deviation. Comparisons between two groups were
performed using the t-test, where P<0.05 was considered to
indicate a statistically significant difference.
Results
ASP and C3 concentrations are
significantly increased in CHD patients compared with healthy
individuals and MS patients
ELISA was used to determine the serum concentrations
of ASP in the control, CHD and MS groups. As shown in Fig. 1A, the levels of ASP in the CHD
group were significantly increased compared with the MS and control
groups (P<0.05). However, the ASP levels in the MS group were
not found to be significantly different when compared with the
control group (P>0.05). These results indicate that the
concentration of ASP in CHD patients was significantly enhanced
compared with the control and MS groups.
 | Figure 1Levels of (A) ASP and (B) C3,
determined by ELISA and immunoturbidimetry, respectively, using
fasting venous blood samples collected from patients in the
control, CHD and MS groups. *P<0.05, vs. healthy
control group; #P<0.05, vs. MS group. ASP, acylation
stimulating protein; C3, complement component 3; CHD, coronary
heart disease; MS, metabolic syndrome; ELISA, enzyme-linked
immunosorbent assay. |
Immunoturbidimetric analysis was performed to
determine the serum concentrations of C3 in the control, CHD and MS
groups (Fig. 1B). As shown in
Fig. 1B, the levels of C3 in the
CHD group were significantly increased compared with the MS and
control groups (P<0.05). However, the difference in the C3
levels between the MS and control groups was not found to be
statistically significant (P>0.05). These results indicate that
the concentration of C3 in CHD patients was significantly increased
compared with the control and MS patients. Thus, ASP and C3 levels
may correlate with the occurrence of CHD, but not MS.
ASP and C3 levels are slightly increased
in MS patients with abnormal baPWV and ABI readings
An arteriosclerosis detection device was used to
determine whether the levels of ASP and C3 were altered in MS
patients with abnormal baPWV and ABI readings. As shown in Table II, the levels of ASP and C3 in the
MS patients with abnormal baPWV and ABI readings were slightly
increased compared with MS patients with normal baPWV and ABI
values. There were no statistically significant differences in the
levels of ASP (P=0.306) and C3 (P=0.416) between patients with
normal and abnormal baPWV values. Additionally, the differences in
the levels of ASP (P=0.406) and C3 (P=0.504) between patients with
normal and abnormal ABI values were not significant. The results
indicate that the ASP and C3 levels were not significantly altered
in MS patients with abnormal baPWV and ABI readings.
 | Table IILevels of ASP and C3 in the MS
group. |
Table II
Levels of ASP and C3 in the MS
group.
| Subgroup | ASP (μg/l) | P-value | C3 (μg/ml) | P-value |
|---|
| baPWV values | | 0.306 | | 0.416 |
| Normal (n=30) | 4.78±1.33 | | 619.4±185.7 | |
| Abnormal (n=32) | 4.86±1.68 | | 639.7±165.5 | |
| ABI values | | 0.406 | | 0.504 |
| Normal (n=28) | 4.99±1.13 | | 631.4±151.6 | |
| Abnormal (n=34) | 5.01±1.23 | | 638.5±145.6 | |
ASP and C3 levels are positively
correlated with the severity of coronary artery disease in the CHD
group
Coronary angiography and Gensini scoring were used
to further evaluate the vascular stenosis degree of CHD patients,
and determine the correlation between ASP levels and the degree of
coronary artery disease. There were 10 cases with a Gensini score
of 0 points and 12 cases with a Gensini score ≤ 20 points. The
Gensini score of 22 cases was between 20 and 40 points.
Additionally, a total of 18 cases had a Gensini score ≥ 40
points.
ELISA was performed to determine the serum
concentrations of ASP in the three CHD subgroups (mild, moderate
and severe) and the healthy control group. As shown in Fig. 2A, the levels of ASP in the moderate
and severe CHD subgroups were significantly higher compared with
the control group and mild CHD subgroup (P<0.05). However, the
ASP levels were not found to be significantly different between the
moderate and severe CHD subgroups. These results indicate that the
levels of ASP in CHD patients correlate with the severity degree of
CHD.
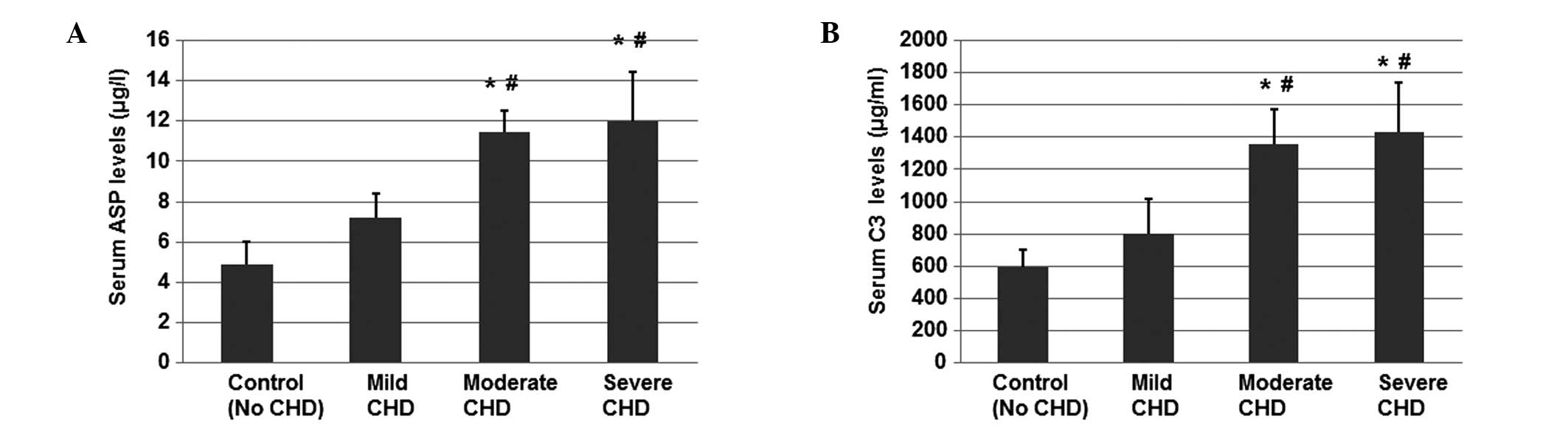 | Figure 2Levels of (A) ASP and (B) C3,
determined by ELISA and immunoturbidimetry, respectively, using
fasting venous blood samples collected from patients in the control
group (n=42) and mild, moderate and severe CHD subgroups (n=62).
*P<0.05, vs. healthy control group;
#P<0.05, vs. mild CHD subgroup. ASP, acylation
stimulating protein; C3, complement component 3; CHD, coronary
heart disease; ELISA, enzyme-linked immunosorbent assay. |
Since C3 is the precursor of ASP, the levels of C3
in the CHD patients were also measured using immunoturbidimetry. As
shown in Fig. 2B, the levels of C3
in the patients with moderate and severe CHD were significantly
higher compared with the control group and the mild CHD subgroup
(P<0.05). In addition, the C3 levels in the moderate and severe
CHD subgroups were similar (P>0.05). These results indicate that
the levels of C3 in CHD patients correlate with the severity of
CHD.
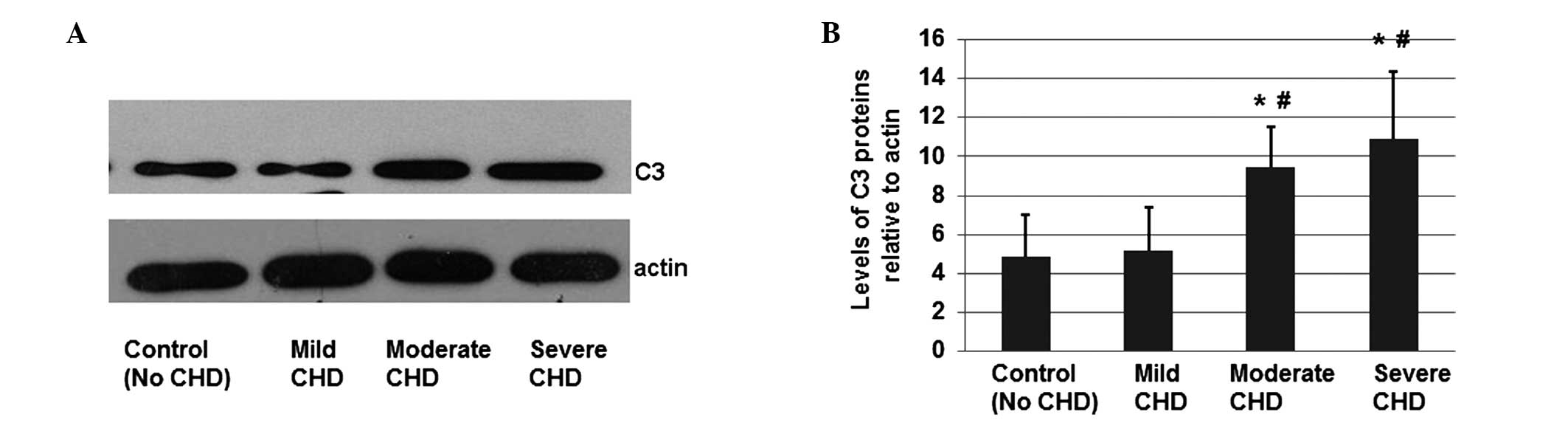
Protein expression levels of C3 in CHD
patients are significantly increased compared with healthy
individuals
Total proteins were extracted from individuals in
the healthy control group and mild, moderate and severe CHD
subgroups, in order to investigate whether the protein expression
of C3 was altered in CHD patients. The protein expression levels of
C3 and actin were determined by western blotting (Fig. 3A), and the mean OD of the C3
protein bands was normalized against the OD of the actin band of
each participant. Error bars in Fig.
3B show the standard error of the mean (mean ± SD;
P<0.05).
As shown in Fig. 3,
the mean protein expression levels of C3 in the moderate and severe
CHD subgroups were significantly higher compared with healthy
individuals and the mild CHD subgroup, indicating that protein
expression of C3 is associated with the development of CHD.
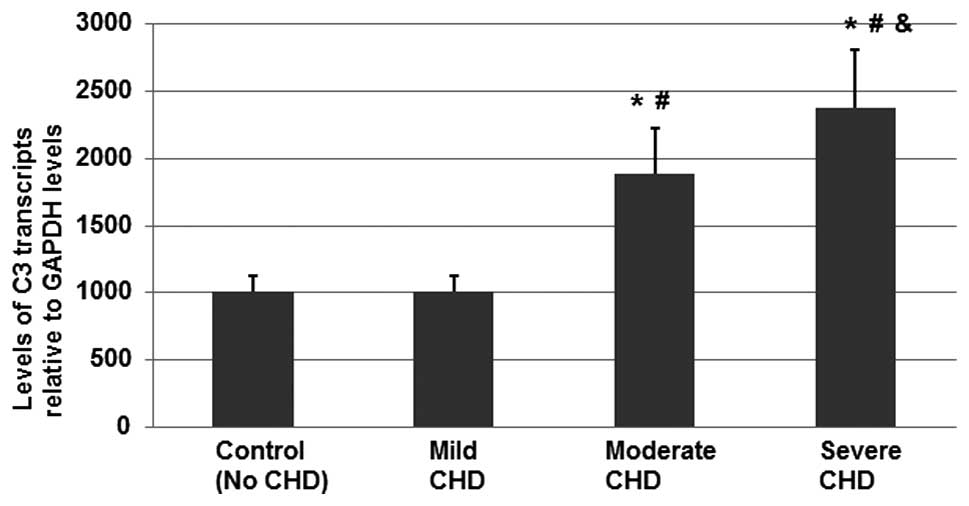
mRNA expression levels of C3 in CHD
patients are significantly increased compared with healthy
individuals
High protein expression levels are often a result of
a high level of gene transcription. Therefore, the serum mRNA
expression levels of C3 in the healthy control group and the three
CHD subgroups were determined using quantitative RT-PCR analysis.
The fold change was calculated by normalizing the level of C3
transcripts to those in the control group. The mean level of the C3
transcripts in the healthy control group was assigned a value of
1,000.
As shown in Fig. 4,
the mean expression levels of C3 mRNA in the moderate and severe
CHD patients were significantly higher compared with the healthy
control group and the mild CHD subgroup (P<0.05). In addition,
the mean mRNA expression levels of C3 in the severe CHD patients
were found to be significantly higher compared with the moderate
CHD subgroup (P<0.05). Therefore, C3 gene transcription was
found to be associated with the development of mild CHD to moderate
CHD.
Discussion
Early diagnosis and treatment of cardiovascular
diseases is important for the improvement of prognosis and the
reduction of morbidity and mortality rates. Decreased arterial
elasticity is an important link that connects the underlying
disease, risk factors and disease endpoints in the cardiovascular
event chain. Timely identification and knowledge of the degree of
arteriosclerosis, and subsequent active intervention, have become a
priority in prevention of morbidity and mortality (12).
baPWV and ABI are frequently used in the evaluation
of early atherosclerosis. A number of studies have confirmed that a
baPWV value of >1,800 cm/sec indicates the occurrence of severe
coronary events (13,14); therefore, baPWV can be used to
diagnose CHD. In addition, ABI and baPWV contribute towards the
early detection of changes in the vascular structure and function.
However, the present study revealed that ASP and C3 were not
correlated with baPWV or ABI. Carotid atherosclerosis is an
indicator of systemic atherosclerosis, and is closely associated
with the incidence of cardiovascular and cerebrovascular diseases
(15). Ultrasound has become the
preferred imaging examination method for patients with carotid
atherosclerosis, with numerous studies confirming that the presence
of carotid atherosclerosis is a valuable independent predictor of
coronary atherosclerosis (16–18).
In the current study, the protein expression levels of C3 in the
moderate and severe CHD patients were found to be significantly
higher compared with the healthy individuals and mild CHD patients.
In addition, quantitative RT-PCR analysis indicated that the levels
of C3 mRNA in the moderate and severe CHD patients were
significantly higher compared with the healthy individuals and the
mild CHD patients. Furthermore, the mean transcript levels of C3 in
the severe CHD patients were found to be higher compared with the
moderate CHD subgroup (P<0.05). In the present study, an
association between ASP and C3 with moderate and severe CHD was
observed, indicating that the factors may be involved in the
occurrence and development of atherosclerosis.
ASP receptor dysfunction is known to exist in
patients with CHD, contributing to the decline of the effect of ASP
(19). In addition, ASP
dysfunction results in reduced triglyceride synthesis in adipose
cells, with the majority of free fatty acids and cholesterol
particles, consisting of triglycerides, being retained in the
circulation for subsequent uptake by the liver (20). In order to transport the increased
fatty acid molecules, an increased number of very-low-density
lipoprotein particles, containing the abundant apolipoprotein B100,
are synthesized and released by the liver, further aggravating the
disorder of lipid metabolism (21).
In conclusion, the present study revealed a
significant correlation between the levels of ASP and C3 with the
severity of coronary arteriosclerosis, indicating that ASP is
involved in the progress of CHD. An in-depth study of the
biological function and metabolic pathways of ASP may aid further
elucidation of the mechanism underlying the occurrence of CHD with
respect to lipid metabolism disorders, and provide a potential
method for the prevention and treatment of cardiovascular
diseases.
Acknowledgements
The study was supported by the Provincial Hospital
of Shandong University, Linyi People’s Hospital and the Qilu
Hospital of Shandong University, China.
References
|
1
|
Elks CM and Francis J: Central adiposity,
systemic inflammation, and the metabolic syndrome. Curr Hypertens
Rep. 12:99–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gremese E and Ferraccioli G: The metabolic
syndrome: the crossroads between rheumatoid arthritis and
cardiovascular risk. Autoimmun Rev. 10:582–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cianflone KM, Sniderman AD, Walsh MJ, et
al: Purification and characterization of acylation stimulating
protein. J Biol Chem. 264:426–430. 1989.PubMed/NCBI
|
|
4
|
Capuano V, D’Arminio T, La Sala G and
Mazzotta G: The third component of the complement (C3) is a marker
of the risk of atherogenesis. Eur J Cardiovasc Prev Rehabil.
13:658–660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engström G, Hedblad B, Eriksson KF, et al:
Complement C3 is a risk factor for the development of diabetes: a
population-based cohort study. Diabetes. 54:570–575.
2005.PubMed/NCBI
|
|
6
|
Weyer C, Tataranni PA and Pratley RE:
Insulin action and insulinemia are closely related to the fasting
complement C3, but not acylation stimulating protein concentration.
Diabetes Care. 23:779–785. 2000. View Article : Google Scholar
|
|
7
|
Gabrielsson BG, Johansson JM, Lönn M, et
al: High expression of complement components in omental adipose
tissue in obese men. Obes Res. 11:699–708. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacLaren R, Cui W and Cianflone K:
Adipokines and the immune system: an adipocentric view. Adv Exp Med
Biol. 632:1–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maslowska M, Wang HW and Cianflone K:
Novel roles for acylation stimulating protein/C3adesArg: a review
of recent in vitro and in vivo evidence. Vitam Horm. 70:309–332.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metabolic syndrome study cooperation group
of Chinese diabetes society. 2004.Suggestions about metabolic
syndrome of Chinese diabetes society. Zhong Hua Tang Niao Bing Za
Zhi. 12:156–161. 2004.
|
|
11
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rubba F, Gentile M, Iannuzzi A, et al:
Vascular preventive measures: the progression from asymptomatic to
symptomatic atherosclerosis management. Evidence on usefulness of
early diagnosis in women and children. Future Cardiol. 6:211–220.
2010. View
Article : Google Scholar
|
|
13
|
Yamashina A, Tomiyama H, Arai T, et al:
Brachial-ankle pulse wave velocity as a marker of atherosclerotic
vascular damage and cardiovascular risk. Hypertens Res. 26:615–622.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Wu Y, Li J, Ma W, Guo X, Luo Y and
Hu D: The predictive value of brachial-ankle pulse wave velocity in
coronary atherosclerosis and peripheral artery diseases in urban
Chinese patients. Hypertens Res. 31:1079–1085. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faxon DP, Creager MA, Smith SC Jr, et al;
American Heart Association. Atherosclerotic vascular disease
conference: Executive summary: Atherosclerotic vascular disease
conference proceeding for healthcare professionals from a special
writing group of the American Heart Association. Circulation.
109:2595–2604. 2004. View Article : Google Scholar
|
|
16
|
Hulthe J, Wikstrand J, Emanuelsson H, et
al: Atherosclerotic changes in the carotid artery bulb as measured
by B-mode ultrasound are associated with the extent of coronary
atherosclerosis. Stroke. 28:1189–1194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yildiz A, Tepe S, Oflaz H, et al: Carotid
atherosclerosis is a predictor of coronary calcification in chronic
haemodialysis patients. Nephrol Dial Transplant. 19:885–891. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurnatowska I, Grzelak P, Stefańczyk L and
Nowicki M: Tight relations between coronary calcification and
atherosclerotic lesions in the carotid artery in chronic dialysis
patients. Nephrology (Carlton). 15:184–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sniderman AD, Cianflone K, Arner P,
Summers LK and Frayn KN: The adipocyte, fatty acid trapping, and
atherogenesis. Arterioscler Thromb Vasc Biol. 18:147–151. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hajer GR, van Haeften TW and Visseren FL:
Adipose tissue dysfunction in obesity, diabetes, and vascular
diseases. Eur Heart J. 29:2959–2971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adiels M, Olofsson SO, Taskinen MR and
Borén J: Overproduction of very low-density lipoproteins is the
hallmark of the dyslipidemia in the metabolic syndrome.
Arterioscler Thromb Vasc Biol. 28:1225–1236. 2008. View Article : Google Scholar : PubMed/NCBI
|