Introduction
Osteoporosis is a major factor restricting adult
orthodontic implant anchorage and tooth implantation. Previous
studies have shown that osteoporosis affects the jawbone and
weakens the osseointegration ability of dental implants (1–3).
A reduction of bone mineral density of between 1 and
2.5 standard deviations is defined as osteopenia. Prior to
osteoporosis, the human body experiences a pathological process in
which bone mineral density is decreased. Osteopenia is a
preclinical state of osteoporosis (4). Therefore, osteopenia is considered as
a risk factor against the success of implantation.
The conventional treatment method for osteoporosis
is drug therapy to prevent bone absorption. However, this modality
is associated with adverse effects, drug resistance and long-term
risks (5–7). Strontium (Sr) is an essential trace
element for human skeletal components. It can promote bone
formation and inhibit bone absorption (8). In vitro and in vivo
assays have demonstrated that Sr can promote osteoblast
proliferation and inhibit osteoclast proliferation, and in
vitro experiments have indicated that Sr has a positive role in
bone tissue engineering (9).
In the present study, Sr coatings were deposited on
the surface of titanium implants by electrochemical deposition
(ECD) and the implants were evaluated in an ovariectomized rat
model. The aim of the study was to identify a new method for
improving the success rate of dental implantation.
Materials and methods
Preparation of Sr coating by ECD
Commercial grade 4 unalloyed titanium plates
(10×10×2 mm) and custom-made screw-type titanium implants (1.5 mm
diameter, 6.5 mm length; 99.8% Ti; National Engineering Research
Center in Biomaterials, Sichuan University, Chengdu, China) were
used as substrates for ECD. Prior to the deposition of coating, the
titanium plates and implants were polished with up to 2,000 grid,
immersed into hydrochloric acid and calcium chloride solutions
successively, ultrasonically cleaned in acetone for 10 min, rinsed
with deionized water and air dried. The ECD experimental setup used
for this study was a two-electrode cell configuration, as
previously described (10). The
working electrode (the coating substrate) was the titanium plate
and the counter electrode was a platinum mesh. The distance between
the electrodes was 2 cm. The constant voltage was 2.5 V, the
temperature was 60°C and the reaction time was 1 h. The
electrolytes were NH4H2PO4 0.036
mol/l, CaCl2 + SrCl2 0.06 mol/l and
NaCl2 0.1 mol/l, pH 4.5.
Morphology and characterization of
coating
The phase composition of the coating was analyzed by
X-ray diffraction (XRD) using a D/MAX2500PC automatic XRD apparatus
(Rigaku, Tokyo, Japan). The surface morphology and coating
thickness were examined by scanning electron microscopy (SEM) using
an S-4800 field emission scanning electron microscope (Hitachi,
Tokyo, Japan). SEM was coupled with energy dispersive X-ray
spectroscopy using a Noran™ system 7 X-ray microanalysis system
(Thermo Fisher, Madison, WI, USA), which was used to determine the
elemental composition of the biomimetic coating.
Animals and ovariectomy (OVX)
A total of 36 adult female Sprague Dawley (SD) rats,
aged 3 months, were obtained from the Military Medical Science
Academy of the PLA (Beijing, China). The in vivo study was
approved by the Animal Care and Use Committee of Hebei United
University [Tangshan, China; License no. SCXK (Jun 2009–003)].
Bilateral OVX was performed in 24 rats through an incision in the
back under general anesthesia with an intraperitoneal injection of
10% chloral hydrate at a dose of 3 ml/kg (Chemical Reagent Co.,
Shanghai, China), while the remaining animals (12 rats) underwent a
sham surgery in which the ovaries were examined and returned to the
original position under the same protocol.
Implantation and animal grouping
Five weeks following OVX or sham surgery, all
animals received simultaneous bilateral tibiae implantation using
pure screw-shaped titanium implants with an ECD-generated Sr
coating. The 12 animals that underwent a sham surgery were
designated as the sham group, and the animals that underwent OVX
were randomly divided into two groups, with 12 animals/group. One
group received implants with a Sr coating (Sr group) and the other
received implants without an Sr coating (OVX group).
Fluorescent labeling
Following implantation, the SD rats were subdermally
injected once a day with tetracycline hydrochloride at 30 mg/kg on
days 13 and 14 and calcein at 6 mg/kg on days 3 and 4.
Histological examination
All rats were sacrificed at 4 weeks following
implantation. Specimens of tibiae were fixed in 10% neutral
buffered formalin for 5 days, dehydrated in increasing gradients of
alcohol, and embedded in methylmethacrylate resin. Undecalcified,
ground 30-μm sections parallel to the long axis of the implant and
vertical to the long axis of tibiae were obtained using an Exakt
saw and grinding equipment (Exakt 300; Exact Advanced Technologies,
GmbH, Norderstedt, Germany). The sections were stained with
toluidine blue. Three sections of each specimen were examined at a
magnification of ×100.
Histomorphometric analysis was performed with a
semi-automated digitizing image analysis system, comprising a Nikon
ECLIPSE E600 stereomicroscope, a computer-coupled Nikon DXM1200
Digital Camera and NIS-Elements F 2.20 image software (Nikon
Corporation, Tokyo, Japan). Histomorphometric indices included the
implant-bone contact rate (IBCR), defined as the direct
implant-bone interface to total implant surface, and the ratio of
calcified bone volume to total bone volume (BV/TV) within 2.0 mm of
the axis of the implant. In each section, five equally spaced sites
of each screw were measured and the mean value of all screws was
accepted as the value of the index of the section (11).
Bone dynamic indices
The distance between double labels (DDL), mineral
apposition rate (MAR) and mineralizing surface/bone surface ratio
(MS/BS) were calculated. The MAR was determined by measuring the
distance between the two fluorescent lines. In each field, five
equally distributed sites of each double fluorescent line were
measured and the mean of the values in the field was accepted as
the value of the field. The measurements were conducted with a
confocal microscope (FV1000; Olympus Corporation, Tokyo, Japan).
The MS/BS value was calculated as follows: MS/BS (%) = (0.5 single
labeled perimeter + double labeled perimeter)/bone perimeter
×100.
Biomechanical testing
The removal torque of the implants was examined with
a method similar to that previously reported (12). Briefly, the specimens were fixed in
10% neutral buffered formalin and embedded in a quadrate metal box
with dental plaster. The testing equipment was a force measuring
device (Asida DZE-5; Zhengye Electronics Co., Ltd., Dongguan,
China) for recording the peak force value in Newtons (N) required
to loosen the implant and a specially made wrench that connected
the implant and the force measuring device. The implant used was
specially designed with a square cap suitable for application of
the wrench. The removal torque was calculated by multiplying the
peak force value with the distance between the force point and the
center of the implant.
Statistical analysis
Data are expressed as mean ± standard deviation (SD)
and statistical analyses were performed using SPSS software,
version 12.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) was used to assess differences between OVX and
sham-operated rats. P<0.05 was considered to be indicate a
statistically significant difference.
Results
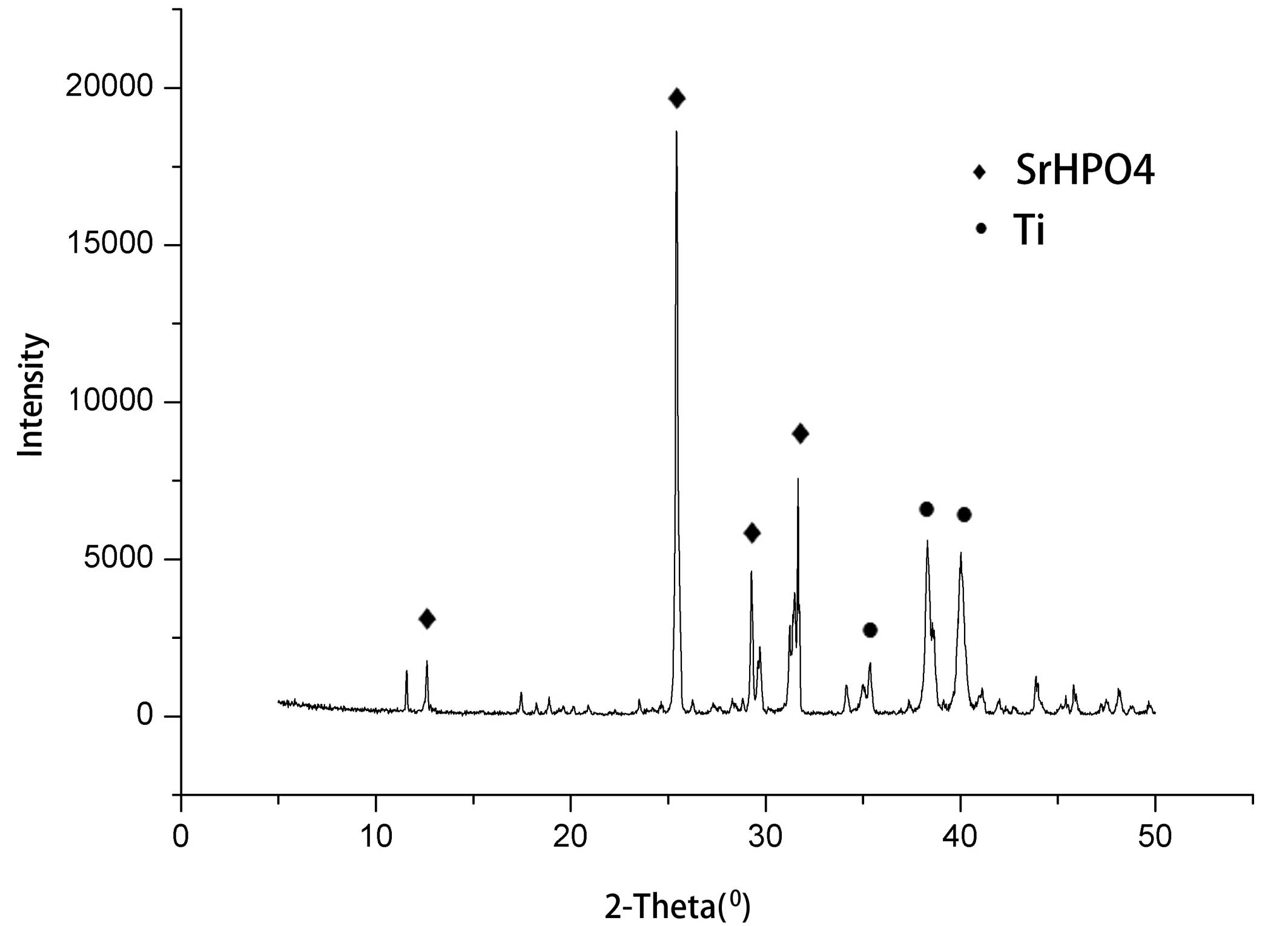
Coating XRD analysis
XRD analysis indicated that the coating comprised
strontium hydrogen phosphate (SrHPO4). The relative
intensities of the diffraction peaks for the SrHPO4
crystals (102) were higher than those for other crystal face
intensities, and revealed a (102) crystal face growth advantage
(Fig. 1).
Analysis of the morphology of the coating
surface
The Sr coating thickness analyzed by SEM was 25 μm.
The Sr coating was observed to consist of lamellar crystals in
aggregated clusters or a petal-like arrangement (Fig. 2).
Histological examination
Histological images obtained from undecalcified
sections are shown in Fig. 3. In
the OVX group, bone formation was diminished with poor bone
continuity. By contrast, in the Sr group, new bone formation was
evident with new thicker laminar bones. Certain laminar bones were
discontinuous. Bone formation was normal in the sham group, with
prominent laminar bone. Table I
shows various histomorphometric indices, namely the IBCR, BV/TV and
values for the thickness of the bone lamellar interface (T). The
IBCR, BV/TV and T in the Sr group were significantly higher than
those of the OVX group (P<0.01), and the OVX group had the
lowest IBCR, BV/TV and T (P<0.05 or P<0.01).
 | Table IHistomorphometric analysis of bone
indices among the three groups (n=12). |
Table I
Histomorphometric analysis of bone
indices among the three groups (n=12).
| Group | IBCR (%) | BV/TV (%) | T (μm) |
|---|
| Sham | 62.71±4.60 | 59.24±5.13 | 67.01±6.66 |
| OVX | 39.34±4.42a | 42.39±5.48a | 49.34±4.49a |
| Sr | 58.72±3.85b,c | 49.39±7.14a,d | 54.27±6.95a,c |
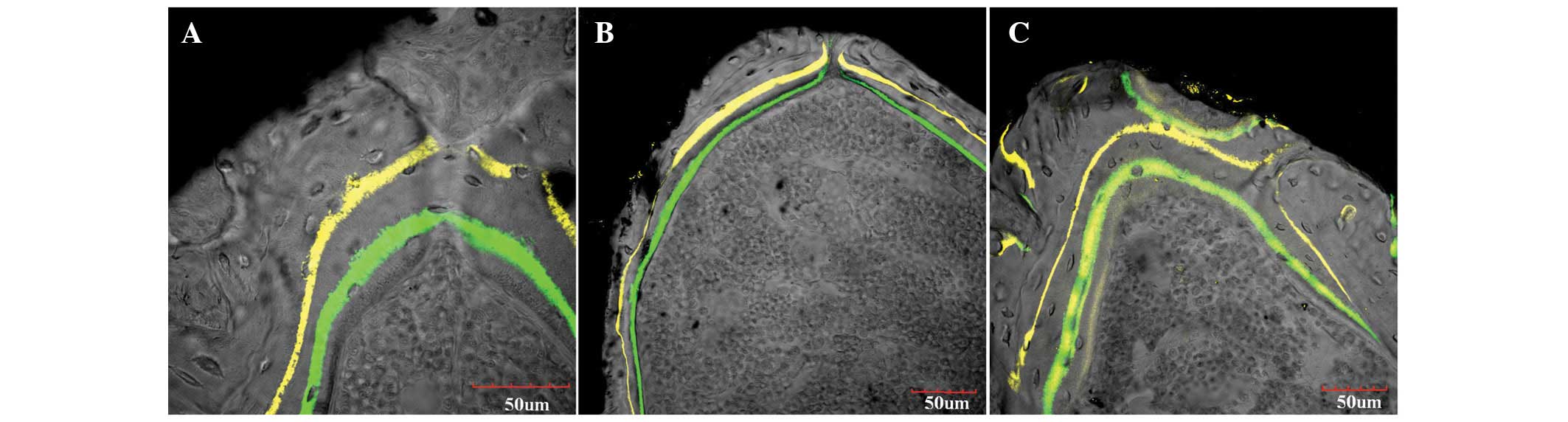
Fluorescent histology
Confocal microscopy showed strong fluorescent
labeling in the OVX and Sr groups, mainly at the trabecular bone
surface and at the interface between the implant and bone (Fig. 4). The sham group showed strong
fluorescent intensity, with thick and continuous fluorescently
labeled lines, and a wide distance between the two fluorescent
lines. However, in the OVX group, the fluorescence intensity was
weak, the fluorescently labeled lines were thin and discontinuous,
and the distance between the two fluorescent lines was narrow. The
appearance of fluorescent labeling was markedly strengthened in the
Sr group and was greater than that in the OVX group, although it
was not as strong as that in the sham group. The aforementioned
changes were also supported by quantitative analysis. The highest
bone dynamic indices, specifically DDL, MAR and MS/BS values, were
found in the sham group, followed in turn by the Sr and OVX groups
(P<0.05 or P<0.01). Significant differences in these indices
were also observed between the Sr and OVX groups (P<0.05 or
P<0.01). Thus, the Sr group exhibited active bone metabolism
(Table II).
 | Table IIDynamic indices of bone metabolism
(n=12). |
Table II
Dynamic indices of bone metabolism
(n=12).
| Group | DDL (μm) | MAR (μm/day) | MS/BS |
|---|
| Sham | 34.99±3.53 | 2.72±0.20 | 0.28±0.05 |
| OVX | 20.59±3.13a | 1.61±0.30a | 0.16±0.03a |
| Sr | 30.41±3.14a,b | 2.30±0.40b,c | 0.20±0.03c,d |
Biomechanical test
The removal torques of the titanium implants in the
sham, OVX and Sr groups were 27.94±1.43, 22.04±2.11 and 25.30±1.38
N.cm respectively. The removal torques in the OVX and Sr groups
were significantly increased when compared with that of the sham
group (P<0.01). The removal torque in the Sr group was higher
than that in the OVX group (P<0.01).
Discussion
Osteoporosis has brought many challenges to
clinicians. Osteoporosis and osteopenia restrict orthodontic
implant anchorage and tooth implantation in adults. A previous
study showed that implant failure in patients with osteoporosis is
associated with the thickness of cortical bone (13). In the present study, it was found
that new bone formation was increased around the Sr coating, and
implant osseointegration was promoted. This suggests that Sr may
improve the stability of implants.
Implant osseointegration in patients with
osteoporosis is affected by reduced bone mass and bone tissue
microstructural changes (3).
However, Motohashi et al discovered that bone formation and
distribution around the implant in rats with osteoporosis are the
same as those in normal rats in the early stage of osteoporosis;
however the absorption rate of new bone is accelerated in the later
stage of osteoporosis (14). The
studies conducted by Mori et al (15) and Fujimoto et al (16) demonstrated that new bone formation
around the implant in osteoporotic animals was delayed compared
with that in healthy animals. Moreover, osteoporosis affects bone
healing around the implant. Due to a reduction of cancellous bone
in patients with osteoporosis, these patients require more frequent
monitoring of implant stability.
After the implant is implanted in bone, the implant
is not able to bear the orthodontic force if healthy bone is
lacking around the mini-implant (17). The success of a mini-implant is
determined by the stability of the connection between the implant
and the bone tissue. Reduced estrogen levels can accelerate the
bone metabolic rate and result in decreased quality and density of
bone, which affects the initial stability of the implant (18). Osteopenia can develop into
osteoporosis (19). Therefore, the
timely intervention of osteopenia is necessary for the management
of osteoporosis.
Sr reduces bone resorption by inhibiting the
proliferation of osteoclasts and improving bone formation by
stimulating the proliferation of osteoblasts (20). Sr has also been shown to enhance
osteogenic cell replication and the activity of osteoblasts,
including the synthesis of bone matrix and the activity of alkaline
phosphatase, in addition to reducing osteoclast markers generated
in the differentiation of bone marrow cells, inhibiting the
differentiation of osteoclasts and reducing the activity of
osteoclasts (21,22). Furthermore, Sr stimulates the
osteogenic differentiation of bone marrow mesenchymal stem cells
and other progenitor cells (23).
Studies have confirmed that Sr-doped hydroxyapatite, calcium
phosphate, calcium silicate, calcium sulfate, boron bioactive glass
and other materials, promote bone tissue reconstruction and new
bone formation (24–26). In the present study, Sr
significantly enhanced bone formation in ovariectomized rats with
Sr-coated implants. This implies that tooth implants with a Sr
coating may be useful in osteopenic patients. However, the
application of Sr coatings requires further investigation for
optimization.
The Sr coatings were deposited on the surfaces of
pure titanium implants by ECD. Four weeks after implantation of the
Sr-coated implants into the tibiae of the rats with osteopenia, the
histomorphometric indices IBCR, BV/TV and T in the Sr group were
observed to be significantly higher than those of the OVX group.
These changes were also supported by quantitative analysis. The
bone dynamic indices DDL, MAR and MS/BS in the Sr group were
significantly higher than those of the OVX group. The Sr group
exhibited active bone metabolism. The histomorphometric indices and
bone dynamic indices confirmed that the presence of Sr at the
surface of the implant promoted osteoblast function and inhibited
osteoclast function. Ultimately, it promoted osseointegration and
increased the removal torque.
In conclusion, Sr coatings are easily made by ECD.
They have a promoting effect on implant osseointegration in animals
with osteopenia, and provide a new proposition for patients with
osteoporosis who require dental implants.
Acknowledgements
The study was partly supported by a National Natural
Science Foundation of China (no. 81270965) and the Beijing Natural
Science Foundation (7112124).
References
|
1
|
Akca K, Sarac E, Baysal U, Fanuscu M,
Chang TL and Cehreli M: Micro-morphologic changes around
biophysically-stimulated titanium implants in ovariectomized rats.
Head Face Med. 3:282007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldhahn J, Seebeck J, Frei R, Frenz B,
Antoniadis I and Schneider E: New implant designs for fracture
fixation in osteoporotic bone. Osteoporos Int. 16(Suppl 2):
S112–S119. 2005. View Article : Google Scholar
|
|
3
|
Marco F, Milena F, Gianluca G and Vittoria
O: Peri-implant osteogenesis in health and osteoporosis. Micron.
36:630–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soen S, Fukunaga M, Sugimoto T, et al:
Japanese Society for Bone and Mineral Research and Japan
Osteoporosis Society Joint Review Committee for the Revision of the
Diagnostic Criteria for Primary Osteoporosis: Diagnostic criteria
for primary osteoporosis: year 2012 revision. J Bone Miner Metab.
31:247–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viera-Negrón YE, Ruan WH, Winger JN, Hou
X, Sharawy MM and Borke JL: Effect of ovariectomy and alendronate
on implant osseointegration in rat maxillary bone. J Oral
Implantol. 34:76–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du Z, Chen J, Yan F and Xiao Y: Effects of
Simvastatin on bone healing around titanium implants in
osteoporotic rats. Clin Oral Implants Res. 20:145–150. 2009.
View Article : Google Scholar
|
|
7
|
Ohkawa Y, Tokunaga K and Endo N:
Intermittent administration of human parathyroid hormone (1–34)
increases new bone formation on the interface of hydroxyapatite
coated titanium rods implanted into ovariectomized rat femora. J
Orthop Sci. 3:533–542. 2008. View Article : Google Scholar
|
|
8
|
Brennan TC, Rybchyn MS, Green W, Atwa S,
Conigrave AD and Mason RS: Osteoblasts play key roles in the
mechanisms of action of strontium ranelate. Br J Pharmacol.
157:1291–1300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fonseca JE: Rebalancing bone turnover in
favour of formation with strontium ranelate: implications for bone
strength. Rheumatology (Oxford). 47(Suppl 4): iv17–iv19. 2008.
View Article : Google Scholar
|
|
10
|
Le Nihouannen D, Hacking SA, Gbureck U,
Komarova SV and Barralet JE: The use of RANKL-coated brushite
cement to stimulate bone remodelling. Biomaterials. 29:3253–3259.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi MC, Zhou XQ, Hu J, et al: Oestrogen
replacement therapy promotes bone healing around dental implants in
osteoporotic rats. Int J Oral Maxillofac Surg. 33:279–285. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi M, Hu J, Li J, et al: Effect of
zoledronate acid treatment on osseointegration and fixation of
implants in autologous iliac bone grafts in ovariectomized rabbits.
Bone. 50:119–127. 2012. View Article : Google Scholar
|
|
13
|
Herrmann I, Lekholm U, Holm S and Kultje
C: Evaluation of patient and implant characteristics as potential
prognostic factors for oral implant failures. Int J Oral Maxillofac
Implants. 20:220–230. 2005.PubMed/NCBI
|
|
14
|
Motohashi M, Shirota T, Tokugawa Y, Ohno
K, Michi K and Yamaguchi A: Bone reactions around
hydroxyapatite-coated implants in ovariectomized rats. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 87:145–152. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mori H, Manabe M, Kurachi Y and Nagumo M:
Osseointegration of dental implants in rabbit bone with low mineral
density. J Oral Maxillofac Surg. 55:351–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujimoto T, Niimi A, Sawai T and Ueda M:
Effects of steroid-induced osteoporosis on osseointegration of
titanium implants. Int J Oral Maxillofac Implants. 13:183–189.
1998.PubMed/NCBI
|
|
17
|
Kang S, Lee SJ, Ahn SJ, Heo MS and Kim TW:
Bone thickness of the palate for orthodontic mini-implant anchorage
in adults. Am J Orthod Dentofacial Orthop. 131(4 Suppl): S74–S81.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohmae M, Saito S, Morohashi T, et al: A
clinical and histological evaluation of titanium mini-implants as
anchors for orthodontic intrusion in the beagle dog. Am J Orthod
Dentofacial Orthop. 119:489–497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozawa S, Ogawa T, Iida K, et al:
Ovariectomy hinders the early stage of bone-implant integration:
histomorphometric, biomechanical, and molecular analyses. Bone.
30:137–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar
|
|
21
|
Ni GX, Yao ZP, Huang GT, Liu WG and Lu WW:
The effect of strontium incorporation in hydroxyapatite on
osteoblasts in vitro. J Mater Sci Mater Med. 22:961–967. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhary S, Halbout P, Alander C, Raisz L
and Pilbeam C: Strontium ranelate promotes osteoblastic
differentiation and mineralization of murine bone marrow stromal
cells: involvement of prostaglandins. J Bone Miner Res.
22:1002–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Capuccini C, Torricelli P, Sima F, et al:
Strontium-substituted hydroxyapatite coatings synthesized by
pulsed-laser deposition: in vitro osteoblast and osteoclast
response. Acta Biomater. 4:1885–1893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caverzasio J and Thouverey C: Activation
of FGF receptors is a new mechanism by which strontium ranelate
induces osteoblastic cell growth. Cell Physiol Biochem. 27:243–250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baier M, Staudt P, Klein R, et al:
Strontium enhances osseointegration of calcium phosphate cement: a
histomorphometric pilot study in ovariectomized rats. J Orthop Surg
Res. 8:162013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan HB, Zhao XL, Zhang X, et al: Strontium
borate glass: potential biomaterial for bone regeneration. J R Soc
Interface. 7:1025–1031. 2010. View Article : Google Scholar :
|