Introduction
Apolipoprotein E (APOE) is an important plasma
protein involved in lipoprotein metabolism and the transport of
cholesterol and triglyceride (1–3).
There are three types of common variant alleles (ɛ2, ɛ3 and ɛ4) in
the world, which result from two single nucleotide polymorphisms
(rs429358 and rs7412) on the APOE gene. These variant alleles can
affect APOE gene transcription and serum levels of cholesterol and
triglyceride (4). Epidemiological
studies have indicated that there is a notable association between
APOE gene polymorphism and a serious risk of cardiovascular disease
or certain infectious diseases (4–6).
Individuals inherit one allele of APOE from each of their parents,
thus yielding six possible genotypes: ɛ2/ɛ2, ɛ2/ɛ3, ɛ2/ɛ4, ɛ3/ɛ3,
ɛ3/ɛ4 and ɛ4/ɛ4 (7). The frequency
of APOE genotypes varies among ethnic groups, but wild-type ɛ3/ɛ3
is the most frequent genotype in all populations (8,9).
Various methods have been developed to detect APOE
genotypes, including allele-specific polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP)
analysis (10,11), PCR-single-strand conformational
polymorphism analysis (12),
microarrays (13), PCR-DNA
sequencing (14) and
allele-specific PCR (15). These
approaches, however, are expensive or time-consuming and are thus
not appropriate for rapid molecular diagnoses in clinical practice
or for molecular screening in large populations; therefore, the
development of a reliable and rapid method of detecting the common
APOE genotypes would be useful for clinical and population genetic
analyses. High-resolution melting (HRM) analysis is a novel, rapid
and powerful mutation screening technique in which PCR and mutation
scanning are performed simultaneously in a single procedure lasting
<30 min. In the present study, an HRM assay was developed to
identify APOE genotypes rapidly and effectively in the Chinese Han
and African Fang populations.
Materials and methods
Population samples
The study subjects were collected from two ethnic
groups: Between February and December 2012, 100 unrelated healthy
Southern Han Chinese individuals (50 male and 50 female) attended
the study in the Chaozhou region of China (Guangdong, China), and
between February and October 2012, 175 unrelated healthy African
Fang individuals (87 male and 88 female) attended the study on
Bioko Island (Equatorial Guinea). Ethical approval to undertake the
survey was obtained from the Ethics Committees of the Malabo
Regional Hospital (Malabo, Equatorial Guinea) and the Chaozhou
Central Hospital Affiliated to Southern Medical University
(Chaozhou, China). The ages of the subjects ranged from 20 to 65
years. Information sheets with nationality, gender, age and
aboriginal status and written consent forms were available in
Chinese or Spanish to ensure comprehensive understanding of the
study objectives, and informed consent was signed or thumb-printed
by the participants. Subsequent to obtaining informed consent, 2-ml
peripheral blood samples were collected into tubes with
EDTA-K2 by the medical laboratories in the Chaozhou
Central Hospital or Malabo Regional Hospital for storage at 4°C
until required.
Strategy for study
A strategy was adopted for detecting the APOE gene
polymorphism (Fig. 1). Firstly,
the heterozygote and homozygote were identified with each of two
paired primers (Table I) by HRM
assay. Secondly, since the melting curve shapes of the homozygous
variants were similar to those of the wild-type, homozygous DNA
samples were mixed with the same amount of reference DNA
(wild-types ɛ3/ɛ3) to generate the heteroduplex, thus making it
easy to separate the homozygous mutations from the wild-types. The
results of the HRM were then analyzed for the identification of the
APOE genotypes. Finally, all amplicons were again ascertained by
DNA sequencing.
 | Table IPrimers for the HRM assay and
polymerase chain reaction-DNA sequencing. |
Table I
Primers for the HRM assay and
polymerase chain reaction-DNA sequencing.
| Name | Primers (5′-3′) | Product (bp) |
|---|
| HRM-rs429358-F |
CGGGCACGGCTGTCCAAG | 91 |
| HRM-rs429358-R |
CGCGGTACTGCACCAGGC | |
| HRM-rs7412-F |
GCAAGCTGCGTAAGCGGCTCC | 112 |
| HRM-rs7412-R |
TCGCGGATGGCGCTGAGG | |
| Sequencing-F |
CCTCCCACTGTGCGACACCCTCC | 532 |
| Sequencing-R |
GTCCGGCTGCCCATCTCCTCCAT | |
DNA isolation
Genomic DNA was extracted from peripheral blood
leukocytes by the DNA blood mini kit (Qiagen Co. Ltd., Shanghai,
China). The DNA concentration was determined using an ultraviolet
spectrophotometer [Unico (Shanghai) Instruments Co., Ltd.,
Shanghai, China] at a wavelength of 260 nm. All DNA templates were
adjusted to 50 ng/μl concentration. The DNA samples were stored at
−80°C until required and would be used for the subsequent HRM
analysis and DNA sequencing.
APOE genotyping by HRM analysis
Oligo 6.64 (Molecular Biology Insights Inc.,
Cascade, CO, USA) and Primer Premier 5.0 (Premier Biosoft, Palo
Alto, CA, USA) software were used for primer design. Two sets of
PCR primers were designed to amplify the regions encompassing
rs7412 [Human genome variation society (HGVS) name:
NC_000019.9:g.45412079C>T] and rs429358 (HGVS name:
NC_000019.9:g.45411941T>C). The amplification length and
localization of all primers are indicated in Table I. The synthesized primers were all
of standard molecular biology quality (Shanghai Invitrogen
Biotechnology Co. Ltd, Shanghai, China).
PCR amplification was carried out with LightCycler
480 II (Roche Diagnostics GmbH, Mannheim, Germany). For the PCR
reaction, each tube contained, in a final volume of 20 μl, 100 ng
genomic DNA, 100 μM each deoxynucleotide triphosphate (dNTP), 0.2
μM each primer, 1.0 μl LC Green Plus® (Idaho Technology
Inc., Salt Lake City, UT, USA), 4.0 μl 5X PCR buffer, 0.5 units
HotStart Taq DNA polymerase (Takara, Dalian, China) and 9.2 μl
double-distilled H2O. The reaction conditions were 95°C
for 5 min, followed by 35 cycles at 98°C for 10 sec and 68°C for 20
sec.
Following amplification, the samples were incubated
at 95°C for 1 min and then at 40°C for 1 min. Melting curve
profiles were generated by increasing the temperature from 65 to
95°C, and fluorescence was continuously acquired at a ramping rate
of 0.05°C/sec with 25 acquisitions per degree. HRM analysis was
performed by the LightCycler 480 SW 1.5 software (Roche Diagnostics
GmbH). The samples with known mutations, which had been validated
by DNA sequencing, were used as standard references. The plots of
samples were identified as the same mutation of the standard when
they were classified into the standard reference.
PCR-DNA sequencing
DNA sequencing of the APOE gene was performed with a
set of primers (Table I). The
reaction mixture (a volume of 50 μl) consisted of 100 ng genomic
DNA, 2.0 mM MgCl2, 1.0 μM each primer, 200 μM dNTP, 5 μl
10X PCR buffer and 2.5 units Taq DNA polymerase (Takara). Reactions
were carried out in an MJ Mini Personal Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with an initial denaturing
step of 95°C for 10 min and then 35 cycles of 95°C for 1 min, 58°C
for 1 min and 72°C for 1.5 min with a final extension at 72°C for
10 min. A total of 10 μl PCR product was subsequently fractionated
on a 1% agar gel to check for the integrity of the products. The
PCR products were then sequenced using an ABI 3730xL DNA Sequencer
(Perkin-Elmer Applied Biosystems, Norwalk, CT, USA).
Statistical analysis
Statistical analyses were performed using SPSS
(version 16.0) statistical software (SPSS Inc., Chicago, IL, USA).
The allele frequencies and genotype distributions were calculated
by the gene-counting method (16).
The χ2 or Fisher’s exact test was used not only to
evaluate the allelic and genotypic frequencies, but also to
estimate the Hardy-Weinberg equilibrium. P<0.05 was considered
to indicate a statistically significant difference.
Results
HRM analysis of APOE genotypes
A total of 275 samples (100 Chinese and 175 African)
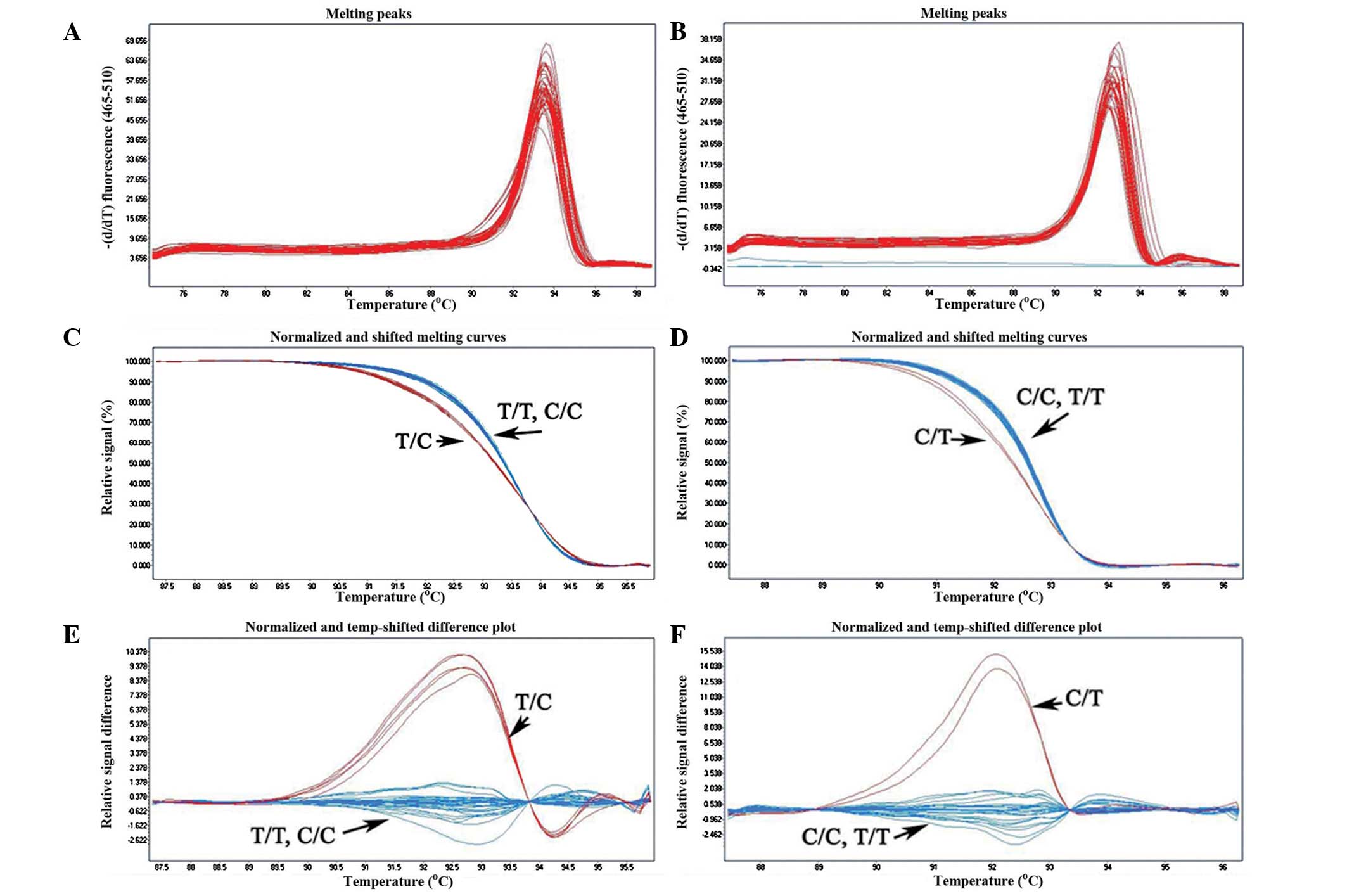
were analyzed by the HRM method. From Fig. 2A and B, only a single sharp peak
was found in the melting curve shapes. This indicated that there
was no nonspecific product during the reaction. Heterozygous
mutation could be easily distinguished from the wild-type, but the
homozygous mutation and wild-type exhibited almost
indistinguishable melting curve profiles (Fig. 2C–F); therefore, a strategy was
formulated to solve the problem (Fig.
1). Wild-type DNA (ɛ3/ɛ3) was added to produce the heteroduplex
DNA, and then the melting curves of the homozygous mutations could
be distinguished from those of the wild-types. Compared with the
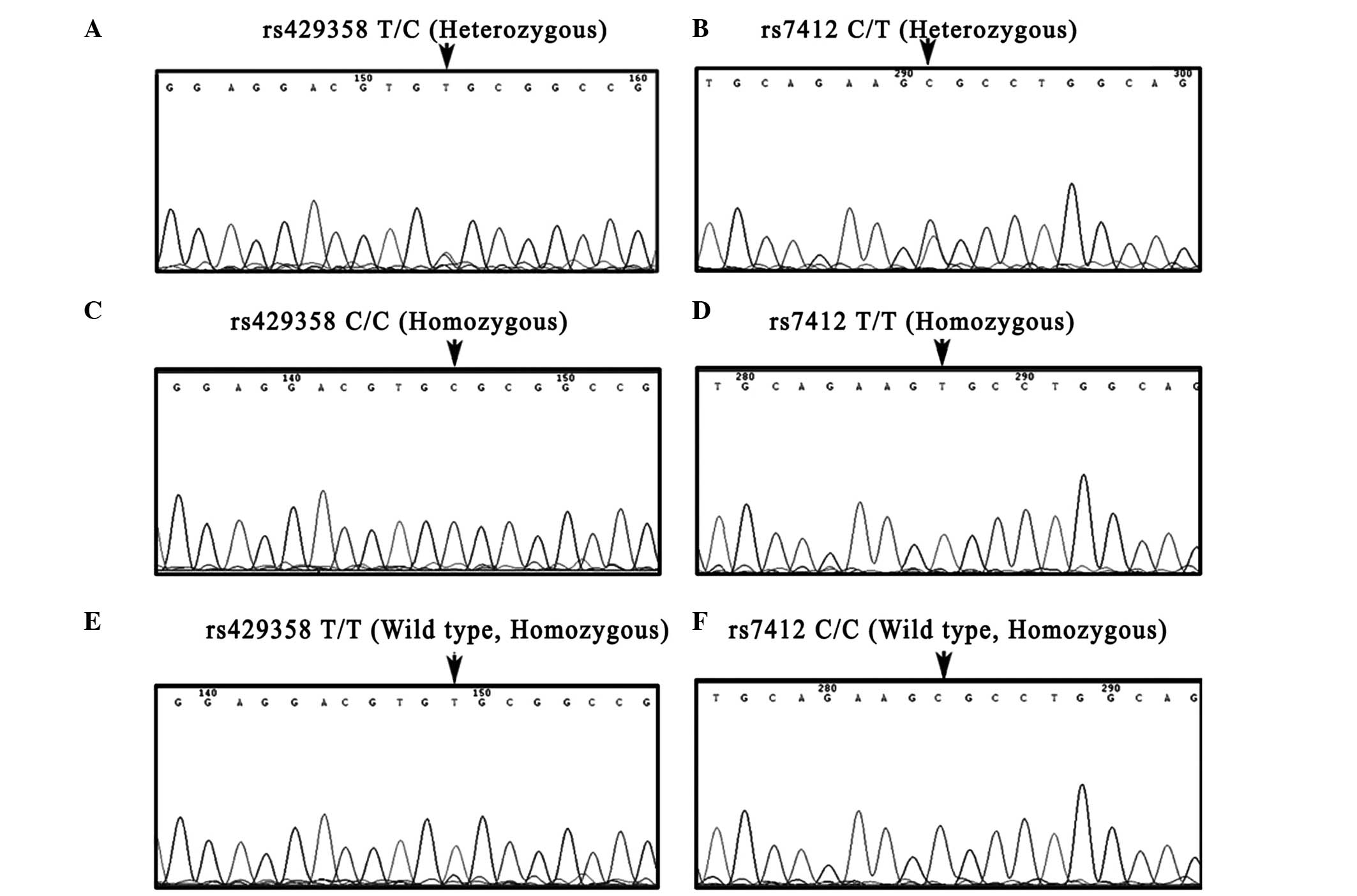
results of the reference method (PCR-DNA sequencing) (Fig. 3), all 275 samples were rapidly and
efficiently identified by HRM analysis. The concordance was
100%.
Frequency distributions of APOE
The frequencies of the APOE genotypes in the
Southern Chinese Han and African Fang populations are shown in
Table II. The genotype
distributions did not deviate from Hardy-Weinberg equilibrium for
the population (P>0.05). Consistent with previous reports
(5–7), ɛ3/ɛ3 was observed to be the most
common genotype in the Southern Han (78%, 78/100) and African Fang
(42.9%, 75/175) populations. In addition, no ɛ4/ɛ4 genotype was
found in the Southern Chinese Han population.
 | Table IIFrequencies of apolipoprotein E
genotypes in the Southern Chinese Han and African Fang
populations. |
Table II
Frequencies of apolipoprotein E
genotypes in the Southern Chinese Han and African Fang
populations.
| Genotypes | Southern Chinese Han,
n (%) | African Fang, n
(%) |
|---|
| ɛ3/ɛ3 | 78 (78.0) | 75 (42.9) |
| ɛ2/ɛ2 | 2 (2.0) | 1 (0.6) |
| ɛ3/ɛ4 | 10 (10.0) | 56 (32.0) |
| ɛ3/ɛ2 | 9 (9.0) | 24 (13.7) |
| ɛ4/ɛ2 | 1 (1.0) | 9 (5.1) |
| ɛ4/ɛ4 | 0 (0.0) | 10 (5.7) |
| Total | 100 (100) | 175 (100) |
The allele frequencies of APOE in the Southern
Chinese Han population were 7.0, 87.5 and 5.5% for ɛ2, ɛ3 and ɛ4,
respectively (Table III). In the
African Fang population, the allele frequencies of APOE were 24.3,
65.7 and 10.0% for ɛ2, ɛ3 and ɛ4, respectively (Table III). A statistically significant
difference was found between the allele frequencies between the
populations (P<0.05).
 | Table IIIAllele frequencies of the
apolipoprotein E gene in various populations. |
Table III
Allele frequencies of the
apolipoprotein E gene in various populations.
| | | Apolipoprotein E
allele frequencies |
|---|
| | |
|
|---|
| First author, year
(ref.) | Population | n | ɛ2 (%) | ɛ3 (%) | ɛ4 (%) |
|---|
| Present data | Han (Chaozhou,
China) | 100 | 7.0 | 87.5 | 5.5 |
| Wang, 2012
(18) | Han (Xinjiang,
China) | 150 | 8.1 | 77.2 | 14.6 |
| Hu, 2011 (19) | Han (Guangxi,
China) | 200 | 9.2 | 81.4 | 9.3 |
| Kao, 1995 (20) | Han (Taiwan,
China) | 564 | 7.6 | 87.5 | 4.9 |
| Wang, 1988
(21) | Han (Beijing,
China) | 95 | 5.3 | 88.3 | 6.4 |
| Wang, 1988
(21) | Han (Hubei,
China) | 113 | 9.3 | 83.2 | 7.5 |
| Wang, 1988
(21) | Han (Hunan,
China) | 102 | 5.3 | 88.4 | 6.3 |
| Wang, 1988
(21) | Han (Jiangsu,
China) | 168 | 7.1 | 86.3 | 6.6 |
| Mayila, 2005
(22) | Uygur (Xinjiang,
China) | 163 | 12.0 | 82.1 | 16.7 |
| Hu, 2011 (19) | Zhuang (Guangxi,
China) | 278 | 15.2 | 79.8 | 4.9 |
| Wang, 2012
(18) | Li (Hainan,
China) | 50 | 9.0 | 76.0 | 15.0 |
| Present data | African Fang
(Equatorial Guinea) | 175 | 24.3 | 65.7 | 10.0 |
| Wozniak, 2003
(26) | African
(Ghana) | 110 | 14.5 | 61.4 | 24.1 |
| Wozniak, 2003
(26) | African (Central
African Rep) | 70 | 5.7 | 53.6 | 40.7 |
| Wozniak, 2003
(26) | African (1,
Nigeria) | 97 | 10.3 | 74.2 | 24.1 |
| Wozniak, 2003
(26) | African (2,
Nigeria) | 781 | 6.4 | 68.4 | 25.2 |
| Wozniak, 2003
(26) | African
(Sudan) | 103 | 8.3 | 62.6 | 29.1 |
| Wozniak, 2003
(26) | African
(Ethiopia) | 164 | 3.0 | 81.1 | 15.8 |
| Wozniak, 2003
(26) | African
(Morocco) | 100 | 6.5 | 85.0 | 8.5 |
| Wozniak, 2003
(26) | African (South
Africa) | 247 | 7.7 | 55.3 | 37.0 |
Discussion
In previous investigations, PCR-RFLP has been the
most common method for APOE polymorphism identification (10,11).
The steps of PCR-RFLP include the PCR reaction, treatment of
amplified fragments by the restriction enzyme HhaI and gel
electrophoresis (10,11). As such, this technique is
time-consuming and costly for a large-scale analysis. In the
present study, an HRM analysis method was adopted for the
identification of APOE genotypes. HRM analysis is a more rapid,
cost-effective and convenient closed-tube genotyping approach for
the screening of genetic disorders (16,17).
This technique could not only reduce the contamination risk, but
also be applied to a high-throughput gene mutation screening of a
large cohort of patients when required (16,17).
The present results showed 100% concordance between HRM analysis
and the reference method (PCR-DNA sequencing). This indicated that
HRM analysis could be used as an accurate and sensitive method for
the rapid screening and identification of APOE genotypes.
The APOE allele frequencies in the Chinese Han
population, which were collected from the Chaozhou region, were
7.0% for ɛ2, 87.5% for ɛ3 and 5.5% for ɛ4. Compared with other
Chinese populations (Table III)
(18–22), the APOE gene allele frequencies of
the study population were most similar to those of a Taiwanese
population (20), but
significantly different from those of the Chinese minority ethnic
groups: The Uygur population in the Xinjiang Uygur Autonomous
Region (22), the Li population on
Hainan Island (18) and the Zhuang
population in the Guangxi Zhuang Autonomous Region (19). A number of factors may be used to
explain this finding. Firstly, the Southern Han population in the
Chaozhou region, known as the Fulao peoples, largely comes from
Henan and Shanxi via Fujian with the well-maintained language and
customs of north-central China. The majority of the Fulao peoples
first settled in Fujian, and then migrated to the Chaoshan region.
Due to geographic isolation and the historical problems of
population migration, the Fulao became a relatively isolated
population. Notable genetic similarities have been found between
the Chaoshan Han and Fujian Han populations (23). Secondly, Fujian faces Taiwan across
the sea. The populations on the two sides of the straits of Taiwan
are closely associated since they have the same ancestors, speak
the same dialect and share the same customs and cultural
traditions. Statistics published in Taiwan (20) have stated that the Taiwanese
population is predominantly (80%) comprised of individuals of
Fujian origin. We therefore hypothesize that the considerable
similarities in APOE allele frequencies are due to the common
genetic background shared between the Chaozhou Han and Taiwan Han
populations.
The samples from individuals of the African Fang
population (an ethnic group of Bantu origin) were collected from
Bioko Island in Equatorial Guinea (24,25).
The APOE allele frequencies of the Fang population were 24.3% for
ɛ2, 65.7% for ɛ3 and 10.0% for ɛ4. The frequency of APOE ɛ2 (24.3%)
in the Fang population was higher than almost all the other known
values for sub-Saharan African populations (the Pygmy, Nigerian,
Sudanese, Ethiopian, Ghanaian and central African populations)
(Table III), but the APOE ɛ4
allele frequency (10.0%) was lower than the values for these
sub-Saharan African populations (26,27)
(Table III). Bioko Island is
characterized as a humid tropical environment with hyper-endemic
malaria transmission (28). As a
significant threat to human life, malaria has exerted the strongest
known selection pressure on the human genome in the past 10,000
years since the origination of agriculture. Previous studies have
reported that there may be a close association between APOE gene
polymorphism and infection with malaria (29–31).
For example, a study of the interactions between the proteins of
Plasmodium falciparum and human APOE indicated a
preferential interaction of the P. falciparum PFE1590w
protein with human APOE ɛ3 and APOE ɛ4, but not APOE ɛ2 (29). This means that individuals carrying
APOE ɛ3 and ɛ4 alleles are more likely to develop severe malaria
(cerebral malaria and severe anemia) (29); therefore, the higher APOE ɛ2 allele
frequency in the Fang population on Bioko Island may be the result
of selection due to malaria. This hypothesis requires future
studies for its confirmation.
In conclusion, the present study provides the first
molecular characterization of the APOE gene polymorphism in the Han
population from Southern China and Fang population from Equatorial
Guinea. These data could be useful for future genetic
investigations of a number of disease risks within the Southern Han
and Fang populations. The present results also indicated that HRM
analysis could be used as an accurate and sensitive method for the
rapid screening and identification of APOE genotypes in prospective
clinical and population genetic analyses.
Acknowledgements
This study was partially supported by the National
Natural Science Foundation of China (contract/grant no. 81101329),
the Social Development Program of Guangdong (contract/grant no.
2011B031800329), the China Postdoctoral Science Foundation funded
project (contract/grant no. 2013M542195) and the Medical Science
Fund of Guangdong (contract/grant no. A2013780).
References
|
1
|
Kaneva AM, Bojko ER, Potolitsyna NN and
Odland JO: Plasma levels of apolipoprotein-E in residents of the
European North of Russia. Lipids Health Dis. 12:432013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahley RW, Innerarity TL, Rall SC Jr and
Weisgraber KH: Plasma lipoproteins: apolipoprotein structure and
function. J Lipid Res. 25:1277–1294. 1984.PubMed/NCBI
|
|
3
|
Eichner JE, Dunn ST, Perveen G, et al:
Apolipoprotein E polymorphism and cardiovascular disease: a HuGE
review. Am J Epidemiol. 155:487–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pilia G, Chen WM, Scuteri A, et al:
Heritability of cardiovascular and personality traits in 6,148
Sardinians. PLoS Genet. 2:e1322006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin YW, Sun QQ, Zhang BB, et al:
Association between apolipoprotein E gene polymorphism and the risk
of coronary artery disease in Chinese population: evidence from a
meta-analysis of 40 studies. PLoS One. 8:e669242013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaisi-Raygani A, Rahimi Z, Nomani H,
Tavilani H and Pourmotabbed T: The presence of apolipoprotein
epsilon4 and epsilon2 alleles augments the risk of coronary artery
disease in type 2 diabetic patients. Clin Biochem. 40:1150–1156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lahiri DK, Sambamurti K and Bennett DA:
Apolipoprotein gene and its interaction with the environmentally
driven risk factors: molecular, genetic and epidemiological studies
of Alzheimer’s disease. Neurobiol Aging. 25:651–660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerdes LU: The common polymorphism of
apolipoprotein E: geographical aspects and new pathophysiological
relations. Clin Chem Lab Med. 41:628–631. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corbo RM and Scacchi R: Apolipoprotein E
(APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’
allele? Ann Hum Genet. 63:301–310. 1999. View Article : Google Scholar
|
|
10
|
El-Tagui MH, Hamdy MM, Shaheen IA, Agha H
and Abd-Elfatah HA: Apolipoprotein E gene polymorphism and the risk
of left ventricular dysfunction among Egyptian β-thalassemia major.
Gene. 524:292–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saidi S, Slamia LB, Ammou SB, Mahjoub T
and Almawi WY: Association of apolipoprotein E gene polymorphism
with ischemic stroke involving large-vessel disease and its
relation to serum lipid levels. J Stroke Cerebrovasc Dis.
16:160–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamruecha W, Chansirikarnjana S, Nimkulrat
E, et al: Rapid detection of apolipoprotein E genotypes in
Alzheimer’s disease using polymerase chain reaction-single strand
conformation polymorphism. Southeast Asian J Trop Med Public
Health. 37:793–797. 2006.PubMed/NCBI
|
|
13
|
Calabretta A, Tedeschi T, Di Cola G, et
al: Arginine-based PNA microarrays for APOE genotyping. Mol
Biosyst. 5:1323–1330. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johansson Å, Enroth S, Palmblad M, et al:
Identification of genetic variants influencing the human plasma
proteome. Proc Natl Acad Sci USA. 110:4673–4678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Darawi MN, Ai-Vyrn C, Ramasamy K, et al:
Allele-specific polymerase chain reaction for the detection of
Alzheimer’s disease-related single nucleotide polymorphisms. BMC
Med Genet. 14:272013. View Article : Google Scholar
|
|
16
|
Pan M, Lin M, Yang L, et al:
Glucose-6-phosphate dehydrogenase (G6PD) gene mutations detection
by improved high-resolution DNA melting assay. Mol Biol Rep.
40:3073–3082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furtado LV, Weigelin HC, Elenitoba-Johnson
KS and Betz BL: A multiplexed fragment analysis-based assay for
detection of JAK2 exon 12 mutations. J Mol Diagn. 15:592–599. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YQ, Wu CJ, Yao M, Zhang YA and Zheng
LL: Study on the apo E gene polymorphism in Li population patients
with cardiovascular and cerebrovascular disease. Jianyan Yixue.
27:308–310. 3152012.(In Chinese).
|
|
19
|
Hu P, Qin YH, Lei FY, et al: Variable
frequencies of apolipoprotein E genotypes and its effect on serum
lipids in the Guangxi Zhuang and Han children. Int J Mol Sci.
12:5604–5615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kao JT, Tsai KS, Chang CJ and Huang PC:
The effects of apolipoprotein E polymorphism on the distribution of
lipids and lipoproteins in the Chinese population. Atherosclerosis.
114:55–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang KQ, Xie YH and He JL: The
investigation of apolipoprotein E polymorphism and genotype
distribution in the Chinese populations (Beijing and Tianjing).
Shengwu Huaxue Zazhi. 18:48–50. 1988.(In Chinese).
|
|
22
|
Mayila W, Fang MW, Cheng ZH and Qiu CC:
Polymorphism of apolipoprotein E gene and natural longevity in the
Xinjiang Uighur people: an association study. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi. 22:462–463. 2005.(In Chinese). PubMed/NCBI
|
|
23
|
Huang H, Su M, Li X, et al: Y-chromosome
evidence for common ancestry of three Chinese populations with a
high risk of esophageal cancer. PLoS One. 5:e111182010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mas J, Yumbe A, Solé N, Capote R and
Cremades T: Prevalence, geographical distribution and clinical
manifestations of onchocerciasis on the Island of Bioko (Equatorial
Guinea). Trop Med Parasitol. 46:13–18. 1995.PubMed/NCBI
|
|
25
|
Calzada P, Suárez I, García S, et al: The
Fang population of Equatorial Guinea characterised by 15 STR-PCR
polymorphisms. Int J Legal Med. 119:107–110. 2005. View Article : Google Scholar
|
|
26
|
Wozniak MA, Faragher EB, Todd JA, et al:
Does apolipoprotein E polymorphism influence susceptibility to
malaria? J Med Genet. 40:348–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerdes LU: The common polymorphism of
apolipoprotein E: geographical aspects and new pathophysiological
relations. Clin Chem Lab Med. 41:628–631. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleinschmidt I, Sharp B, Benavente LE, et
al: Reduction in infection with Plasmodium falciparum one year
after the introduction of malaria control interventions on Bioko
Island, Equatorial Guinea. Am J Trop Med Hyg. 74:972–978.
2006.PubMed/NCBI
|
|
29
|
Vignali M, McKinlay A, LaCount DJ, et al:
Interaction of an atypical Plasmodium falciparum ETRAMP with human
apolipoproteins. Malar J. 7:2112008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wozniak MA, Riley EM and Itzhaki RF:
Apolipoprotein E polymorphisms and risk of malaria. J Med Genet.
41:145–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rougeron V, Woods CM, Tiedje KE, et al:
Epistatic interactions between apolipoprotein E and hemoglobin S
genes in regulation of malaria parasitemia. PLoS One. 8:e769242013.
View Article : Google Scholar : PubMed/NCBI
|