Introduction
Cluster of differentiation (CD) 40 is a member of
the tumor necrosis factor (TNF) receptor superfamily. It is
expressed on the surface of immune cells, including B cells,
monocytes, macrophages, dendritic cells (DCs) and activated T
cells, as well as on the surface of non-immune cells, such as
epithelial, endothelial and mesenchymal cells (including
fibroblasts, myofibroblasts, synoviocytes and stellate cells)
(1,2).
CD154, a CD40 ligand, is preferentially expressed by
activated T cells, activated DCs and activated platelets, although
it can also be variably expressed by monocytes and mononuclear
phagocytes, as well as natural killer, B, CD8+ T, human
vascular endothelial and smooth muscle cells (1–4). The
CD40-CD154 interaction plays a critical role in the regulation of
humoral immunity, cell-mediated immunity and inflammation, and
results in the production of numerous chemokines and cytokines, the
upregulation of adhesion molecules, the secretion of matrix
metalloproteinases (MMPs) and the induction of apoptosis (3). An impaired CD40-CD154 interaction
leads to humoral and cellular immunodeficiency; thus, the
CD40-CD154 co-stimulatory pathway is associated with the
pathogenesis of several diseases, including autoimmune thyroiditis,
type 1 diabetes, inflammatory bowel disease, psoriasis, multiple
sclerosis, rheumatoid arthritis and systemic lupus erythematosus
(4,5).
Soluble CD40 (sCD40) comprises the extracellular
domain of CD40 and is generated via proteolytic cleavage from the
surface of CD40-expressing cells (6,7). The
binding of CD40 to its receptor on CD40-expressing cells can lead
to enhanced sCD40 release (7).
sCD40, as a CD40 antagonist, is able to bind to CD154 and inhibit
CD40-CD154-mediated immune responses by blocking the interaction of
CD40 itself with CD154 (8–10). Circulating sCD40 levels are
elevated in patients with chronic renal failure, chronic liver
diseases, Alzheimer’s disease, systemic sclerosis and hematological
malignancies (10–14).
The CD40-CD154 co-stimulatory pathway is associated
with liver injury and hepatocyte apoptosis (15–17).
Kupffer cells and hepatocytes can express elevated levels of CD40
in hepatitis C virus-associated chronic liver disease (18,19).
CD40-activated B cells and macrophages produce inflammatory
cytokines and contribute to the pathogenesis of necroinflammatory
liver disease (20). Although
CD40-expressing cells have been studied extensively in patients
with liver diseases, limited information is available regarding the
serum levels of sCD40 in these conditions. Schmilovitz-Weiss et
al (14) reported that sCD40
levels were significantly higher in patients with liver disease
than those in controls; however, in their study, only a few
patients with chronic hepatitis B (CHB) were enrolled, and these
patients were not analyzed as a separate group.
Since the pathogenesis in different liver diseases
varies, and the role of sCD40 in CHB has not been clarified, the
levels of sCD40 in sera from patients with CHB were retrospectively
measured in the present study, and their association with
biochemical abnormalities and liver histological characteristics
were analyzed in detail.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Beijing 302 Hospital (Beijing, China), and written
informed consent was obtained from each subject.
Subjects
The patients enrolled in this study had been
admitted to Beijing 302 Hospital between December 2001 and December
2005. The diagnoses were based on standard clinical, biochemical
and histological criteria, according to the guidelines for CHB
(21). Patients with CHB had been
hepatitis B virus surface antigen (HBsAg)-positive for at least six
months and exhibited symptoms of viral hepatitis and abnormal
hepatic function during this period. All the patients were infected
solely with hepatitis B virus (HBV), and no other cause of liver
disease (such as other virus infections, autoimmune disease, drug
hypersensitivity, significant alcohol intake, hemochromatosis or
Wilson’s disease) had been diagnosed in any of the patients.
Patients were excluded from this study if they had other diseases,
such as heart disease, nephritis, cholecystitis and gastritis, or
if they had received antiviral or immunomodulatory treatment. Blood
samples from healthy donors that had reported to the hospital for
physical examination in the corresponding period were used as
controls.
Detection of sCD40
At the time of admission, sera from the healthy
individuals and patients with CHB were collected and stored at
−70°C. In the present study, serum sCD40 concentrations were
simultaneously measured using an ELISA according to the protocol
for the sCD40 Module Set (Bender MedSystems GmbH, Vienna,
Austria).
Laboratory data
Data on laboratory indices, including the serum
levels of alanine transaminase (ALT), aspartate transaminase (AST),
total bilirubin, direct bilirubin (Dbil), globulin, cholinesterase,
alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γGT), total
bile acids (TBA) and hepatitis B virus extracellular antigen
(HBeAg), which had been measured on the date of serum collection,
were retrospectively obtained from the hospital records.
Histology and immunohistochemistry
The patients who had CHB and whose data were
included in the study had undergone a liver biopsy with a Menghini
needle within one week of the date of serum collection. These liver
biopsy specimens were evaluated by a hepatic pathologist who was
unaware of the patients’ clinical and biochemical data or sCD40
levels. The specimens were graded according to the modified
histological activity index (HAI) scoring system described by Ishak
et al (22). The modified
HAI grading and staging scores provided a semi-quantitative
assessment of the observed histological features. The grading
described the intensity of necroinflammatory activity, while the
staging denoted the degree of fibrosis and architectural changes
that occurred in chronic hepatitis (22).
The expression of HBsAg and HBV core antigen (HBcAg)
was determined in formalin-fixed, paraffin-embedded tissue
specimens by indirect immunoperoxidase staining, with
semi-quantitative scoring (0, negative; 1, <25%; 2, 25–49%; 3,
50–74% and 4, ≥75%). Briefly, the liver tissue (5 μm) was incubated
with mouse anti-human HBsAg or HBcAg antibodies (MS-314 and
RB-1413, respectively; 1:50; Maixin Biotech; Fuzhou, China)
overnight at 4°C following the blocking of endogenous peroxidase
activity with 0.3% H2O2.
3,3′-diaminobenzidine was used as the substrate followed by
counterstaining with hematoxylin for single staining.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 12.0; SPSS Inc., Chicago, IL, USA). Quantitative
variables were statistically tested for normality of distribution.
Normal quantitative variables are presented as the mean ± standard
deviation and were analyzed using parametric tests. The values of
serum sCD40 concentration were transformed to their natural log
values and analyzed by one-way analysis of variance and the
Student’s t-test. Skewed quantitative variables are expressed as
the median and interquartile range (IQR) and analyzed using the
Kruskal-Wallis or Mann-Whitney tests. Associations between sCD40
concentrations and other variables were tested using Spearman’s
rank correlation coefficient. χ2 or Fisher’s exact tests
were used for categorical variables. Multiple regression and
comparison of the areas under the receiver operating characteristic
(ROC) curves for sCD40, ALT and AST were performed using MedCalc
software (version 12.0.4; MedCalc Corp., Mariakerke, Belgium).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
sCD40 concentrations were measured in 132 patients
with CHB and 33 healthy individuals, with median ages of 21.7 years
(IQR, 15.3 years) and 21.8 years (IQR, 5.3 years), respectively. No
significant differences in age were observed between the patients
with CHB and healthy individuals (P=0.157). The association between
serum sCD40 concentration and age was insignificant, with a
correlation coefficient of −0.090 for patients with CHB (P=0.306)
and −0.005 for healthy individuals (P=0.979). In addition, the
proportion of male subjects was similar in the two groups (77.3 vs.
87.9%; Pearson χ2 value, 2.266; P=0.312).
Correlations between sCD40 concentration
and laboratory indices
The laboratory data of patients with CHB were
correlated with serum sCD40 levels using Spearman’s rank
correlation coefficient (Table I).
The sCD40 levels in patients with CHB correlated positively with
serum levels of ALT, AST, Dbil, globulin, ALP, γGT and TBA.
 | Table ICorrelations between soluble cluster
of differentiation 40 concentration and laboratory indices in
patients with chronic hepatitis B. |
Table I
Correlations between soluble cluster
of differentiation 40 concentration and laboratory indices in
patients with chronic hepatitis B.
| Variables | N | Valuea | Correlation
coefficient | P-value |
|---|
| ALT (U/l) | 130 | 59 (101)a | 0.487 | <0.001 |
| AST (U/l) | 130 | 45 (66)a | 0.492 | <0.001 |
| Total bilirubin
(μmol/l) | 130 | 10.2 (6.6)a | 0.170 | 0.053 |
| Direct bilirubin
(μmol/l) | 130 | 2.4 (3.6)a | 0.226 | 0.010 |
| Globulin (g/l) | 130 | 24.9 (3.9)b | 0.239 | 0.006 |
| Cholinesterase
(U/l) | 122 | 8321 (2453)b | −0.131 | 0.150 |
| ALP (U/l) | 122 | 96 (156)a | 0.232 | 0.010 |
| γGT (U/l) | 122 | 29 (34)a | 0.499 | <0.001 |
| TBA (μmol/l) | 122 | 7 (6)a | 0.327 | <0.001 |
sCD40 concentration is elevated with
aggravated liver injury in patients with CHB
The sCD40 concentrations in patients with CHB are
shown in Table II. sCD40 levels
in patients with CHB were higher than those in healthy controls
(P<0.001). The difference in sCD40 concentrations between serum
HBeAg-positive and HBeAg-negative CHB patients was not significant
(P=0.488). sCD40 concentrations in patients with CHB correlated
positively with the Ishak score (Spearman correlation coefficient,
0.506; P<0.001).
 | Table IISoluble cluster of differentiation 40
concentrations in patients with chronic hepatitis B. |
Table II
Soluble cluster of differentiation 40
concentrations in patients with chronic hepatitis B.
| Grouping | N | Geometric mean
(pg/ml) |
|---|
| Chronic hepatitis
B | 132 | 82.8 |
|
HBeAg-positive | 87 | 78.7 |
|
HBeAg-negative | 45 | 92.2 |
| Necroinflammatory
grading scorea |
| 0–4 | 66 | 61.8 |
| 5–8 | 43 | 91.7 |
| 9–12 | 15 | 139.0 |
| 13–18 | 8 | 203.2 |
| Fibrosis staging
scorea |
| 0 | 5 | 59.0 |
| 1–2 | 67 | 66.1 |
| 3–4 | 44 | 96.2 |
| 5–6 | 16 | 157.2 |
| Healthy
controls | 33 | 32.8 |
To investigate the sCD40 levels in patients with
different intensities of liver inflammation, the patients with CHB
were distributed into four groups based on their Ishak scores:
Minimal inflammation, scores 1–4; mild inflammation, scores 5–8;
moderate inflammation, scores 9–12; and marked inflammation, scores
13–18. The correlation between these groups and the sCD40 levels
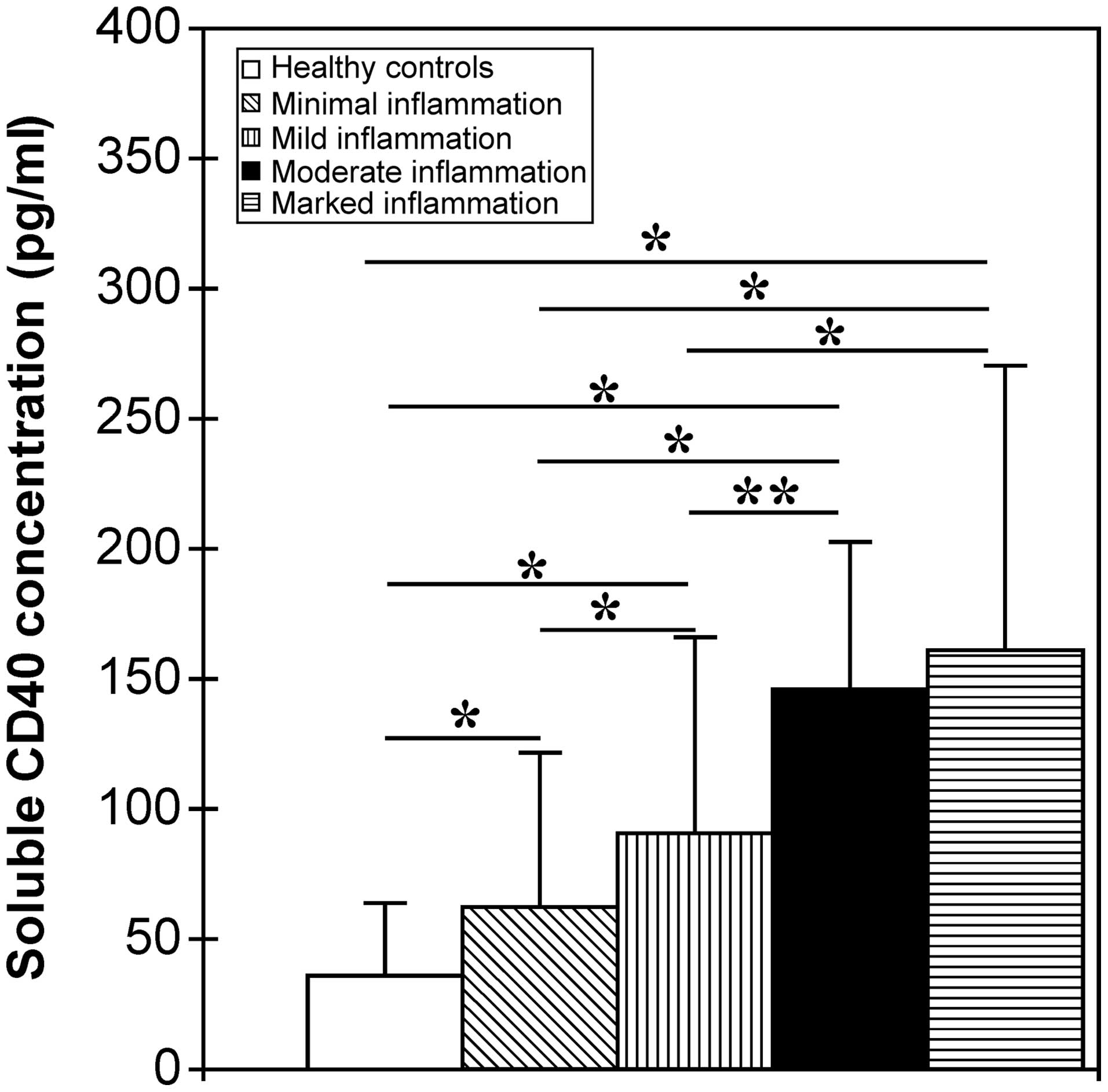
was then assessed. It was found that sCD40 concentrations gradually
rose with increasing liver necroinflammation. The
liver-inflammation groups showed a significantly higher sCD40
concentration than did the healthy control group (P<0.001,
Fig. 1). The sCD40 concentration
in patients with CHB with minimal inflammation was significantly
lower than that in patients with mild, moderate and marked
inflammation (P<0.01), and the sCD40 concentration in patients
with CHB with mild inflammation was significantly lower than that
in patients with moderate and marked inflammation (P<0.05,
Fig. 1). The difference in sCD40
concentrations, however, between individuals with moderate
inflammation and those with marked inflammation was not significant
(P=0.186, Fig. 1).
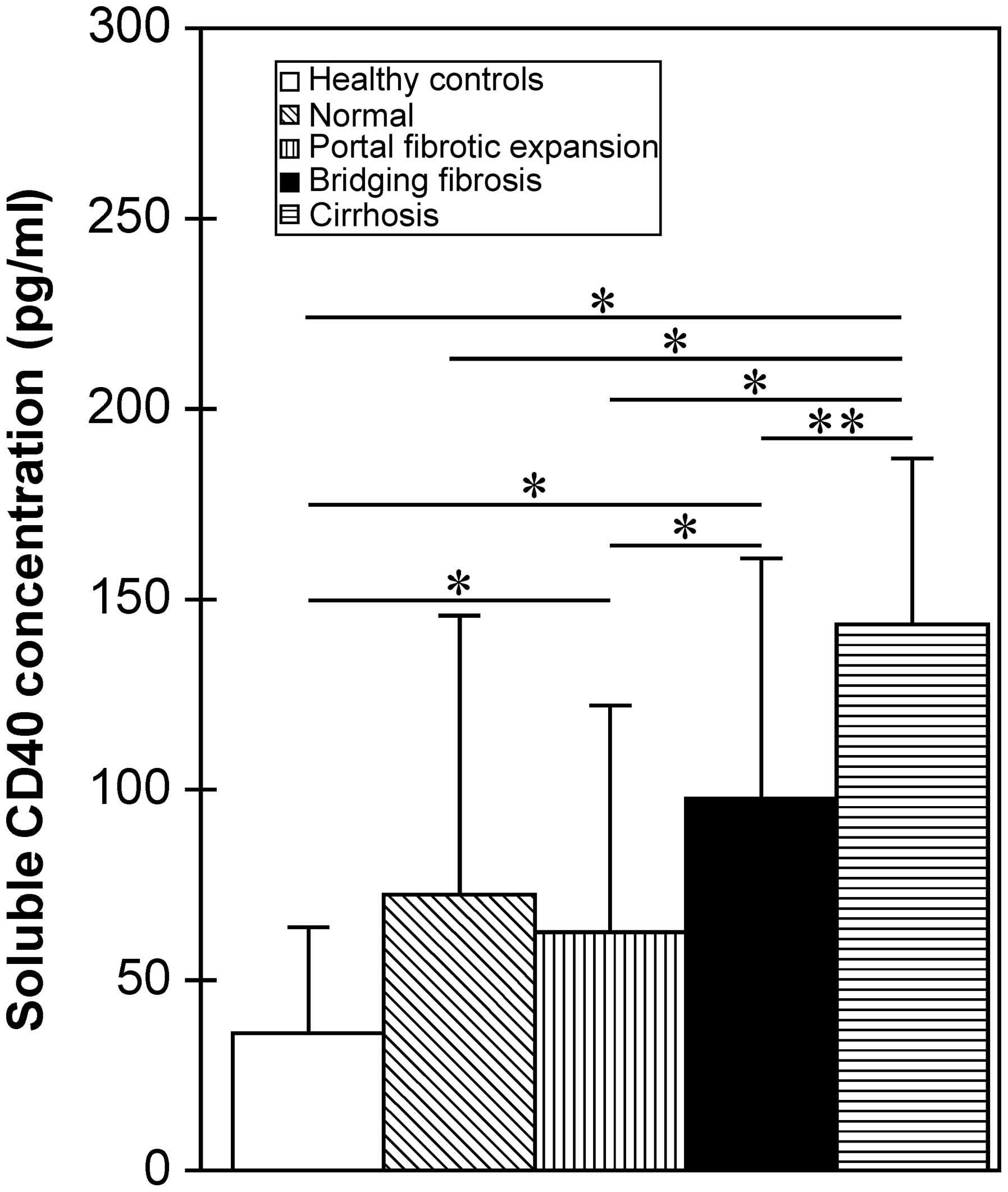
The sCD40 concentration in patients with CHB also
positively correlated with the Ishak fibrosis staging score
(Spearman correlation coefficient, 0.395; P<0.001). Patients
with CHB with different fibrosis staging scores were distributed
into four groups: normal, score 0; portal fibrotic expansion,
scores 1–2; bridging fibrosis, scores 3–4; and cirrhosis, scores
5–6. The correlation between sCD40 level and these groups was then
investigated. It was found that the sCD40 concentration gradually
increased with the aggravation of liver fibrosis (Table II). The difference in sCD40 levels
between patients with CHB without fibrosis (normal group) and the
healthy controls was not significant (P=0.072), whereas groups with
portal fibrotic expansion, bridging fibrosis and cirrhosis showed
significantly higher sCD40 concentrations than did healthy controls
(P<0.001, Fig. 2). The sCD40
concentration in patients with CHB with portal fibrotic expansion
was significantly lower than that in patients with bridging
fibrosis or cirrhosis (P<0.01), and the sCD40 concentration in
patients with CHB with cirrhosis was significantly higher than that
in patients with bridging fibrosis (P<0.05; Fig. 2).
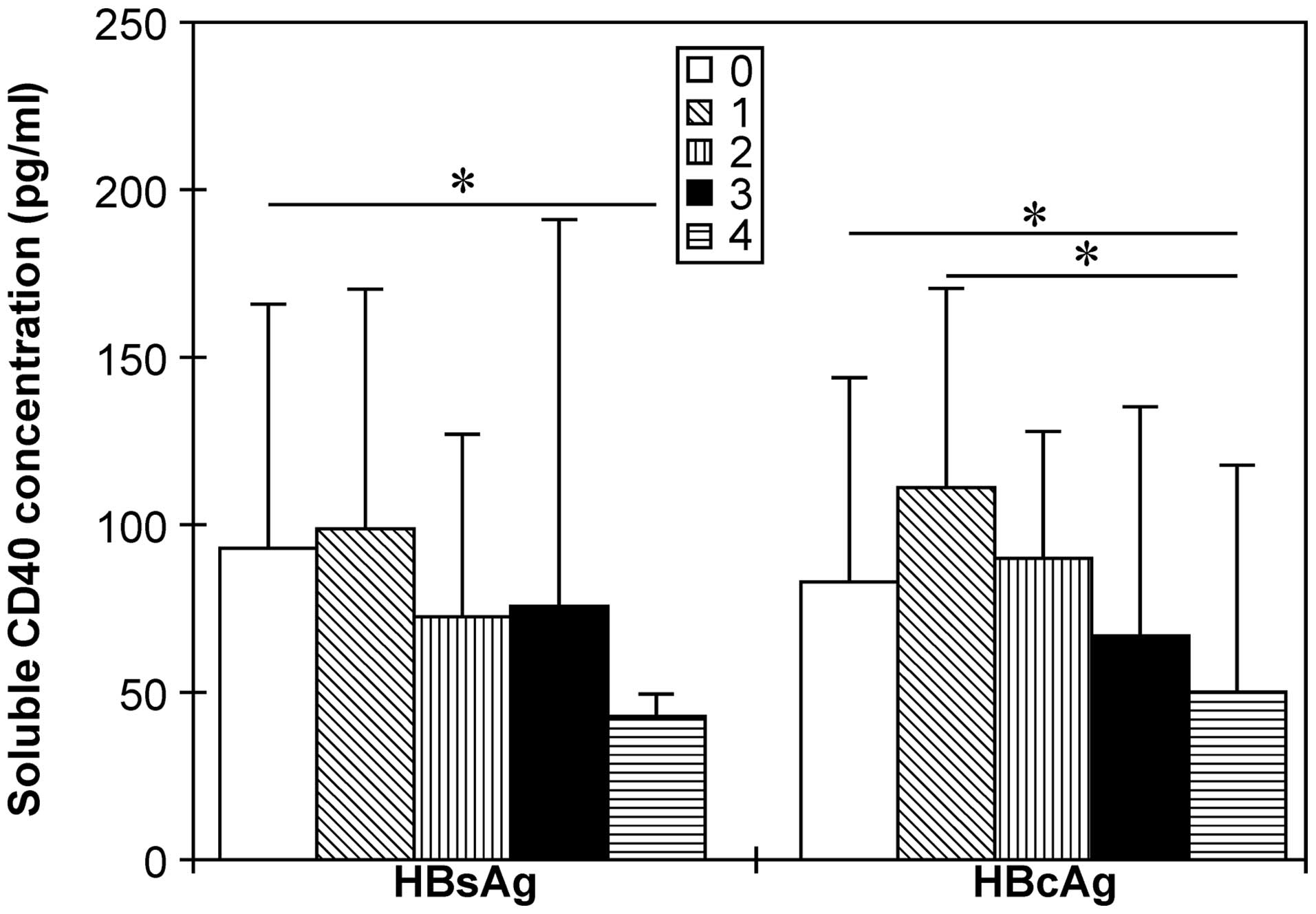
sCD40 concentration is reduced with an
increase in hepatic HBV antigen expression
sCD40 concentration correlated negatively with HBsAg
(r=−0.194; P=0.053) and HBcAg (r=−0.212; P<0.05) expression in
the liver. The sCD40 concentration in patients with HBsAg
expression present in >75% of liver tissue was significantly
lower than that in patients without detectable HBsAg expression.
Furthermore, sCD40 levels in patients with HBcAg expression in
>75% of liver tissue were significantly lower than those in
patients with HBcAg expression in <25% of liver tissue (Fig. 3).
sCD40 concentration has high a diagnostic
accuracy for detecting severe liver injury in patients with
CHB
To further investigate the diagnostic value of sCD40
levels in liver injury, ROC analysis was performed (Table III). The area under the curve
(AUC) of sCD40 for discriminating patients with CHB from healthy
individuals was 0.843 (P<0.001), with a sensitivity,
specificity, positive-predictive value and negative-predictive
value (NPV) of 0.712, 0.848, 0.949 and 0.424, respectively. The AUC
of sCD40 for diagnosing patients with CHB with moderate and marked
inflammation (necroinflammatory grading score >9), patients with
marked inflammation (necroinflammatory grading score >13) and
patients with cirrhosis (fibrosis staging score >5) was 0.820,
0.855 and 0.783, respectively, with a high sensitivity, specificity
and NPV (Table III).
 | Table IIIDiagnostic accuracy of soluble
cluster of differentiation 40 for the detection of different
degrees of liver injury in patients with CHB. |
Table III
Diagnostic accuracy of soluble
cluster of differentiation 40 for the detection of different
degrees of liver injury in patients with CHB.
| Detection
subject | AUC | P-value | Cut-off value
(pg/ml) | Sensitivity | Specificity | PPV | NPV |
|---|
| CHB patients | 0.843 | <0.001 | 57.5 | 0.712 | 0.848 | 0.949 | 0.424 |
| CHB patients with
grading score ≥5a | 0.737 | <0.001 | 103.3 | 0.606 | 0.818 | 0.769 | 0.675 |
| CHB patients with
grading score ≥9a | 0.820 | <0.001 | 116.7 | 0.870 | 0.761 | 0.435 | 0.965 |
| CHB patients with
grading score ≥13a | 0.855 | <0.001 | 118.4 | 1.000 | 0.710 | 0.182 | 1.000 |
| CHB patients with
staging score ≥3b | 0.705 | <0.001 | 103.3 | 0.583 | 0.764 | 0.673 | 0.688 |
| CHB patients with
staging score ≥5b | 0.783 | <0.001 | 116.7 | 0.812 | 0.716 | 0.283 | 0.965 |
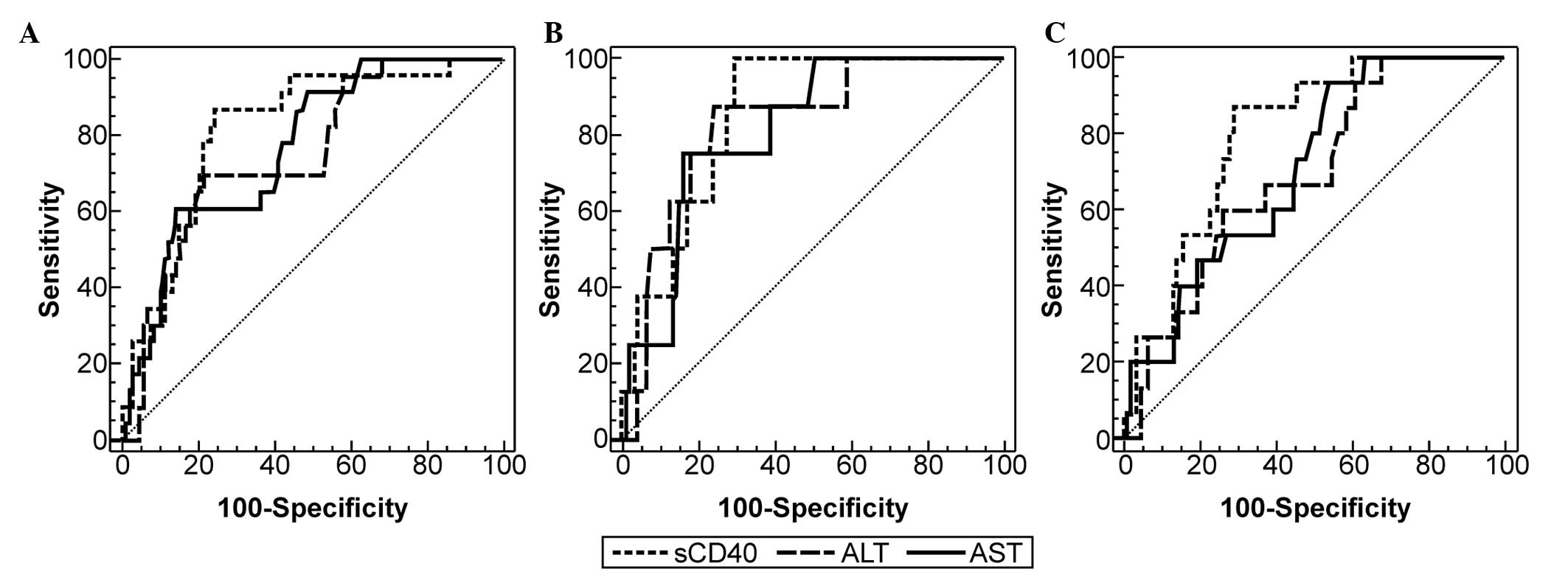
Comparisons of ROC curves of serum sCD40, ALT and
AST to detect different degrees of liver injury in patients with
CHB are shown in Fig. 4. Although
the differences between the AUC of sCD40 and ALT or sCD40 and AST
were not statistically significant, the AUC of sCD40 was greater
than the AUC of ALT and AST, when used to diagnose patients with
CHB with moderate and marked inflammation (0.817 vs. 0.752 and
0.769, respectively), patients with marked inflammation (0.852 vs.
0.829 and 0.816, respectively) and patients with cirrhosis (0.799
vs. 0.694 and 0.709, respectively). This indicated that sCD40 may
have a slightly higher diagnostic accuracy than ALT and AST for
detecting severe liver injury in patients with CHB.
The necroinflammatory grading score, fibrosis
staging score and levels of sCD40, ALT, and AST were introduced as
variables into a stepwise multiple regression analysis. The
regression equation used was as follows: necroinflammatory grading
score = −0.408 + 2.031 × fibrosis-staging score + 0.008 × sCD40
level. The finding that the ALT and AST variables were excluded
from the regression equation further supported our finding of a
stronger association between liver inflammation and sCD40 level
than between liver inflammation and ALT and AST levels.
Discussion
Hepatitis B is the most common chronic liver disease
in China. The CD40-CD154 co-stimulatory pathway is involved in the
pathogenesis of CHB (16).
Intrahepatic CD40 expression has been shown to be upregulated on
the surface of hepatocytes in CHB and to cause liver injury
(23,24). Furthermore, activation of CD40 on
hepatocytes and cholangiocytes is critical for amplifying
Fas-mediated apoptosis in the human liver (25). The CD40 molecules on the cell
surface that are activated by CD154 can trigger sCD40 release
(12,13), and it is known that an increased
expression of CD40 on the membrane is associated with abundant
release of its soluble form (6).
The elevated serum levels of sCD40 in patients with CHB observed in
the present study may therefore be the result of increased shedding
of this peptide from CD40-expressing cells and decreased
elimination by the impaired liver.
Serum ALT, AST, Dbil, ALP, γGT and TBA are markers
of liver dysfunction and are associated with liver injury. Positive
associations between sCD40 levels and these biochemical indices, as
well as liver necroinflammatory grading scores, in patients with
CHB observed in the present study suggested an involvement of sCD40
in liver inflammation. The observation that the already-elevated
sCD40 concentration in patients with CHB gradually increased with
increasing severity of liver necroinflammation or fibrosis also
supported this finding.
CD40-CD154 interactions in the liver can induce
immune responses, inflammatory injury and hepatocyte apoptosis
(26,27). The elevated levels of sCD40 in CHB
can compete with membrane CD40 for binding to CD154, thereby
inhibiting CD40-CD154 interactions and ultimately achieving
effective negative feedback control of the CD40-CD154-mediated
immune response and hepatocyte apoptosis (7,10,12,13,28).
Thus, we speculated that inhibition of the immune responses
mediated by sCD40 shedding would prevent liver tissue from
excessive injury (10,13). ROC and multiple regression analysis
of sCD40 showed that sCD40 levels have higher diagnostic accuracy
than do those of ALT and AST when used to detect severe liver
inflammation in patients with CHB. This suggested that serum sCD40
levels could serve as a novel immunological marker of hepatic
tissue injury in such patients.
Liver fibrosis represents a pathological
accumulation of extracellular matrix (ECM) components, which are
mainly degraded by the MMPs, e.g. MMP-1, MMP-2, MMP-3 and MMP-9
(29–31). CD40 ligation on
monocytes/macrophages and endothelial cells by CD154 can increase
the release of MMP-1, MMP-3, MMP-9 and activated MMP-2 (32,33).
Low activity of MMPs may contribute to the excess deposition of
intrahepatic ECM and may thus play an important role in the process
of liver fibrosis. Previously, it was found that the serum levels
of MMP-1, MMP-2 and MMP-9 in patients with CHB were significantly
lower than those in healthy controls, and serum MMP-1 levels
negatively correlated with fibrosis stage and inflammation grade
(34–37). Although the tissue inhibitors of
MMP-1 and -2 are considered to be the major reasons for inhibition
of MMP activity, sCD40 may also reduce MMP expression by blocking
the CD40-CD154 interaction. This could reduce hepatic degradation
of ECM and result in liver fibrosis. This hypothesis is also
supported by the positive correlation between serum sCD40 levels
and hepatic fibrosis observed in the present study (38); however, the mechanism underlying
the role played by sCD40 in liver fibrosis requires further
clarification.
The CD40-CD154 interaction represents a critical
co-stimulatory pathway that modulates the immune response. CD40
binding to intrahepatic antigen-presenting cells has been shown to
induce the secretion of antiviral cytokines, such as interleukin-12
and TNF-α, and then to inhibit HBV replication in the liver of
HBV-transgenic mice (16). sCD40
can inhibit the production of antiviral cytokines and may therefore
weaken the CD40-CD154-mediated antiviral immune response (39). The results from the present study,
however, suggest that the elevation of serum sCD40 levels in
patients with CHB is associated with downregulation of intrahepatic
HBV antigen expression. The mechanism by which sCD40 elevation
inhibits intrahepatic HBV antigen expression is unknown. It is
possible that a CD40-CD154-mediated antiviral immune response
contributes to both the inhibition of HBV antigen expression and
the shedding of sCD40. These two consequences have no direct
association, as patients with serum HBeAg-positive and
HBeAg-negative CHB showed similar sCD40 levels.
In conclusion, the present results suggest that
sCD40 plays an important role in the pathogenesis of CHB. sCD40 may
serve as a diagnostic and immunological marker of liver injury and
can act as a negative regulator of the CD40-CD154 interaction in
patients with CHB.
Acknowledgements
This study was funded by the 12th Five-Year Plan for
Medical Science and Technology in China (project cod:
CWS11J166).
References
|
1
|
Quezada SA, Jarvinen LZ, Lind EF and
Noelle RJ: CD40/CD154 interactions at the interface of tolerance
and immunity. Annu Rev Immunol. 22:307–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma DY and Clark EA: The role of CD40 and
CD154/CD40L in dendritic cells. Semin Immunol. 21:265–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suttles J and Stout RD: Macrophage CD40
signaling: a pivotal regulator of disease protection and
pathogenesis. Semin Immunol. 21:257–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danese S, Sans M and Fiocchi C: The
CD40/CD40L costimulatory pathway in inflammatory bowel disease.
Gut. 53:1035–1043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peters AL, Stunz LL and Bishop GA: CD40
and autoimmunity: the dark side of a great activator. Semin
Immunol. 21:293–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Paoli P, Cozzi M, Tedeschi R, Gloghini
A, Cilia AM, van Kooten C, Gaidano G and Carbone A: High CD40
membrane expression in AIDS-related lymphoma B cell lines is
associated with the CD45RA+, CD45RO+,
CD95+ phenotype and high levels of its soluble form in
culture supernatants. Cytometry. 30:33–38. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Contin C, Pitard V, Itai T, Nagata S,
Moreau JF and Déchanet-Merville J: Membrane-anchored CD40 is
processed by the tumor necrosis factor-alpha-converting enzyme.
Implications for CD40 signaling. J Biol Chem. 278:32801–32809.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuang Y, Huang J, Zhou Z, et al: A novel
blocking monoclonal antibody recognizing a distinct epitope of
human CD40 molecule. Tissue Antigens. 65:81–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fanslow WC, Anderson DM, Grabstein KH,
Clark EA, Cosman D and Armitage RJ: Soluble forms of CD40 inhibit
biologic responses of human B cells. J Immunol. 149:655–660.
1992.PubMed/NCBI
|
|
10
|
Contin C, Pitard V, Delmas Y, et al:
Potential role of soluble CD40 in the humoral immune response
impairment of uraemic patients. Immunology. 110:131–140. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ait-ghezala G, Abdullah L, Volmar CH,
Paris D, Luis CA, Quadros A, Mouzon B, Mullan MA, Keegan AP,
Parrish J, Crawford FC, Mathura VS and Mullan MJ: Diagnostic
utility of APOE, soluble CD40, CD40L, and Abeta1-40 levels in
plasma in Alzheimer’s disease. Cytokine. 44:283–287. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komura K, Fujimoto M, Matsushita T, et al:
Increased serum soluble CD40 levels in patients with systemic
sclerosis. J Rheumatol. 34:353–358. 2007.PubMed/NCBI
|
|
13
|
Hock BD, McKenzie JL, Patton NW, et al:
Circulating levels and clinical significance of soluble CD40 in
patients with hematologic malignancies. Cancer. 106:2148–2157.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmilovitz-Weiss H, Belinki A, Pappo O,
et al: Role of circulating soluble CD40 as an apoptotic marker in
liver disease. Apoptosis. 9:205–210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ke B, Shen XD, Gao F, et al: The
CD154-CD40 T-cell co-stimulation pathway in liver ischemia and
reperfusion inflammatory responses. Transplantation. 79:1078–83.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura K, Kakimi K, Wieland S, Guidotti LG
and Chisari FV: Activated intrahepatic antigen-presenting cells
inhibit hepatitis B virus replication in the liver of transgenic
mice. J Immunol. 169:5188–5195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura K, Nagaki M, Takai S, Satake S and
Moriwaki H: Pivotal role of nuclear factor kappaB signaling in
anti-CD40-induced liver injury in mice. Hepatology. 40:1180–1189.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burgio VL, Ballardini G, Artini M,
Caratozzolo M, Bianchi FB and Levrero M: Expression of
co-stimulatory molecules by Kupffer cells in chronic hepatitis of
hepatitis C virus etiology. Hepatology. 27:1600–1606. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiraki K, Sugimoto K, Okano H, et al:
CD40 expression in HCV-associated chronic liver diseases. Int J Mol
Med. 18:559–563. 2006.PubMed/NCBI
|
|
20
|
Kimura K, Moriwaki H, Nagaki M, et al:
Pathogenic role of B cells in anti-CD40-induced necroinflammatory
liver disease. Am J Pathol. 168:786–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinese Society of Hepatology, Chinese
Medical Association; Chinese Society of Infectious Diseases,
Chinese Medical Association. Guideline on prevention and treatment
of chronic hepatitis B in China (2005). Chin Med J (Engl).
120:2159–2173. 2007.
|
|
22
|
Ishak K, Baptista A, Bianchi L, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan J, Jie Z, Hou L, et al: Parenchymal
expression of CD40 exacerbates adenovirus-induced hepatitis in
mice. Hepatology. 53:1455–1467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Connolly MK, Bedrosian AS, Mallen-St Clair
J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey
AB and Miller G: In liver fibrosis, dendritic cells govern hepatic
inflammation in mice via TNF-alpha. J Clin Invest. 119:3213–3225.
2009.PubMed/NCBI
|
|
25
|
Williams KT, Young SP, Negus A, Young LS,
Adams DH and Afford SC: C4b binding protein binds to CD154
preventing CD40-mediated cholangiocyte apoptosis: a novel link
between complement and epithelial cell survival. PLoS One.
2:e1592007. View Article : Google Scholar
|
|
26
|
Zhou F, Ajuebor MN, Beck PL, Le T,
Hogaboam CM and Swain MG: CD154-CD40 interactions drive hepatocyte
apoptosis in murine fulminant hepatitis. Hepatology. 42:372–380.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitz V, Dombrowski F, Prieto J, et al:
Induction of murine liver damage by overexpression of CD40 ligand
provides an experimental model to study fulminant hepatic failure.
Hepatology. 44:430–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lunsford KE, Koester MA, Eiring AM, Horne
PH, Gao D and Bumgardner GL: Targeting LFA-1 and CD154 suppresses
the in vivo activation and development of cytolytic
(CD4-Independent) CD8+ T cells. J Immunol.
175:7855–7866. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Overall CM, Wrana JL and Sodek J:
Independent regulation of collagenase, 72-kDa progelatinase, and
metalloendoproteinase inhibitor expression in human fibroblasts by
transforming growth factor-beta. J Biol Chem. 264:1860–1869.
1989.PubMed/NCBI
|
|
30
|
Takahara T, Furui K, Funaki J, et al:
Increased expression of matrix metalloproteinase-II in experimental
liver fibrosis in rats. Hepatology. 21:787–795. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murphy G and Docherty AJ: The matrix
metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol.
7:120–125. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mach F, Schönbeck U, Fabunmi RP, et al: T
lymphocytes induce endothelial cell matrix metalloproteinase
expression by a CD40L-dependent mechanism: implications for tubule
formation. Am J Pathol. 154:229–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mach F, Schönbeck U, Bonnefoy JY, Pober JS
and Libby P: Activation of monocyte/macrophage functions related to
acute atheroma complication by ligation of CD40: induction of
collagenase, stromelysin, and tissue factor. Circulation.
96:396–399. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuo WH, Chou FP, Lu SC, Chu SC and Hsieh
YS: Significant differences in serum activities of matrix
metalloproteinase-2 and -9 between HCV- and HBV-infected patients
and carriers. Clin Chim Acta. 294:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Flisiak R, Al-Kadasi H, Jaroszewicz J,
Prokopowicz D and Flisiak I: Effect of lamivudine treatment on
plasma levels of transforming growth factor beta1, tissue inhibitor
of metalloproteinases-1 and metalloproteinase-1 in patients with
chronic hepatitis B. World J Gastroenterol. 10:2661–2665.
2004.PubMed/NCBI
|
|
36
|
Ljumovic D, Diamantis I, Alegakis AK and
Kouroumalis EA: Differential expression of matrix
metalloproteinases in viral and non-viral chronic liver diseases.
Clin Chim Acta. 349:203–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang BB, Cai WM, Weng HL, et al:
Diagnostic value of platelet derived growth factor-BB, transforming
growth factor-beta1, matrix metalloproteinase-1, and tissue
inhibitor of matrix metalloproteinase-1 in serum and peripheral
blood mononuclear cells for hepatic fibrosis. World J
Gastroenterol. 9:2490–2496. 2003.PubMed/NCBI
|
|
38
|
Flisiak R, Maxwell P, Prokopowicz D, Timms
PM and Panasiuk A: Plasma tissue inhibitor of metalloproteinases-1
and transforming growth factor beta 1 - possible non-invasive
biomarkers of hepatic fibrosis in patients with chronic B and C
hepatitis. Hepatogastroenterology. 49:1369–1372. 2002.PubMed/NCBI
|
|
39
|
Shu U, Kiniwa M, Wu CY, et al: Activated T
cells induce interleukin-12 production by monocytes via CD40-CD40
ligand interaction. Eur J Immunol. 25:1125–1128. 1995. View Article : Google Scholar : PubMed/NCBI
|