Introduction
General anesthesia with a secured airway is
recommended for patients undergoing uvulopalatopharyngoplasty for
obstructive sleep apnea (OSA) (1).
Patients with OSA are at increased perioperative risk due to their
susceptibility to the respiratory depressant and airway effects of
sedatives, opioids and inhaled anesthetics, which can be attributed
to their propensity for airway collapse and sleep deprivation.
Therefore, securing the airway and reducing the postoperative
respiratory compromise should be considered when selecting
anesthetics.
Dexmedetomidine, a highly selective α2-adrenergic
receptor agonist, provides arousable sedation similar to that of
natural sleep and preserves spontaneous respiration even at large
doses, making it suitable for sedation in awake intubation
procedures such as fiberoptic intubation (FOI) (2–4). In
addition, dexmedetomidine may be a useful adjuvant during general
anesthesia, particularly for OSA patients, by promoting hemodynamic
stability and decreasing the doses of anesthetics and analgesics,
which may therefore allow early recovery and reduce potential
postoperative respiratory compromise (5,6).
However, studies have shown that dexmedetomidine delays recovery
from propofol or propofol-remifentanil anesthesia (7,8).
Propofol and propofol-remifentanil are commonly used anesthetics
for patients with OSA undergoing uvulopalatopharyngoplasty
(9,10).
Doxapram is a respiratory and central nervous system
(CNS) stimulant, which has comprehensive effects on peripheral and
central chemoreceptors and could potentially hasten the recovery
from several types of anesthetics (11–19).
Therefore, it was hypothesized that doxapram may accelerate the
recovery following dexmedotomidine-propofol-remifentanil
anesthesia. In this study, the aim was to determine whether
doxapram hastens the recovery in OSA patients following
dexmedetomidine-propofol-remifentanil anesthesia.
Patients and methods
Patient eligibility
This double-blind, randomized prospective study was
approved by the Ethics Committee of Qilu Hospital of Shandong
University (Jinan, China) and registered as a clinical trial
(http://www.chictr.org/; identifier,
ChiCTR-TRC-13003346). Written, informed consent was obtained from
60 adult patients with American Society of Anesthesiologists (ASA)
class I and II physical status scheduled for elective
uvulopalatopharyngoplasty for OSA. Polysomnograms were performed in
all patients (Alice 4™; Respironics Inc., Pittsburgh, PA, USA) and
the records were staged manually according to standard criteria by
the same skilled technician. Respiratory events were scored
according to the American Academic Sleep Medicine (AASM) criteria:
Apnea was defined as complete cessation of airflow lasting for ≥10
sec; hypopnea was defined as either a ≥50% reduction in airflow for
≥10 sec, or a <50% but discernible reduction in airflow
accompanied either by a reduction in oxyhemoglobin saturation of
≥4% or an arousal. The apnea-hypopnea index (AHI) was defined as
the number of events of apnea and hypopnea per hour during sleep
time, based on the results of the overnight polysomnographs (PSGs).
If the AHI was ≥5/h, the patient was diagnosed as positive for OSA.
The patients were excluded if they had bradycardia [<50 beats
per min (bpm)], hypotension (systolic blood pressure <90 mmHg),
hepatic impairment, had taken a α2-adrenoceptor agonist or
antagonist within the previous 14 days, were contraindicated for
nasal intubation, intolerant or allergic to the study drug, or
refused to be involved in the study.
Patient grouping
Patients were randomized into two groups according
to a computer-generated table of random numbers. The doxapram group
(n=30) received doxapram (Jiangsu Nhwa Pharmaceutical Corporation
Ltd., Xuzhou, China) 1 mg/kg intravenously (i.v.), and the control
group (n=30) received isovolumic normal saline i.v. Study drugs
were prepared by an anesthesia nurse. The anesthesiologist and the
subjects were unaware of group identities.
Surgical anesthesia
Atropine (0.5 mg; Minsheng Pharmaceutical Group Co.,
Ltd., Hangzhou, China) was administered intramuscularly as
premedication 30 min prior to the patient’s arrival in the
operating room. On arrival in the operating room, routine monitors
were applied to each subject, including continuous
electrocardiogram, peripheral pulse oximeter, non-invasive blood
pressure monitor, end-tidal CO2 monitor and bispectral
index (BIS) monitor (S/5; GE Healthcare Finland Oy, Helsinki,
Finland). Following the recording of baseline vital signs and BIS
values, dexmedetomidine (Jiangsu Hengrui Medicine Co. Ltd.,
Lianyungang, China) was administered as a loading dose of 1.0 μg/kg
over 10 min followed by a continuous infusion of 0.7 μg/kg/h until
a Ramsey score of 3 was achieved. Topical lidocaine (Shanghai
Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) was administered
for an uneventful FOI.
Following the identification of exhaled
CO2 by infrared spectroscopy, general anesthesia was
induced with 2 mg/kg propofol (AstraZeneca, London, UK) i.v., and
initial muscle relaxation was achieved with 50 mg atracurium
(Jiangsu Hengrui Medicine Co., Ltd.) i.v. Ventilation was adjusted
to maintain end-tidal CO2 values between 35 and 40 mmHg
using an inspiratoy O2 fraction of0.5.
Anesthesia was titrated with propofol to maintain
the BIS scores in the range of 50±10, with remifentanil infusion
(0.1–0.25 μg/kg/min; Yichang Renfu Pharmaceutical Co., Ltd.,
Yichang, China) to maintain the heart rate (HR) between 50 and 80
bpm and atracurium (5–10 μg/kg/min) to maintain a train-of-four
(TOF) stimulation value of 0 [TOF-Watch® SX; Organon
(Ireland) Ltd., Dublin, Ireland]. Neostigmine (0.05 mg/kg; Shandong
Tianfu Pharmaceutical Factory, Zibo, China) and atropine 0.01 mg/kg
i.v.) were administered 10 min prior to the end of surgery to allow
for the return to spontaneous breathing. Propofol and remifentanil
were discontinued at the end of surgery and then the study drug
(doxopram or saline) was administered i.v. over 1 min.
Recovery procedure
The name of the patient was called every 30 sec, and
the patient was asked ‘Are you awake? Open your eyes.’ The time
from the end of the general anesthesia to eye opening was measured.
Tracheal extubation was performed when the patients achieved a
regular breathing pattern and were able to follow the verbal
command to squeeze the anesthesiologist’s hand.
The following parameters were evaluated by an
anesthesiologist who was unaware of study group allocations: Time
to return to spontaneous breathing, eye opening on verbal command,
hand squeezing in response to verbal command, and time to
extubation of the trachea from the end of general anesthesia. HR,
systolic blood pressure, BIS values and SpO2 values were
determined prior to surgery, at 5-min intervals during surgery, and
then at each minute after the injection of the study drugs for 16
min. The respiratory rate (RR) was also recorded from the time of
study drug injection to the time of extubation. Modified Aldrete
scores (20) were evaluated every
5 min until a score of 9 was reached, and then the patient was
discharged from the operating room.
The anesthesia process and the treatment of patients
are schematically illustrated in Fig.
1.
Recall, awareness during anesthesia or abnormal
psychological feeling during emergence was recorded at 24 h after
surgery. The incidences of reintubation, hypoxemia, myocardial
infarction, arrhythmia, delirium, thromboembolism were also
recorded at 24 h after surgery.
Statistical analysis
The time to achieve eye opening on verbal command
was defined as the primary end-point of this study. The time to
return to eye opening in the control group was 15.0±6.2 min in a
pilot study of 10 patients, and it was assumed that the standard
deviation (SD) in the test group was equal to that of the control
group. A difference of 5 min to eye opening was set between groups.
At least 25 patients per group were required to provide 80% power
to detect this difference at α=0.05. Assuming the possibility of
patients being excluded from the study, 30 patients were enrolled
per group. For continuous variables, the distribution of the data
was first evaluated using the Kolmogorov-Smirnov test for
normality. The normally distributed data are presented as the mean
± SD, and significance was tested via the Student’s t-test. The
non-normally distributed data were analyzed via the Mann-Whitney U
test. Descriptive variables were subjected to Chi-square analysis.
In all tests, P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient and treatment variables
There were no significant differences in demographic
characteristics, duration of anesthesia and doses between the
doxapram and control groups (Table
I).
 | Table IDemographic and clinical data. |
Table I
Demographic and clinical data.
| Variable | Control | Doxapram |
|---|
| Age (years) | 38.6±9.7 | 37.6±9.8 |
| Body height (cm) | 179.5±16.7 | 179.2±17.0 |
| Body weight (kg) | 86.9±17.6 | 87.6±16.9 |
| Anesthesia
durationa (min) | 90.8±20.8 | 91.5±21.7 |
| Dexmedetomidine
(μg) | 136.6±31.8 | 135.3±32.6 |
| Propofol (mg) | 577.8±130.6 | 584.8±142.0 |
| Remifentanil
(μg) | 646.5±110.2 | 672.4±98.4 |
| Atracurium (mg) | 96.7±20.4 | 97.5±25.7 |
Recovery parameters
The time to return to spontaneous breathing, eye
opening, hand squeeze on command, and extubation were observed to
be significantly shorter in the doxapram group (P<0.05) compared
with those in the control group (Table II).
 | Table IIRecovery parameters. |
Table II
Recovery parameters.
| Time for recovery
(min) | | |
|---|
|
| | |
|---|
| Recovery
parameter | Control | Doxopram | t-value | P-value |
|---|
| Spontaneous
breathing | 11.7±3.4 | 5.2±2.9a | 7.9668 | <0.001 |
| Eye opening | 15.9±6.3 | 9.3±4.7a | 4.5992 | <0.001 |
| Response to
command | 17.6±7.7 | 11.8±6.5a | 3.1526 | 0.0026 |
| Extubation of
trachea | 19.2±9.6 | 14.2±7.8a | 2.2140 | 0.0308 |
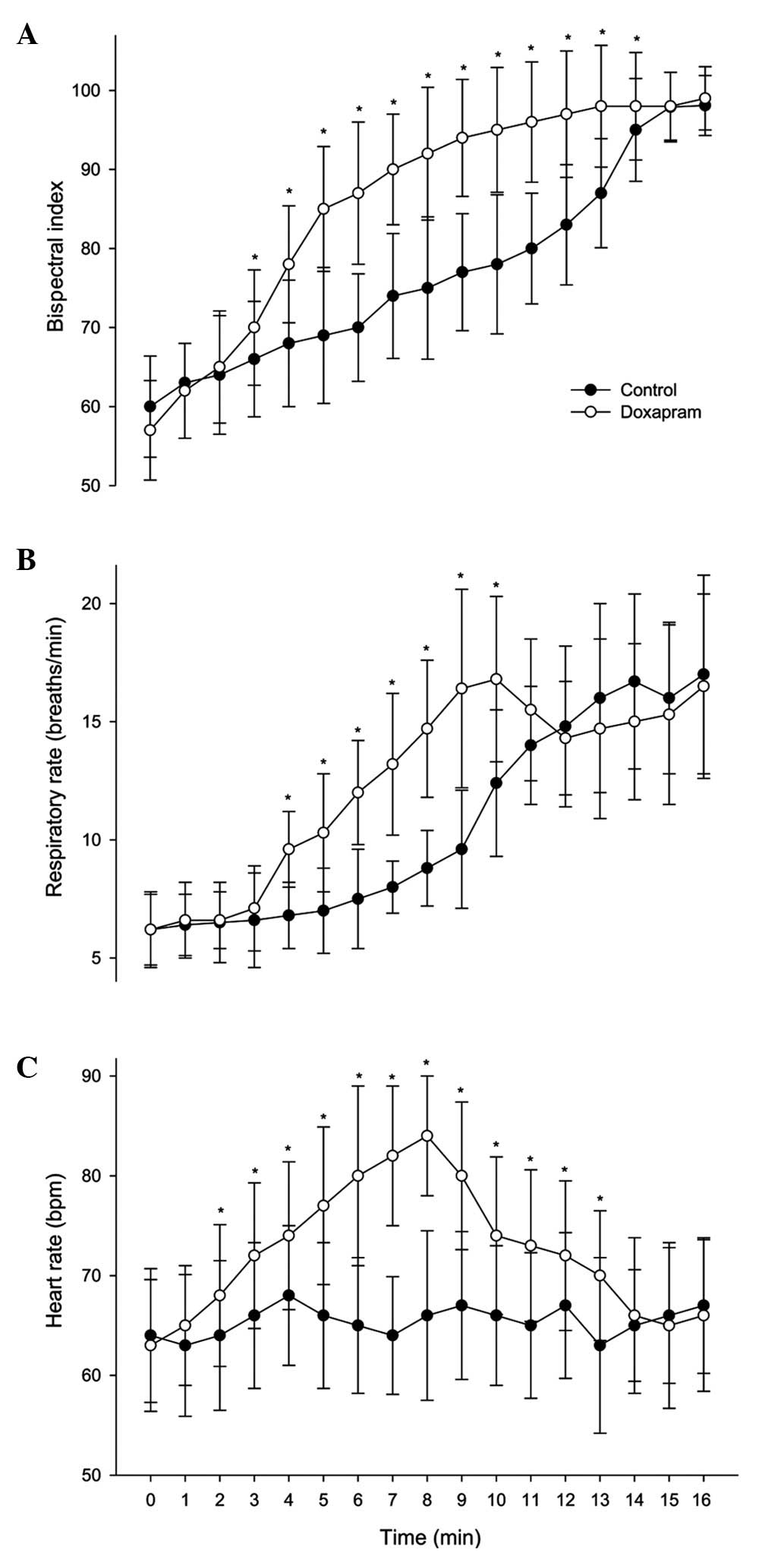
Following treatment, the BIS scores were
significantly higher in the doxapram group at 3–14 min compared
with those in the control group (P<0.05; Fig. 2A). The doxapram group had
significantly higher RR and HR values compared with those in the
control group at 4–10 min (in RR) and at 2–13 min (in HR)
(P<0.05; Fig. 2B and C). No
significant differences were identified in BIS values, end-tidal
CO2, HR and systolic blood pressure during the
anesthesia and post-anesthesia periods between the two groups.
Three patients in the control group and two in the
doxapram group experienced a HR <50 bpm, and all these patients
achieved a HR of 50 bpm within 5 min without treatment during
dexmedetomidine infusion. All patients who received doxapram
recovered from anesthesia smoothly without unpleasant psychological
effects, remained calm and could respond well to commands. There
were no differences in the modified Aldrete score between the two
groups.
Hypoxemia (SpO2 <90%) occurred in four
patients in the control group and in five in the doxapram group but
only one patient from the control group required reintubation. No
other adverse effects were observed at 24 h after surgery.
Discussion
The main finding of this study is that the recovery
from dexmedetomidine-propofol-remifentanil anesthesia in patients
with OSA undergoing elective uvulopalatopharyngoplasty is hastened
by doxapram (1 mg/kg i.v.). Rapid and complete recovery from
general anesthesia benefits patients with OSA following
uvulopalatopharyngoplasty (6).
However, dexmedetomidine has been demonstrated to delay the
recovery from propofol anesthesia when used to maintain the
anesthesia or to induce anesthesia in minor surgery due to its
longer half-life (≥2 h) (7–9).
Doxapram has been used to treat drug-induced post-anesthetic CNS
depression, including that arising from the use of opioids and
propofol (17,18). A 1-mg/kg dose of doxapram is
commonly recommended for intravenous injection (18,19).
The present study indicated that doxapram is effective in reversing
the anesthetic effects of
dexmedetomidine-propofol-remifentanil.
The present study also demonstrated that doxapram
administration caused a rapid recovery of BIS, similar to that in
the reversal of sevoflurane and propofol-remifentanil anesthesia
(18,19). Wang et al demonstrated that
a loading dose of dexmedetomidine of 1.0 μg/kg over 10 min followed
by infusion at 0.5 μg/kg/h decreased the BIS values under stepwise
propofol target-controlled infusion (21). Previous studies have also indicated
that BIS correlates well with the hypnotic and sedative effects of
various anesthetic agents, including isoflurane, sevoflurane,
midazolam and propofol (22,23).
The data from the present study demonstrated that BIS correlated
well with the levels of consciousness under the circumstance of
co-administration of dexmedetomidine with propofol-remifentanil.
The recovery effect of doxapram may be associated with rapid
recovery of BIS values resulting from its nonspecific and extensive
CNS stimulant properties.
Some adverse effects of doxapram have been reported,
including tachycardia, cardiac arrhythmia, hypertension, anxiety
reactions, hallucinations, excitation, panic attacks and even
cerebrovascular accident (14,16,17,24).
In the present study, 1 mg/kg doxapram was used, a dose that has
previously been demonstrated to be effective in reversing the
depressant effects of anesthetic without adverse responses
(15–19). All patients who received doxapram
remained calm following extubation of the trachea, in a similar
manner to those in the control group. It appears that the sedative
effect of dexmedetomidine continues beyond the time of extubation.
The complications associated with anesthesia in patients with OSA
undergoing uvulopalatopharyngoplasty were not different between
groups in the present study.
There are several limitations in this study.
Firstly, a dexmedotomidine-propofol-remifentanil group was not
provided as a negative control in this study to demonstrate the
more prolonged recovery time associated with dexmedetomidine
co-administration. Secondly, serum dexmedetomidine, propofol or
remifentanil concentrations were not measured, precluding the
ability to distinguish pharmacokinetic interactions from
pharmacodynamic interactions. Thirdly, the data could not
distinguish which part of the co-administration of dexmedetomidine,
propofol and remifentanil was the main target of doxapram action.
The inclusion of a control group without dexmedetomidine will be
used in future studies to clarify the mechanism by which doxapram
accelerates the recovery of patients with OSA following total
intravenous anesthesia (TIVA).
In conclusion, a single dose administration of
doxapram (1 mg/kg i.v.) at the end of TIVA hastens the early
recovery from dexmedetomidine-propofol-remifentanil anesthesia in
OSA patients undergoing uvulopalatopharyngoplasty without
appreciable side-effects. Considering the benefits resulting from
rapid and clear-headed emergence in OSA patients, this study
provides helpful guidance on the clinical management of patients
with OSA undergoing uvulopalatopharyngoplasty.
Acknowledgements
This study was supported by Shandong Provincial
Natural Science Foundation, China (Y2007C115 and ZR2011HM028 to
Huan-Liang Wang and ZR2009CM060 to Wei-Fu Lei).
References
|
1
|
Gross JB, Bachenberg KL, Benumof JL, et
al; American Society of Anesthesiologists Task Force on
Perioperative Management. Practice Guidelines for the Perioperative
Management of Patients with Obstructive Sleep Apnea. Practice
guidelines for the perioperative management of patients with
obstructive sleep apnea: a report by the American Society of
Anesthesiologists Task Force on Perioperative Management of
patients with obstructive sleep apnea. Anesthesiology.
104:1081–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnston KD and Rai MR: Conscious sedation
for awake fibreoptic intubation: a review of the literature. Can J
Anaesth. 60:584–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cattano D, Lam NC, Ferrario L, Seitan C,
Vahdat K, Wilcox DW and Hagberg CA: Dexmedetomidine versus
remifentanil for sedation during awake fiberoptic intubation.
Anesthesiol Res Pract. 2012:7531072012.PubMed/NCBI
|
|
4
|
Hu R, Liu JX and Jiang H: Dexmedetomidine
versus remifentanil sedation during awake fiberoptic nasotracheal
intubation: a double-blinded randomized controlled trial. J Anesth.
27:211–217. 2013. View Article : Google Scholar
|
|
5
|
Hofer RE, Sprung J, Sarr MG and Wedel DJ:
Anesthesia for a patient with morbid obesity using dexmedetomidine
without narcotics. Can J Anaesth. 52:176–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ankichetty S, Wong J and Chung F: A
systematic review of the effects of sedatives and anesthetics in
patients with obstructive sleep apnea. J Anaesthesiol Clin
Pharmacol. 27:447–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohtani N, Kida K, Shoji K, Yasui Y and
Masaki E: Recovery profiles from dexmedetomidine as a general
anesthetic adjuvant in patients undergoing lower abdominal surgery.
Anesth Analg. 107:1871–1874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turgut N, Turkmen A, Ali A and Altan A:
Remifentanil-propofol vs. dexmedetomidine-propofol - anesthesia for
supratentorial craniotomy. Middle East J Anesthesiol. 20:63–70.
2009.
|
|
9
|
Ryu JH, Lee SW, Lee JH, Lee EH, Do SH and
Kim CS: Randomized double-blind study of remifentanil and
dexmedetomidine for flexible bronchoscopy. Br J Anaesth.
108:503–511. 2012. View Article : Google Scholar
|
|
10
|
Wong GL and Morton NS: Total intravenous
anesthesia (TIVA) in pediatric cardiac anesthesia. Paediatr
Anaesth. 21:560–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moser KM, Luchsinger PC, Adamson JS,
McMahon SM, Schlueter DP, Spivack M and Weg JG: Respiratory
stimulation with intravenous doxapram in respiratory failure. A
double-blind cooperative study. N Engl J Med. 288:427–431. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitchell RA and Herbert DA: Potencies of
doxapram and hypoxia in stimulating carotid-body chemoreceptors and
ventilation in anesthetized cats. Anesthesiology. 42:559–566. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scott RM, Whitwan JG and Chakrabarti MK:
Evidence of a role for the peripheral chemoreceptors in the
ventilator response to doxapram in man. Br J Anaesth. 49:227–231.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Po BT, Watson RL and Hansen HR: Arousal
time following intravenous anesthetic agents, methohexital and
thiopental: effect of doxapram hydrochloride. Anesth Analg.
47:446–451. 1968.PubMed/NCBI
|
|
15
|
Gupta PK and Dundee JW: Hastening of
arousal after general anaesthesia with doxapram hydrochloride. Br J
Anaesth. 45:493–496. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riddle PL and Robertson GS: Use of
doxapram as an arousal agent in outpatient general anaesthesia. Br
J Anaesth. 50:921–924. 1978. View Article : Google Scholar
|
|
17
|
Ramamurthy S, Steen NS and Winnie AP:
Doxapram antagonism of meperidine-induced respiratory depression.
Anesth Analg. 54:352–356. 1975.PubMed/NCBI
|
|
18
|
Kim DW, Joo JD, In JH, et al: Comparison
of the recovery and respiratory effects of aminophylline and
doxapram following total intravenous anesthesia with propofol and
remifentanil. J Clin Anesth. 25:173–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu CC, Mok MS, Chen JY, Wu GJ, Wen YR and
Lin CS: Doxapram shortens recovery following sevoflurane
anesthesia. Can J Anaesth. 53:456–460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aldrete JA: The post-anesthesia recovery
score revisited. J Clin Anesth. 7:89–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Ge S, Xiong W, Zhou P, Cang J and
Xue Z: Effects of different loading doses of dexmedetomidine on
bispectral index under stepwise propofol target-controlled
infusion. Pharmacology. 91:1–6. 2013. View Article : Google Scholar
|
|
22
|
Bard JW: The BIS monitor: a review and
technology assessment. AANA J. 69:477–483. 2001.
|
|
23
|
Mourisse J, Lerou J, Zwarts M and Booij L:
Electromyographic assessment of blink reflexes correlates with a
clinical scale of depth of sedation/anaesthesia and BIS during
propofol administration. Acta Anaesthesiol Scand. 48:1174–1179.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosenberg J, Kristensen PA, Pedersen MH
and Overgaard H: Adverse events with continuous doxapram infusion
against late postoperative hypoxaemia. Eur J Clin Pharmacol.
50:191–194. 1996. View Article : Google Scholar : PubMed/NCBI
|