Introduction
Inflammation and inappropriate immune activity may
be closely associated with the development of type 2 diabetes
mellitus (T2DM) and diabetic nephropathy (DN) (1–4). In
T2DM, monocytes demonstrate pro-inflammatory characteristics and an
increase in the expression of inflammatory factors (3–7).
Vitamin D can prevent the development of numerous
chronic diseases, such as diabetes (8,9),
infectious diseases (10,11) and autoimmune diseases (12,13).
Vitamin D may have a preventative effect on T2DM, since it is known
that the concentration of a key metabolite, 1,25-dihydroxyvitamin
D3 (1,25-(OH)2D3), is
independently associated with insulin sensitivity and β-cell
function among individuals at risk of T2DM (9). 1,25-(OH)2D3
suppresses the expression of toll-like receptor (TLR) 2 and TLR4
proteins and mRNA in human monocytes in a time- and dose-dependent
manner, and reduces the effectiveness of the monocyte response to
bacterial cell wall components in line with a vitamin D receptor
(VDR)-dependent mechanism, presumably due to the reductions in the
levels of TLR2 and TLR4 (11).
1,25-(OH)2D3 downregulates the expression of
TLR by monocytes and triggers hyporesponsiveness to
pathogen-associated molecular patterns (12). Due to these factors, vitamin D has
attracted attention from researchers.
1,25-(OH)2D3 is an active
metabolite of vitamin D. Its interaction with the VDR in target
cells regulates calcium phosphate metabolism, exerts an
anti-inflammatory effect, controls cell proliferation, induces cell
differentiation, affects immunoregulation and enhances glucose
metabolism (14–16). In a pilot study, it was found that
1,25-(OH)2D3 had an anti-inflammatory effect
on human monocytes incubated with sera from patients with T2DM and
DN with uremia, and that it may exert an anti-inflammatory effect
by regulating the signal transduction pathways that control VDR and
signal transducer and activator of transcription 5 (STAT5)
expression (1). Considering that
these findings were obtained from the preliminary research phase,
however, as well as that reports on the anti-inflammatory effect of
1,25-(OH)2D3 are rare, this anti-inflammatory
effect requires further validation. Furthermore, the mechanism
underlying the effect remains to be explored.
Based on the aforementioned results, the present
study aimed to further validate the effect of
1,25-(OH)2D3 on the expression of VDR and
phosphorylated STAT5 (p-STAT5) in human monocytes, as well as
cytoskeletal rearrangement, and to explore the possible interaction
between VDR and p-STAT5. The results of this study may shed new
light on the multiple functions of vitamin D and lay a theoretical
foundation for further exploration in related fields.
Materials and methods
Materials and reagents
Rabbit polyclonal VDR antibody (cat. no. ab3508) was
the purchased from Abcam (Cambridge, UK) and rabbit polyclonal
p-STAT5 antibody (cat. no. 9351) was obtained from Cell Signaling
Technology, Inc. (Denver, MA, USA). Mouse monoclonal STAT5 (cat.
no. sc-377069) and p-STAT5 (cat. no. sc-81524) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
p-STAT5 was fluorescein isothiocyanate (FITC)-labeled and VDR was
tetramethylrhodamine (TRITC)-labeled. In addition, horseradish
peroxidase-labeled goat anti-mouse immunoglobulin (Ig)G (cat. no.
ZB-2305); horseradish peroxidase-labeled goat anti-rabbit IgG (cat.
no. ZB-2301); FITC-labeled goat anti-mouse IgG (cat. no. ZF-0312);
TRITC-labeled goat anti-rabbit IgG (cat. no. ZF-0316) were
purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China); and mouse monoclonal anti-β-actin (cat. no.,
ICM001-100) was purchased from Beijing 4A Biotech Co., Ltd.
(Beijing, China). 1,25-(OH)2D3,
lipopolysaccharide (LPS) and F-actin were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Recombinant human interleukin
(IL)-15 was manufactured by Peprotech (Rocky Hill, NJ, USA).
THP-1 cells have been widely used in investigations
of human monocytes and macrophages in vitro (17,18). The
THP-1 cell line (TcHu 57) used in this study was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cell culture and grouping
The THP-1 cells were re-suspended in RPMI-1640
culture medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco Life Technologies),
penicillin and streptomycin (HyClone Laboratories, Inc., Logan, UT,
USA) both at 100 U/ml, and flask-cultured in 5% (v/v)
CO2 at 37°C. Prior to each experiment, the cells were
allowed to grow in serum-free medium for 24 h to ensure that all
cells were synchronized at the G0 phase.
The synchronized cells were divided into the
control, LPS + IL-15 and 1,25-(OH)2D3 groups
according to their differing treatment. The control group was
treated with phosphate-buffered saline (PBS) only. In the LPS +
IL-15 group, LPS at 1 µg/ml and IL-15 at 100 ng/ml were added for 4
h of incubation. In the 1,25-(OH)2D3 group,
the cells were pre-treated with 1,25-(OH)2D3
at 1×10−7 mol/l for 48 h, followed by 4 h of incubation
with 1 µg/ml LPS and 100 ng/ml IL-15.
Western blot analysis
The protein expression of STAT5 and p-STAT5 was
observed using western blot analysis. Following treatment according
to the grouping method, ice-cold protein extraction buffer (Nanjing
KeyGen Biotech Co. Ltd., Nanjing, China), supplemented with 1%
protease inhibitor and 1% phosphorylation inhibitor (Nanjing KeyGen
Biotech Co. Ltd.), was added for protein extraction. Protein
concentrations were determined according to the instructions
indicated in the bicinchoninic acid (BCA) protein concentration
detection kit (Nanjing KeyGen Biotech Co. Ltd.). The protein sample
was mixed with sample-loading buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and heated to 100°C for 5 min of
loading. Proteins were separated in 6 or 10% Tris-glycine
polyacrylamide gradient gels (Beyotime Institute of Biotechnology).
The obtained proteins were transferred onto a nitrocellulose
membrane (Invitrogen Life Technologies, Carlsbad, CA, USA) and then
blocked with Tris-buffered saline-Tween® containing 5% bovine serum
albumin (Cell Signaling Technology, Inc.) for 1 h. The membrane was
incubated overnight at 4°C with the primary rabbit polyclonal
anti-STAT and anti-p-STAT5 antibodies (1:500 dilution) or β-actin
(1:5,000 dilution). Following washing, the membrane was incubated
at room temperature for 2 h with horseradish peroxidase-labeled
goat anti-rabbit secondary antibody (ZSGB-BIO, Beijing, China)
(1:3,000 dilution with blocking buffer, Abcam). Protein expression
was then detected according to the instructions provided in the
chemiluminescent staining reagent kit (Beyotime Institute of
Biotechnology). The average pixel density was analyzed with
UN-SCAN-IT gel analysis software (Silk Scientific Inc., Orem, UT,
USA).
Cell slide preparation
Following treatment, the THP-1 cells were applied
onto adhesive polylysine-coated glass slides (Abcam) and fixed in
4% paraformaldehyde at ambient temperature for 20–30 min for
immunofluorescence and laser confocal experiments.
Laser confocal microscopy
Monocytic cytoskeletons were characterized using
laser confocal microscopy. The cells were washed in PBS, fixed in
4% paraformaldehyde, and permeabilized with 0.5% Triton X-100 for
10 min. Following PBS washing, they were incubated with 1:20
rhodamine-labeled phalloidin (Sigma-Aldrich). Specific conjugation
to F-actin was allowed at 25°C (room temperature) for 40 min. The
cells were washed with PBS and then mounted in an
anti-photobleaching mounting medium (Santa Cruz Biotechnology,
Inc.). Photomicrographs were captured with an Axio LSM 710 laser
confocal microscope (Carl Zeiss GmbH, Jena, Germany) at a
magnification of ×2,000.
Fluorescence microscopy
VDR and p-STAT5 proteins were localized using
fluorescence microscopy. The slides were washed thrice with PBS.
Triton™ X-100 (0.3%) was added for 10 min of membrane
permeabilization. Following another three washes with PBS (5–10 min
per wash), they were blocked with bovine serum for 30 min.
Primary antibodies (rabbit polyclonal anti-VDR and
mouse monoclonal anti-p-STAT5 antibodies, 1:200 dilution) were
applied for incubation at 4°C overnight. Following three washes
with PBS, the slides were dried in air. The samples were incubated
with secondary antibodies (goat anti-mouse secondary antibody and
goat anti-rabbit secondary antibody, 1:50 dilution) at room
temperature away from light for 1–2 h. Following washing, the
samples were incubated with 4′,6-diamidino-2-phenylindole (DAPI)
solution at room temperature for 5–10 min. The slides were washed
thrice and then covered with coverslips. Observation was performed
under an Axio Observer A1 fluorescence microscope (magnification,
×10–40; Carl Zeiss AG, Oberkochen, Germany) and images were
captured.
Enzyme-linked immunosorbent assay
(ELISA)
The IL-6 secretion in the cell culture supernatant
was detected using an ELISA kit (PeproTech). The procedures were
conducted in accordance with the manufacturer's instructions. The
samples were measured in duplicate.
Co-immunoprecipitation
The interaction between VDR and p-STAT5 was
validated using the co-immunoprecipitation technique.
The cells in the different groups were collected
following treatment. After washing twice with pre-cooled PBS, the
cells were dissociated in 1 ml pre-cooled nuclear protein
extraction solution (Nanjing KeyGen Biotech Co. Ltd.) and then
centrifuged at 700 × g for 15 min. The supernatant was collected.
Approximately 5 µg TRITC-labeled rabbit anti-VDR polyclonal
antibody (1:100 dilution; cat. no. ab3508; Abcam) was added and
agitation was performed at 4°C for 4 h. Protein A-Sepharose beads
(Pierce Biotechnology, Inc., Rockford, IL, USA) were added,
followed by agitation at 4°C for 30 min. The sample was then
centrifuged at 700 × g for 15 min. The beads bearing
antigen-antibody complexes were collected. Following thrice washing
with pre-cooled lysis buffer (Sangon Biotech Co., Ltd., Shanghai,
China), the beads were transferred into a fresh electrophoresis
tube and loading buffer was added. The suspension was boiled for 4
min, and then SDS-PAGE gradient gel electrophoresis was performed.
The proteins were transferred to polyvinylidene fluoride membranes
(Sigma-Aldrich) and incubated with the FITC-labeled mouse
monoclonal anti-p-STAT5 antibody (1:50 dilution; cat. no. sc-81524;
Santa Cruz Biotechnology, Inc.) overnight at 4°C. Following
washing, the secondary antibody (goat anti-rabbit and anti-mouse
IgG; 1:50 dilutions) was added and incubation proceeded at room
temperature for 1 h. The membranes were washed and chromogenic
developmental reagent (Beyotime Institute of Biotechnology) was
added in the absence of light. Each experiment was repeated
thrice.
Statistical analysis
Measurement data were presented as mean ± standard
error of the mean. Statistical analysis was carried out using SPSS
software (version 15.0; SPSS Inc., Chicago, IL, USA) using a t-test
for comparisons between groups and one-factor analysis of variance
for comparisons among groups. Differences of P<0.05 were
considered to be statistically significant.
Results
STAT5 and p-STAT5 protein
expression
To assess the response of
l,25-(OH)2D3 pretreated monocytes to LPS +
IL-15, the protein expression of STAT5 and p-STAT5 was observed
using western blot analysis. The results are shown in Fig. 1.
 | Figure 1.STAT5 and p-STAT5 protein expression
determined by western blot analysis. Data are expressed as mean ±
standard error of the mean. Lane/bar 1, the control group; 2, the
LPS + IL-15 group; 3, the 1,25-(OH)2D3 group.
*P<0.05 vs. control; #P<0.05 vs. the LPS +IL-15
group. STAT5, signal transducer and activator of transcription 5;
p, phosphorylated; LPS, lipopolysaccharide; IL, interleukin;
1,25-(OH)2D3, 1,25-dihydroxyvitamin
D3. |
In the LPS + IL-15 group, the cells exhibited a
significantly higher level of p-STAT5 expression compared with that
in the control group (0.481±0.16 vs. 0.086±0.024; P=0.016). In the
1,25-(OH)2D3 group, however, p-STAT5
expression did not show a significant difference compared with that
in the control group (0.092±0.028 vs. 0.086±0.024; P>0.05). No
significant differences in the level of STAT5 expression were
observed among the three groups (the expression levels in the
control, LPS + IL-15 and 1,25-(OH)2D3 groups
were 0.580±0.098, 0.594±0.086 and 0.568±0.105, respectively;
P>0.05).
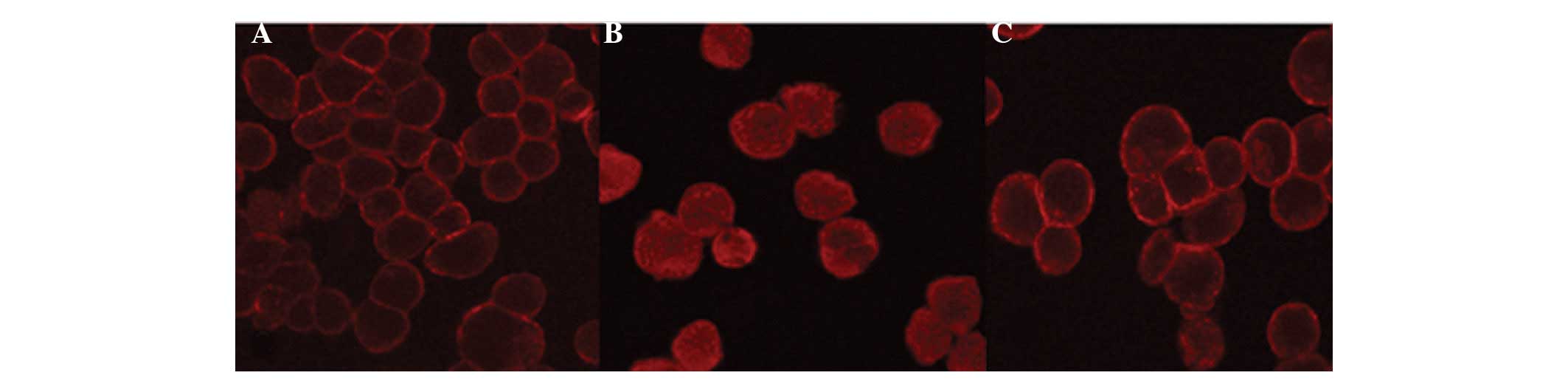
Monocytic cytoskeletons
Monocytic cytoskeletons in the different groups were
characterized using laser confocal microscopy. The results are
shown in Fig. 2. In the control
group, THP-1 cytoskeletons were primarily distributed at the
periphery of the cytoplasm (Fig.
2A). In the LPS + IL-15 group, the cell morphology altered, the
actins were remodeled, the actin bands at the cytoplasmic periphery
disappeared and the actin masses noticeably shrank. In addition,
the lysis of microfilaments was observed in certain cells (Fig. 2B). In the
1,25-(OH)2D3 group, the pretreatment with
1,25-(OH)2D3 partially prevented the effects
caused by LPS + IL-15 (Fig. 2C).
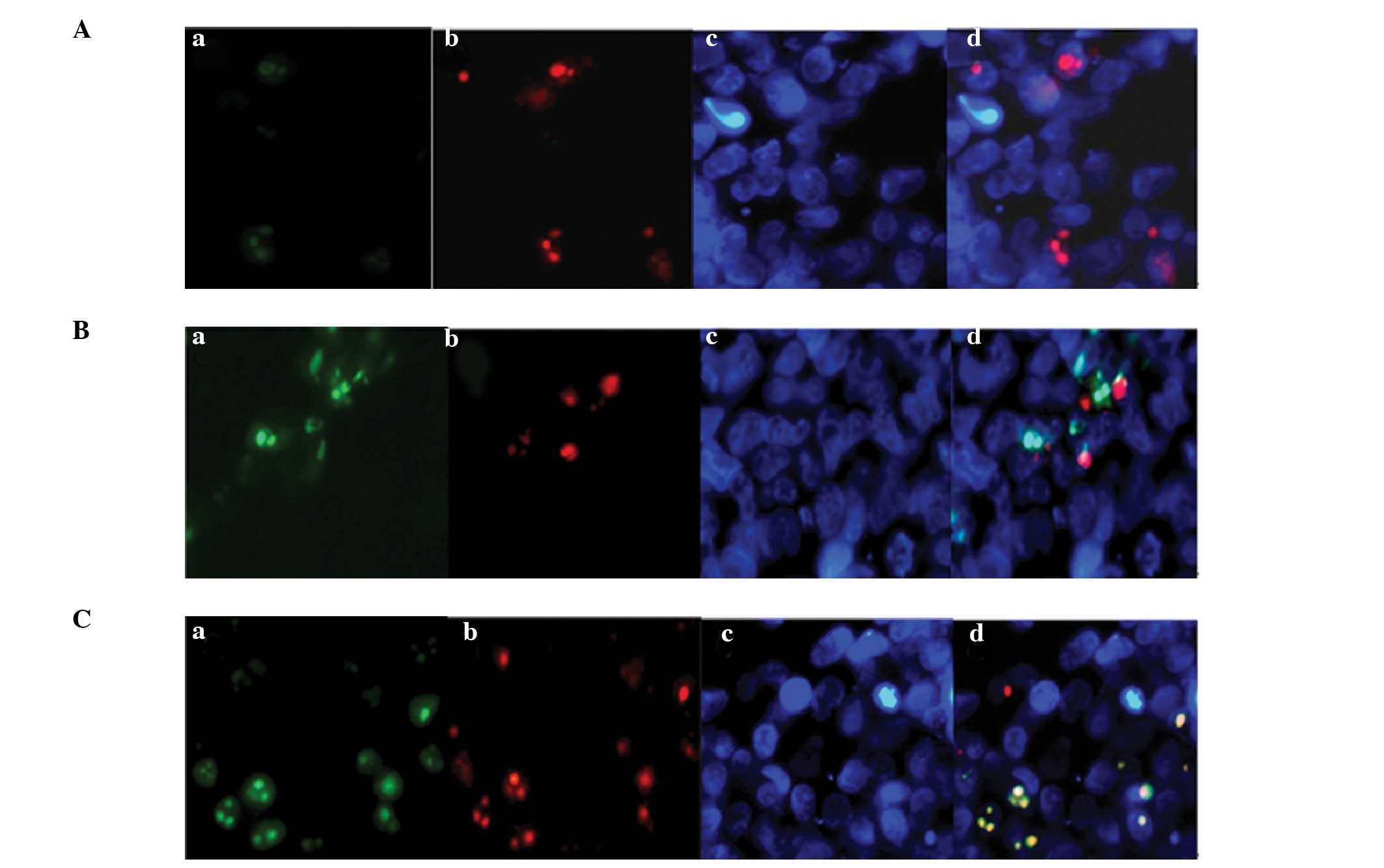
Co-localization of VDR and
p-STAT5
To detect the interaction between VDR and p-STAT5,
co-localization was performed using immunofluorescence. The cells
were grouped and treated according to the method described
previously. Prior to stimulation with LPS + IL-15, STAT5 was
expressed in the cytoplasm. Subsequent to LPS + IL-15 stimulation,
STAT5 was phosphorylated and expressed in the nucleus. In all three
groups, VDR was mostly expressed in the nucleus with a small amount
expressed in the membrane. p-STAT5 was almost undetectable in the
control group (Fig. 3A). Compared
with the control group, the LPS + IL-15 group exhibited a greater
amount of nuclear p-STAT5 and some co-expressed VDR + p-STAT5
complexes (Fig. 3B; indicated in
yellow). The pretreatment with 1,25-(OH)2D3
markedly enhanced the nuclear expression levels of VDR and p-STAT5;
their co-localization was more noticeable than that in the LPS +
IL-15 group (stained in yellow in Fig.
3C).
IL-6 level
As shown in Fig. 4,
the IL-6 level in the LPS + IL-15 group was significantly higher
compared with that in the control and
1,25-(OH)2D3 groups (53.122±17.756 vs.
0.063±0.006 and 13.472±5.056 pg/ml, respectively; both
P<0.01).
Co-immunoprecipitation
The possible interaction between VDR and p-STAT5 was
further tested using the co-immunoprecipitation method. VDR and
proteins interacting with p-STAT5 were precipitated from the cell
extracts, and western blot analysis was performed (Fig. 5). In the control group, when LPS and
IL-15 were absent, p-STAT5 was not observed. In the LPS + IL-15
group, p-STAT5 became noticeable in VDR-containing protein
complexes. In the 1,25-(OH)2D3 group, the
association between VDR and p-STAT5 became more evident compared
with that in the LPS + IL-15 group. These data, as well as the
immunofluorescence results, provided evidence of the interaction
between VDR and p-STAT5. Each experiment was repeated three times
and yielded a consistent result.
 | Figure 5.Possible intranuclear interaction of
VDR with p-STAT5 as indicated by immunofluorescence. Lane 1, the
control group; 2, the LPS + IL-15 group; and 3, the
1,25-(OH)2D3-pretreated group. VDR, vitamin D
receptor; p-STAT, phosphorylated signal transducer and activator of
transcription. LPS, lipopolysaccharide; IL, interleukin; IB,
immunoblotting; IP, immunoprecipitation; IgG, immunoglobulin G. |
Discussion
1,25-(OH)2D3 is an active
metabolite of vitamin D that has multiple activities (14–16). It
exerts action via the VDR; therefore, the effect of vitamin D is
dependent on the VDR level. Our previous study (5) demonstrated that
1,25-(OH)2D3 and LPS + IL-15 influenced the
expression of VDR and STAT5 in serum-incubated monocytes from
patients with T2DM and uremia caused by DN: LPS + IL-15 upregulated
the expression of p-STAT5, whereas pretreatment with
1,25-(OH)2D3 significantly inhibited this
effect. Considering the wide potential clinical application of
vitamin D, however, a further step was taken in the present study
to validate the anti-inflammatory effect of
1,25-(OH)2D3 and to explore the mechanism
underlying this effect.
The results of the present study showed that THP-1
cells exhibited a distorted morphology following stimulation with
LPS + IL-15; the cytoskeletons became depolymerized and remodeled,
actin bands disappeared at the periphery, and actin masses emerged.
Furthermore, LPS + IL-15 significantly strengthened the DNA binding
activity of nuclear p-STAT5 and increased the level of IL-6 in the
supernatant. These changes were significantly inhibited, however,
through 1,25-(OH)2D3 pretreatment. These
results were consistent with those found previously (5), strengthening the evidence of the
effects of 1,25-(OH)2D3 on the expression of
VDR and p-STAT5 as well as cytoskeletal rearrangement in human
monocytes.
According to the literature (19), 1,25-(OH)2D3
promotes the formation of STAT1-VDR complexes in THP-1 monocytes;
it significantly weakens the transcriptional activity of VDR but
enhances STAT1 transcription. The signaling pathways controlled by
VDR and STAT may, therefore, be interconnected and the
anti-inflammatory effect of 1,25-(OH)2D3 may
be associated with the effects of vitamin D on these pathways.
The Janus kinase (JAK)/STAT signaling pathway is one
of the essential signal transduction channels involved in multiple
cell behaviors, such as growth, development, division,
differentiation, apoptosis and functional synchronization (20). IL-15 is a JAK/STAT signaling-mediated
soluble cytokine that is able to promote inflammation (21). During chronic micro-inflammation in
patients with T2DM and uremia caused by DN, LPS affects the
production of IL-6, IL-15, IL-18 and IL-10 by its action on
intracellular TLR4 or TLR2 (22–26). It
promotes the secretion of pro-inflammatory cytokines, such as IL-6
and IL-15. These cytokines bind to cell-borne receptors to activate
the tyrosine kinase, JAK (22,27,28),
which, in turn, activates STAT5 to form p-STAT5 via
phosphorylation. p-STAT5 takes the form of homo- or hetero-dimers
or oligomers. It enters the nucleus, where it binds to promoter VDR
DNA to regulate VDR transcription. This process can be partially
prevented, however, through 1,25-(OH)2D3
pretreatment. Following the binding of
1,25-(OH)2D3 to the VDR,
1,25-(OH)2D3/VDR/retinoid X receptor
complexes are formed and VDR DNA-binding sites are exposed. These
sites are bound by p-STAT5 and VDR-STAT5 complexes are formed. The
formed complexes induce the production of anti-inflammatory
cytokines and inhibit the secretion of pro-inflammatory cytokines,
thereby preventing the occurrence of inflammation to a certain
degree. Such an effect of 1,25-(OH)2D3 on
monocytes may reflect the interaction between
1,25-(OH)2D3 with the VDR and the JAK/STAT
signaling pathway. To test whether a crosstalk between VDR and
STAT5 occurs, monocytes were incubated with
1,25-(OH)2D3 prior to their treatment with
LPS and IL-15 in this study. The results obtained from the
immunofluorescence and co-immunoprecipitation experiments suggest
that crosstalk between these two proteins does exist in THP-1
cells; it is this crosstalk that further induces cytoskeletal
rearrangement.
This study has a limitation: The results would be
more convincing if an animal or human in vivo study or a
study on monocytes directly isolated from patients with diabetes
was conducted.
In conclusion, 1,25-(OH)2D3
may exert an anti-inflammatory action by influencing the crosstalk
between STAT5 and VDR to a certain extent. The present study sheds
new light on the mechanism behind the effect of vitamin D in its
wide application and provides a new therapeutic target in the
treatment of diseases, such as T2DM and uremia caused by DN.
Acknowledgements
This study was supported by the Science and
Technology Department of Guizhou Province [Guizhou Branch of Social
Research Programs SY word (2012) No. 3116], the Science and
Technology Department of Guizhou, Zunyi Medical College, Zunyi
Municipal Science and Technology Bureau of Science and Technology
Joint Fund [Guizhou Branch of J word (2013) No. 53], and the Zunyi
Medical College Foundation for Doctors (Project No. F-588).
References
|
1
|
Duncan BB, Schmidt MI, Pankow JS, et al:
Low-grade systemic inflammation and the development of type 2
diabetes: the atherosclerosis risk in communities study. Diabetes.
52:1799–1805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donath MY and Shoelson SE: Type 2 diabetes
as an inflammatory disease. Nat Rev Immunol. 11:98–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco
O, et al: Suppressors of cytokine signaling abrogate diabetic
nephropathy. J Am Soc Nephrol. 21:763–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang M, Shen Z, Chen D, Gan H, Shen Q,
Yang B and Du X: Effects of 1,25-(OH)(2)D(3) on the expressions of
vitamin D receptor, STAT5 and cytoskeletal rearrangement in human
monocytes incubated with sera from type 2 diabetes patients and
diabetic nephropathy patients with uremia. Inflamm Res. 61:511–520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pickup JC: Inflammation and activated
innate immunity in the pathogenesis of type 2 diabetes. Diabetes
Care. 27:813–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ko GJ, Kang YS, Han SY, et al:
Pioglitazone attenuates diabetic nephropathy through an
anti-inflammatory mechanism in type 2 diabetic rats. Nephrol Dial
Transplant. 23:2750–2760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathieu C, Gysemans C, Guilietti A and
Bouillon R: Vitamin D and diabetes. Diabetologia. 48:1247–1257.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kayaniyil S, Vieth R, Retnakaran R, et al:
Association of vitamin D with insulin resistance and beta-cell
dysfunction in subjects at risk for type 2 diabetes. Diabetes Care.
33:1379–1381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu PT, Stenger S, Li H, et al: Toll-like
receptor triggering of a vitamin D-mediated human antimicrobial
response. Science. 311:1770–1773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sadeghi K, Wessner N, Laggner U, Ploder M,
et al: Vitamin D3 down-regulates monocyte TLR expression and
triggers hyporesponsiveness to pathogen-associated molecular
patterns. Eur J Immunol. 36:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baeke F, van Etten E, Gysemans C, et al:
Vitamin D signaling in immune-mediated disorders: Evolving insights
and therapeutic opportunities. Mol Aspects Med. 29:376–387. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams JS and Hewison M: Unexpected actions
of vitamin D: new perspectives on the regulation of innate and
adaptive immunity. Nat Clin Pract Endocrinol Metab. 4:80–90. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stubbs JR, Idiculla A, Slusser J, et al:
Cholecalciferol supplementation alters calcitriol-responsive
monocyte proteins and decreases inflammatory cytokines in ESRD. J
Am Soc Nephrol. 21:353–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Norman AW, Frankel JB, Heldt AM and
Grodsky GM: Vitamin D deficiency inhibits pancreatic secretion of
insulin. Science. 209:823–825. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitri J, Muraru MD and Pittas AG: Vitamin
D and type 2 diabetes: a systematic review. Eur J Clin Nutr.
65:1005–1015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reddy PV, Puri RV, Khera A and Tyagi AK:
Iron storage proteins are essential for the survival and
pathogenesis of Mycobacterium tuberculosis in THP-1 macrophages and
the guinea pig model of infection. J Bacteriol. 194:567–575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marcil V, Lavoie JC, Emonnot L, et al:
Analysis of the effects of iron and vitamin C co-supplementation on
oxidative damage, antioxidant response and inflammation in THP-1
macrophages. Clin Biochem. 44:873–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vidal M, Ramana CV and Dusso AS:
Stat1-vitamin D receptor interactions antagonize
1,25-dihydroxyvitamin D transcriptional activity and enhance
stat1-mediated transcription. Mol Cell Biol. 22:2777–2787. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaoka K, Otsuka K, Niiro H, et al:
Activation of STAT5 by lipopolysaccharide through
granulocyte-macrophage colony-stimulating factor production in
human monocytes. J Immunol. 160:838–845. 1998.PubMed/NCBI
|
|
21
|
Alleva DG, Kaser SB, Monroy MA, et al:
IL-15 functions as a potent autocrine regulator of macrophage
proinflammatory cytokine production: evidence for differential
receptor subunit utilization associated with stimulation or
inhibition. J Immunol. 159:2941–2951. 1997.PubMed/NCBI
|
|
22
|
Kimura A, Naka T, Muta T, et al:
Suppressor of cytokine signaling-1 selectively inhibits LPS-induced
IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci USA.
102:17089–17094. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neely GG, Robbins SM, Amankwah EK, Epelman
S, et al: Lipopolysaccharide-stimulated or granulocyte-macrophage
colony-stimulating factor-stimulated monocytes rapidly express
biologically active IL-15 on their cell surface independent of new
protein synthesis. J Immunol. 167:5011–5017. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foster N, Andreadou K, Jamieson L, et al:
VIP inhibits P. gingivalis LPS-induced IL-18 and IL-18BPa in
monocytes. J Dent Res. 86:883–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Merendino RA, Arena A, Gangemi S, Ruello
A, et al: In vitro effect of lithium chloride on interleukin-15
production by monocytes from IL-breast cancer patients. J
Chemother. 12:252–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moue M, Tohno M, Shimazu T, et al:
Toll-like receptor 4 and cytokine expression involved in functional
immune response in an originally established porcine intestinal
epitheliocyte cell line. Biochim Biophys Acta. 1780:134–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaoka K, Otsuka T, Niiro H, et al:
Activation of STAT5 by lipopolysaccharide through
granulocyte-macrophage colony-stimulating factor production in
human monocytes. J Immunol. 160:838–845. 1998.PubMed/NCBI
|
|
28
|
Musikacharoen T, Matsuguchi T, Kikuchi T
and Yoshikai Y: NF-kappa B and STAT5 play important roles in the
regulation of mouse Toll-like receptor 2 gene expression. J
Immunol. 166:4516–4524. 2001. View Article : Google Scholar : PubMed/NCBI
|