Introduction
Diabetes mellitus is caused by absolute insulin
deficiency due to autoimmune destruction of insulin secreting
pancreatic β-cells (type 1 diabetes, T1D) or by relative insulin
deficiency due to decreased insulin sensitivity (type 2 diabetes,
T2D). Treatment for T1D and T2D often involves regular insulin
injections and oral medication with sulfonylurea. However, this
treatment method neither precisely controls the blood sugar levels,
nor prevents complications associated with diabetes. Reduction of
β-cell mass in the pancreas is the hallmark of the development of
diabetes. Regeneration and maintenance of pancreatic endocrine
tissue following the onset of islet destruction can have a
considerable therapeutic impact on diabetes (1). Transplantation therapies for T1D
include whole organ transplantation, islet transplantation and
regeneration therapy (2–5). The success of the Edmonton protocol for
pancreatic islet transplantation has sparked new interests in the
treatment of T1D (3). However, the
requirement for immunosuppression and the scarcity of organs
available for processing and transplantation has hindered the
widespread use of this therapy. Therefore, the search for
alternative approaches is of paramount clinical interest.
Mesenchymal stem cells (MSCs) have already made
their mark in the field of regenerative medicine, since they have
the capacity of proliferation and differentiation into the
mesenchymal lineage, secreting a variety of cytokines and growth
factors, as well as a profound immunosuppressive capability in
vitro (6–8). MSCs can be easily derived from various
tissues, and their therapeutic worth has already been validated for
a number of clinical conditions (9).
Unlike embryonic stem cells, neither their procurement nor their
use is deemed controversial (10).
Thus, there has been great interest in the potential clinical
applications of MSCs (11,12). There is increasing evidence from
animal studies and clinical trials indicating the therapeutic
effects of MSC transplantation in a number of diseases, such as
myocardial infarction (13,14), stroke (15), spinal cord injury (16), brain injury (17), refractory systemic lupus
erythematosus (18–20), liver disease (21), diabetes mellitus (10,22,23) and
acute and chronic graft-versus-host disease (GVHD) (24,25).
Human umbilical cord-derived mesenchymal stem cells (UCMSCs)
possess greater pluripotency compared with adult stem cells,
expressing the pluripotency markers, Oct-4, Sox-2 and c-Myc
(26), and share similar in
vitro immunosuppressive properties as MSCs obtained from the
bone marrow. Unlike embryonic stem cells, however, UCMSCs do not
form tumors when transplanted (27,28).
Furthermore, UCMSCs are immune-privileged, immune-suppressive and
readily available as a cell source with few ethical disputes
(29,30). Thus, UCMSCs may provide a novel
source of cell therapy for diabetes. However, to date, few clinical
investigations have focused on the treatment of T2D with UCMSCs.
Therefore, the present study reports the preliminary experience of
the application of an intravenous infusion of UCMSCs in six
patients with T2D.
Materials and methods
Patient enrollment
In total, six male patients with different histories
of T2D were enrolled in the study between April 2010 and December
2011 at Weifang People's Hospital (Weifang, China). Their clinical
characteristics are outlined in Table
I. The mean age at infusion was 40.5±3.76 years (range, 27–51
years), and the mean duration of time from the symptoms of
hyperglycemia to transplantation (first infusion) was 64.7±23.8
weeks (range, 4–157 weeks). All the recipients had previously been
treated with insulin injections. Despite this treatment, their
blood glucose and glycated hemoglobin (HbA1c) levels were poorly
controlled, and insulin secretion was relatively insufficient under
the condition of insulin resistance prior to infusion. The study
protocol was evaluated and approved by the Medical Ethics Board of
Weifang People's Hospital affiliated to Weifang Medical College,
under the auspices of the Development Plan Project of Science and
Technology of Weifang. Prior to transplantation, all the patients
were informed of the process concerning the UCMSC infusion and
volunteered to receive this treatment. Written informed consent was
obtained from all the involved diabetic patients. Exclusion
criteria were as follows: i) Severe comorbidity, such as
cardiopulmonary, renal or liver dysfunction, or systemic infection;
ii) leukocyte, platelet or hemoglobin levels of
<3.0×109/l, 75×109/l and 100 g/l,
respectively; iii) serum positive serum for HIV, the surface
antigen of hepatitis B (HBV; HBsAg), hepatitis C (HCV) or tumor
markers; iv) unwillingness to participate in the clinical trial; v)
previous enrollment in other clinical trials in the last three
months; vi) lactating or pregnant females; vii) prior history of
severe allergic reactions; and viii) other clinical conditions that
the investigators considered not appropriate for enrollment in this
study.
 | Table I.Administered UCMSC dose and
pretreatment characteristics of the type 2 diabetes patients. |
Table I.
Administered UCMSC dose and
pretreatment characteristics of the type 2 diabetes patients.
|
|
|
|
|
|
|
|
| Pretreatment
characteristic |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient | Age (years) | Duration
(months) | First dose
(×106cells/kg) | Second dose
(×106cells/kg) | Interval
(days) | Follow-up
(months) | Insulin-free period
(months) | BMI
(kg/m2) | HbA1c (%) | Fasting C-peptide
(ng/ml) | Cmax
(ng/ml) | Insulin dose
(IU/kg/day) | Diabetic
complications |
|---|
| 1 | 47 | 4 | 0.88 | 0.85 | 16 | 44 | 43 | 24.25 | 10.1 | 1.08 | 5.07 | 0.225 | None |
| 2 | 42 | 36 | 0.95 | 0.94 | 14 | 36 | RI | 24.31 | 8.9 | 1.26 | 4.03 | 0.296 | None |
| 3 | 51 | 84 | 0.86 | 0.87 | 17 | 35 | RI | 23.60 | 7.4 | 0.56 | 1.32 | 0.779 | None |
| 4 | 27 | 12 | 0.90 | 0.87 | 16 | 32 | 29 | 23.24 | 6.3 | 0.91 | 3.61 | 0.405 | None |
| 5 | 32 | 48 | 0.81 | 0.79 | 14 | 28 | 25 | 22.50 | 9.8 | 1.42 | 5.69 | 0.267 | None |
| 6 | 44 | 72 | 0.88 | 0.90 | 15 | 24 | RI | 24.10 | 8.8 | 0.96 | 2.16 | 0.619 | None |
|
|
Averagea | 40.5±3.76 | 42.7±13.02 | 0.88±0.02 | 0.87±0.02 | 15.3±0.49 | 33.2±2.82 |
| 23.7±0.29 | 8.55±0.59 | 1.03±0.12 | 3.65±0.68 | 0.43±0.09 | None |
Preparation of UCMSCs
Umbilical cord samples were collected from full-term
cesarean section patients with their consent, and approval was
granted by Weifang People's Hospital. All procedures were conducted
in accordance with the guidelines of the Medical Ethics Committee
of the Health Bureau (31). UCMSCs
were isolated from the gelatinous tissue surrounding the vein and
the artery. In addition, a 10-ml sample of cord blood was analyzed
for communicable diseases, including HBV, HCV, HIV, cytomegalovirus
(CMV) and syphilis. Virus-free umbilical cords were transferred for
cell preparation in a good manufacturing practice laboratory.
Briefly, the primary culture was initiated by seeding chopped
tissue (2–3-mm3 sections) onto 100-mm dishes in growth
medium, containing α-MEM (Gibco Life Technologies, Carlsbad, CA,
USA), supplemented with 10% fetal bovine serum (FBS), 10 ng/ml
basic fibroblast growth factor (Invitrogen Life Technologies,
Carlsbad, CA, USA), 50 I.U./ml penicillin and 50 µg/ml
streptomycin. UCMSCs were incubated at 37°C in a humidified, 5%
CO2 atmosphere. When the cells reached 70–80% confluence
(∼10 days), they were harvested with 0.25% trypsin (Gibco Life
Technologies) and subcultured with the same culture medium. In
addition, a 30-ml sample of venous blood was collected from the
participants for serum extraction (5810R; Eppendorf, Hamburg,
Germany). The blood sample was allowed to clot by leaving it
undisturbed at room temperature for 15–30 min, and then, the serum
was extracted by spinning at 2,000 × g for 10 min in a refrigerated
centrifuge.
After two passages of culture, the FBS was removed
from the culture medium and replaced with 3% autologous serum.
UCMSCs at passages 3–5 were used in the study. To ensure the
quality of the UCMSCs, cell growth was regularly monitored during
the cultivation. The cell viability of harvested UCMSCs was
determined by trypan blue testing. Briefly, the cell suspension was
diluted as 1:1 using a 0.4% trypan blue solution (Bio-Rad
Laboratories, Shanghai, China) and incubated at room temperature
for 1–2 min. The percentage of non-viable cells (blue) was assessed
by counting cells under the microscope.
UCMSCs used for treatment were subject to pass
quality control tests, including immunophenotype identification and
microbiological analysis. The cluster of differentiation (CD)
marker expression was identified for the immunophenotype
identification of UCMSCS, including CD34, CD44, CD45 and CD90. The
fluorescein isothiocyanate (FITC) conjugated CD antibodies and
their corresponding isotypes were purchased from eBiosciences (San
Diego, CA, USA) and used according to the manufacturer's
instructions. The cells were detected by flow cytometry with
FACScan EPICS XL-ADC (Beckman Coulter, USA) as previously described
(32). Routine microbiological tests
were also performed before cell transplantation, including tests
for endotoxin, aerobic and anaerobic bacteria and fungus. Any
contaminated cell preparation was eliminated upon
identification.
UCMSCs transplantation
USMSCs used for transplantation were collected
between passages 3 and 5, depending on the cell growth status of
the individual sample. Approximately 106 UCMSCs/kg body
weight were suspended in saline (40 ml) and filtered through a cell
strainer (40 µm; BD Falcon, BD Biosciences, Bedford, MA, USA). The
infusion was administered intravenously through the cubital vein
over 15 min, and the patients were discharged after 6 h of
observation. Each patient received treatment with UCMSCs two times
at a two-week interval. The patients were gender-matched with the
infant whom the umbilical cord was obtained.
Assessment of efficacy and
follow-up
Fasting plasma glucose (FPG) levels were checked
daily for 3 months, followed by weekly check-ups for an additional
3 months. Exogenous insulin requirements were timely adjusted
according to the levels of FPG. The daily insulin dose and duration
were monitored and recorded for all the patients throughout the
entire study period. The oral glucose tolerance test was performed
using a standard 75-g glucose load, and blood samples were
collected at 0, 0.5, 1 and 2 h after the test load. The area under
the curve (AUC), relating to the C-peptide level, during the 2-h
oral glucose tolerance test was calculated using the following
formula: AUC = 0.25 × (fasting value) + 0.5 × (0.5-h value) + 0.75
× (1-h value) + 0.5 × (2-h value) (33). All associated parameters were
routinely monitored, including the fasting and 2-h C-peptide level,
the HbA1c level, the peak value of C-peptide during the 75-g oral
glucose tolerance test (Cmax), the AUC and the levels of
glutamic acid decarboxylase antibody (GAD-Ab), islet cell antibody
(ICA) and insulin autoantibody (IAA). The temporal changes of
exogenous insulin requirement were recorded at the baseline (prior
to therapy) and at 1, 3, 6, 12, 18 and 24 months
post-transplantation. In addition, transplant complications were
recorded following the procedure. Clinical characteristics,
including the body mass index, duration of disease and diabetic
complications were also recorded (Table
I). The follow-up period was different for each patient due to
different times of patient enrollment, however, a minimum of a
24-month follow-up period was required for the present study.
Blood glucose was measured using a glucometer
(OneTouch Ultra2, LifeScan, Milpitas, CA, USA), and the serum
C-peptide levels were detected using a chemoluminescent immunoassay
with a commercially available kit (DiaSorin S.P.A., Saluggia,
Italy). HbA1c levels were determined using high-pressure liquid
chromatography (Shanghai Huachen Medical Technology, Co., Ltd.,
Shanghai, China). Detection of the serum levels of GAD-Ab, ICA and
IAA were conducted using a combinatorial islet autoantibody
workshop kit (Shenzhen Sciarray BioTech Co., Ltd., Shenzhen, China)
at the clinical endocrinology laboratories of Weifang People's
Hospital.
Safety evaluation of UCMSC
therapy
Clinical, laboratory and radiographic measurements
were used to assess the safety of the UCMSC transplantation
therapy. Immediate reactions included fever, respiratory failure,
headache and systemic complications (systemic infections), while
delayed reactions included tumor formation. Examinations were
conducted prior to and following transplantation at 1, 3, 6, 12, 18
and 24 months. The assessments included routine blood and urine
tests, liver and kidney function assessment and blood lipid content
analysis. In addition, the presence of tumor markers in the blood
serum was assessed, including α-fetoprotein, carcinoembryonic
antigen, carbohydrate antigen 125 and carbohydrate antigen 199. The
presence of HBV, HCV, HIV, CMV and syphilis infections was also
analyzed. Finally, an electrocardiogram, chest X-ray examination
and abdominal color Doppler ultrasound examination were
performed.
Statistical analysis
Clinical and biochemical data are expressed as the
mean ± standard error, and comparisons of the changes from the
baseline conditions were analyzed using the Student's t-test
(two-tailed, paired). Differences among groups and follow-up times
after transplantation (1, 3, 6, 12, 18 and 24 months) were
determined with one-way analysis of variance. The baseline data
were reported individually in a tabular form. All data were
analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA),
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In total, six patients ranging in age between 27 and
51 years (average, 40.5±3.76 years) were enrolled in the study. All
the patients were diagnosed with T2D based on the World Health
Organization criteria (34), with a
mean diesease duration of 42.7±13.02 months. The mean UCMSC dose
(including the first and second infusion) was
0.88±0.05×106 cells/kg (range,
0.79–0.95×106/kg). The mean interval between the first
and second infusion was 15.3±0.49 days and the follow-up time
varied between 24 and 44 months (mean, 33.2±2.82 months). Patients
5 and 6, who tested serum positive for GAD-Ab prior to treatment,
were found to test negative at 1 month after the transplantation,
which was maintained for 24 months. Serum ICA and IAA levels were
negative in all the patients prior to and following
transplantation. Patients 1, 4 and 5, who presented with diabetic
ketoacidosis at diagnosis, overcame this condition at 1 month after
the UCMSC transplantation. Table I
outlines the diabetes-associated parameters of all the involved
patients prior to USMSC administration.
Properties of the UCMSCs
Using an umbilical cord tissue block culture
attachment method, primary UCMSCs were successfully isolated from
the Wharton's jelly of human umbilical cords. The UCMSCs exhibited
typical fibroblast-like morphology (Fig.
1A), and the cell viability was ≥85%, as determined with trypan
blue testing. Each preparation was negative for pathogenic
microorganisms, including aerobic and anaerobic bacteria and
fungus. Endotoxin was ≤0.25 EU/ml in the supernatants of each cell
preparation. HBsAg, HCV-Ab, HIV-Ab, human CMV-IgM and syphilis
antibody tests were all negative. At passage 3, the cultured cells
showed positive expression of CD44 and CD90 (>90%) and negative
expression of CD34 and CD45 (<3.0%; Fig. 1B), exhibiting similar characteristics
to those of previously reported bone marrow-derived MSCs (17). These results were consistent with the
immunophenotypic characteristics of UCMSCs, which had been reported
in previous studies (35,36).
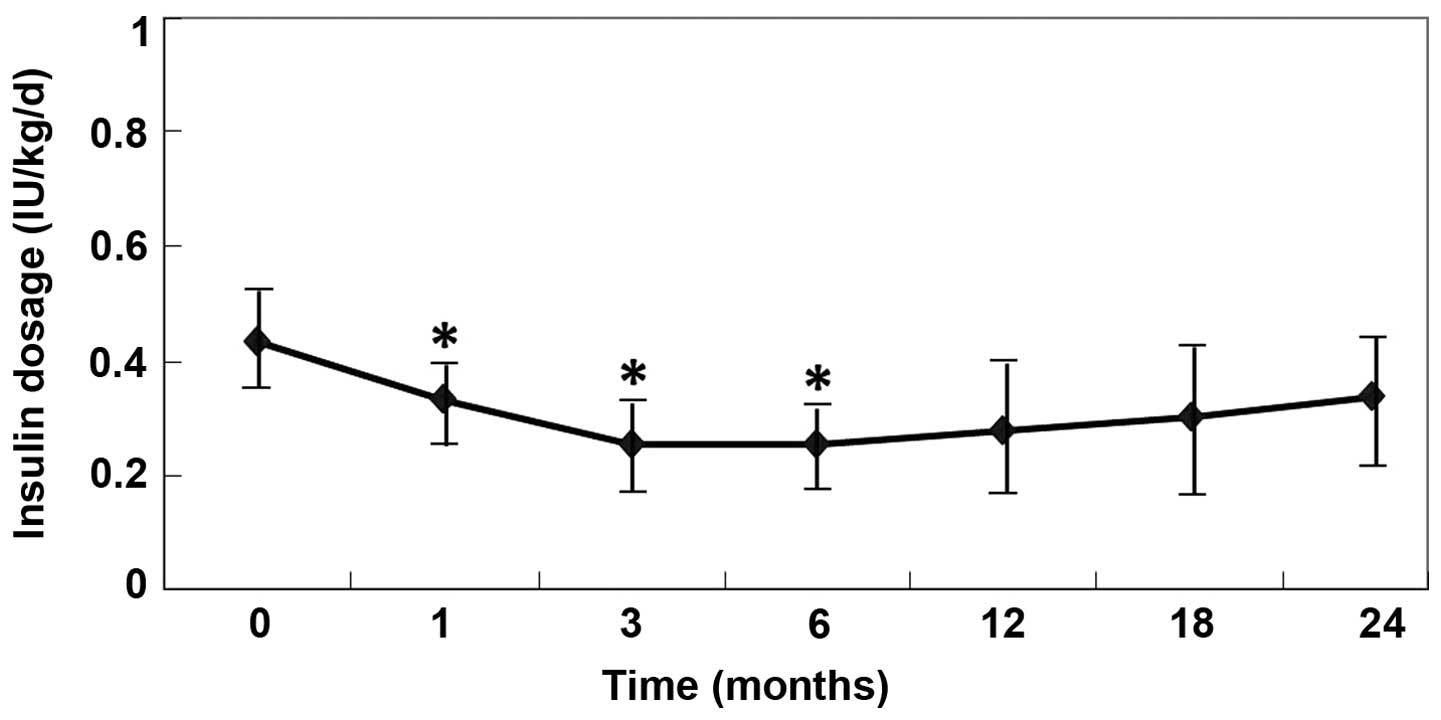
Therapeutic effects of UCMSCs
Table II outlines
the insulin dosages administered for all the studied patients. The
average daily insulin requirements were significantly decreased
following the UCMSC transplantation (P<0.05, vs. pretreatment;
Fig. 2). Three of six patients (1, 4
and 5; 50.0%) became insulin free (defined as the insulin-free
group) for the whole follow-up period (>24 months). The insulin
requirement of the additional three patients (2, 3 and 6) reached
the lowest levels at 3 months (0.252 IU/kg/day) after the
transplantation, but then gradually increased between 12 and 24
months (defined as the insulin-dependent group). Notably, the
insulin-free patients had presented with diabetic ketoacidosis at
diagnosis, and the insulin-dependent patients continued to require
insulin injections; however, their insulin requirement was
significantly reduced for a short period (6 months; Fig. 2).
 | Table II.Insulin dosage of the individual
patients (IU/kg/day). |
Table II.
Insulin dosage of the individual
patients (IU/kg/day).
| Patients | Month 0 | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 |
|---|
| 1 | 0.225 | 0.17 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0.296 | 0.26 | 0.2 | 0.24 | 0.25 | 0.279 | 0.298 |
| 3 | 0.779 | 0.602 | 0.56 | 0.71 | 0.78 | 0.8 | 0.82 |
| 4 | 0.405 | 0.28 | 0.16 | 0 | 0 | 0 | 0 |
| 5 | 0.267 | 0.14 | 0.03 | 0 | 0 | 0 | 0 |
| 6 | 0.619 | 0.52 | 0.56 | 0.58 | 0.633 | 0.74 | 0.915 |
|
Averagea | 0.432±0.090 | 0.329±0.077 | 0.252±0.102 | 0.255±0.130 | 0.277±0.143 | 0.303±0.154 | 0.339±0.174 |
In the insulin-free group (Fig 3A-C), the C-peptide production, AUC and
Cmax were significantly increased at 1, 3, 6, 12 and 24
months after the transplantation (P<0.05, vs. pretreatment),
while there was no statistically significant changes observed in
the insulin-dependent group (P>0.05, vs. pretreatment).
Furthermore, the insulin-free patients exhibited significantly
higher levels of fasting C-peptide, AUC and Cmax at
different follow-up time points after the infusion of UCMSCs. The
HbA1c level was shown to decrease significantly 3 months after the
transplantation, and was maintained at a stable reduced level for
24 months of the follow-up period (P<0.01, vs. pretreatment). In
addition, the FPG and 2 h postprandial blood glucose levels
obtained 3 months after the transplantation were more stable
compared with those observed 7 days prior to the treatment. In the
insulin-dependent group, the HbA1c level significantly decreased
only at 3 months after the transplantation (P=0.014, vs.
pretreatment; Fig. 3D).
Evaluation of the feasibility and
safety of MSC administration
Clinical and laboratory evaluations of the
UCMSC-treated T2D patients revealed no mortality or cell-related
adverse side effects. In addition, there was no immediate or
delayed toxicity associated with UCMSC administration within the
24-month follow-up period. Results of the physiological
examinations are presented in Table
III. There was no positive detection with regard to the tested
viruses, including HIV, HBV, HCV, CMV and syphilis, and no
infections were observed during the follow-up period. Tumor markers
were not found to be positively detectable following UCMSC
transplantation (Table IV), and no
tumor was identified via imaging examinations in any of the
patients. Throughout the entire procedure, no immunosuppressive
drugs were administered for the UCMSC transplantation and GVHD was
not observed.
 | Table III.Results of the predominant laboratory
examination. |
Table III.
Results of the predominant laboratory
examination.
| Items | Baseline | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 | P-value |
|---|
| WBC
(×109/l) | 6.83±0.41 | 6.47±0.36 | 6.70±0.35 | 6.63±0.38 | 6.77±0.40 | 6.70±0.39 | 6.72±0.37 | >0.05 |
| N
(×109/l) | 5.21±0.22 | 5.08±0.20 | 5.30±0.32 | 5.19±0.30 | 5.32±0.27 | 5.26±0.23 | 5.31±0.33 | >0.05 |
| L
(×109/l) | 2.30±0.13 | 2.11±0.10 | 2.32±0.12 | 2.25±0.11 | 2.31±0.13 | 2.33±0.14 | 2.22±0.12 | >0.05 |
| Hb (g/l) | 132.1±2.9 | 133.3±2.7 | 130.6±2.5 | 134.1±2.8 | 132.8±2.6 | 134.3±2.2 | 133.5±3.0 | >0.05 |
| PLT
(×109/l) | 218.8±14.2 | 220.6±10.7 | 217.3±12.1 | 212.9±11.1 | 222.3±14.4 | 220.7±13.5 | 221.9±11.2 | >0.05 |
| ALT (U/l) | 22.5±2.0 | 22.0±1.7 | 23.4±2.3 | 21.9±1.6 | 23.0±2.1 | 22.4±1.9 | 23.1±2.2 | >0.05 |
| AST (U/l) | 21.6±1.7 | 22.5±2.1 | 23.0±2.0 | 22.5±2.2 | 21.8±1.9 | 23.0±2.3 | 22.6±2.1 | >0.05 |
| BUN (mnol/l) | 5.23±0.18 | 5.30±0.13 | 4.97±0.16 | 4.99±0.17 | 5.05±0.18 | 5.20±0.15 | 4.98±0.14 | >0.05 |
| sCr (µmol/l) | 68.5±3.3 | 67.0±2.8 | 64.8±3.0 | 67.5±3.9 | 66.0±3.1 | 65.4±3.1 | 66.1±3.4 | >0.05 |
 | Table IV.Results of the blood tumor
markers. |
Table IV.
Results of the blood tumor
markers.
| Items | Baseline | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 | P-value |
|---|
| AFP (ng/ml) | 4.52±0.50 | 4.61±0.42 | 4.31±0.33 | 4.67±0.40 | 3.94±0.26 | 4.11±0.44 | 4.45±0.39 | >0.05 |
| CEA (ng/ml) | 3.32±0.16 | 3.15±0.26 | 2.85±0.18 | 3.03±0.27 | 2.95±0.20 | 3.35±0.17 | 3.22±0.15 | >0.05 |
| CA125 (U/ml) | 18.5±1.5 | 17.4±1.3 | 18.0±1.6 | 16.9±1.8 | 17.8±1.5 | 18.1±1.7 | 16.8±1.2 | >0.05 |
| CA199 (U/ml) | 20.5±0.7 | 21.2±0.9 | 20.0±0.6 | 21.1±0.8 | 20.9±1.0 | 21.3±0.8 | 20.3±0.6 | >0.05 |
Discussion
Diabetes has become a major cause of mortality in
individuals aged <60 years (37).
Type 2 diabetes (T2D) accounts for the majority of the diabetic
population (>90%). Insulin resistance and defects in pancreatic
β-cell function have been recognized as major pathophysiological
abnormalities underlying the majority of T2D cases. T2D is a
complex disorder that is affected by multiple genetic and
environmental factors. Although complexity and heterogeneity exist
in the pathogenesis of T2D, the disease is characterized by
progressive and inexorable β-cell dysfunction, which subsequently
leads to insulin deficiency. β-cell failure is central to the
development and progression of T2D, particularly during the later
stages of the disease (38). Insulin
secretion decreases due to the glucotoxicity and lipotoxicity
effects on pancreatic β-cells (39).
Thus, intense research has focused on possible mechanisms to
promote the expansion of existing pancreatic β-cells and β-cell
development from either endogenous or exogenous bone
marrow-residing stem cells (40,41).
C-peptide levels are considered to be a critical indicator of the
efficacy of novel therapies for T2D. Since genetic and
environmental risk factors are involved in human T2D,
transplantation of endogenous or exogenous stem cells may be
beneficial for patients with diabetes (42,43).
A number of studies have suggested that bone marrow
cells (BMCs) and mesenchymal stem cells (MSCs) are able to
differentiate into islet β-cells and influence their regeneration
in diabetic animals (23,44,45).
Dong et al (23) reported
that allogeneic MSC transplantation can reduce blood glucose levels
in recipient rats. A relatively small number of transplanted MSCs
survived and subsequently transdifferentiated into
insulin-producing cells in the pancreas of the recipient rats.
Furthermore, Banerjee et al (46) successfully treated streptozotocin
(STZ)-induced diabetes with multiple infusions of unfractionated
BMCs; however, the authors did not suggest a mechanism for the
recovery. In the study by Lee et al (47), repeated transplantation with a high
number of MSCs (2.5×106) induced the repair of
pancreatic islets and renal glomeruli in non-obese diabetic/scid
mice suffering from STZ-induced diabetes. Urbán et al
(22) reported that
cotransplantation of syngeneic unfractionated BMCs and syngeneic or
allogeneic culture-expanded MSCs was able to reverse STZ-induced
diabetes in mice; however, neither BMC nor MSC transplantation was
effective alone. In 2008, Tsai et al reported that UCMSCs
were capable of differentiating into pancreatic lineage cells in
vitro and functioning as insulin-producing cells in
vitro and in vivo. Furthermore, after the
transplantation of differentiated cells into the portal vein of the
diabetic rats using a Port-A catheter, blood sugar levels were
shown to decrease. Insulin-producing cells containing human
C-peptide and human nuclei were observed in the liver (48). In the study by Trivedi et al
(49), five patients with type 1
diabetes mellitus were successfully infused with unfractionated
cultured bone marrow plus human adipose-tissue-derived,
insulin-making MSCs. There were not any untoward effects observed,
and a 30–50% decrease in insulin requirements with a 4-26-fold
increase in serum C-peptide levels was achieved, with a mean
follow-up period of 2.9 months.
To the best of our knowledge, the present study was
the first to use UCMSC-based therapy for T2D patients. Of the six
patients included in the current study, three patients achieved
insulin independence that remained controlled for a median time of
29 months, with the longest insulin dependence observed in one
patient for 43 months ongoing. The insulin-free group presented an
increase in C-peptide levels (fasting, C-peptide AUC and
Cmax) for up to 2 years during the follow-up period. A
marked increase in C-peptide production indicates an improved
β-cell function. However, the six patients with T2D enrolled in the
present study were heterogeneous, and a differential clinical
response was identified with three patients continuing to require
insulin injections, although C-peptide levels were found to
increase in the first month after the UCMSC transplantation.
However, these levels decreased quickly after the third month (Fig.
4A). Previous studies have demonstrated that the self-renewal,
survival and differentiation abilities of stem cells are
predominantly regulated by their microenvironment (12,50). In
insulin-dependent patients, a long diabetic history, older age,
lower serum C-peptide level at pretreatment and any other
complications may indicate the presence of a less conducive
microenvironment for the transplanted cells, leading to the
short-term recovery of β-cell function, which impacts the treatment
efficiency of UCMSC transplantation. Although a prolonged follow-up
is required, the results of the present study indicate that an
intravenous infusion of UCMSCs is beneficial for the restoration of
β-cell function in patients with T2D.
In the insulin-free patients, the serum C-peptide
levels were shown to continuously improve, which may have been due
to a mild immune injury and a better microenvironment for the
transplanted UCMSCs in these patients. The possible explanations
for the success of this treatment are two-fold. Firstly, the
transplanted UCMSCs may induce the regeneration of
recipient-derived pancreatic insulin-secreting cells. Secondly,
UCMSCs can inhibit T-cell-mediated immune responses against newly
formed β-cells, which in turn, are able to survive in this altered
immunological milieu (10,22,51). The
administration of UCMSCs for the treatment of systemic lupus
erythematosus has provided additional evidence for their
immunoregulatory role (52),
supporting their use in controlling autoimmunity and triggered
inflammation. However, the mechanisms underlying the regenerative
process and the appropriate conditions for UCMSC therapy in
diabetes remain poorly understood. Furthermore, the efficacy of
this treatment may be associated with the duration of diabetes, the
microenvironment conditions, the remaining β-cells, the severity of
complications, times of infusion and the UCMSC delivery method.
Therefore, further well-controlled studies with an increased number
of cases are required to clarify the efficacy and safety of UCMSC
intravenous infusion for the treatment of T2D.
Patient safety and the potential benefit to risk
ratio are always the foremost considerations in clinical practice.
In the present study, a novel approach for the treatment of T2D was
attempted using the transplantation of UCMSCs. Systemic follow-ups
were undertaken to evaluate the safety and efficacy of UCMSC
therapy in human subjects. The safety measurements indicated that
UCMSC administration via intravenous infusion was well-tolerated at
the indicated doses without immediate effects. During the follow-up
period, there were no immunological reactions and tumor formation
was not identified. Thus, the results indicated that the
risk-benefit ratio of UCMSC-based therapy in T2D appears to be
favorable. This observation is in accordance with previous studies
performed by Shi et al (53)
and Lv et al (54), where the
results also suggested that transplantation with UCMSCs may be a
safe approach. However, further observations of possible transplant
complications are required.
In conclusion, the present clinical trial-based
study transplanted allogeneic UCMSCs in patients with T2D. After
two UCMSC infusions, all the studied patients exhibited a
significant improvement in the diabetic status, as indicated by
changes in the C-peptide and HbA1c levels. In addition, a reduction
in the insulin requirement or insulin independence were achieved
during the follow-up period. Cell infusion-related immediate and
long-term side effects were not observed during the treatment.
Therefore, the present study provided a novel cell therapeutic
protocol for the treatment of T2D with allogeneic UCMSC
transplantation, without the application of immunosuppressive
drugs.
Acknowledgements
This study was supported by a grant from the Science
and Technology Development Foundation of Weifang (no. 201102009).
The authors thank Dr Chang-Shan Liu and Dr Lin Liu for their
helpful suggestions and are grateful to Li Gao, Zhi-Cui Liu, Nan
Jia, Feng-Fei Yu, Na Li and Gui-Lan Ma for their technical
assistance.
References
|
1
|
Jung KY, Kim KM and Lim S: Therapeutic
approaches for preserving or restoring pancreatic β-cell function
and mass. Diabetes Metab J. 36:426–436. 2014. View Article : Google Scholar
|
|
2
|
Larsen JL: Pancreas transplantation:
indications and consequences. Endocr Rev. 25:919–946. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shapiro AM, Lakey JR, Ryan EA, et al:
Islet transplantation in seven patients with type 1 diabetes
mellitus using a glucocorticoid-free immunosuppressive regimen. N
Engl J Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan EA, Lakey JR, Rajotte RV, et al:
Clinical outcomes and insulin secretion after islet transplantation
with the Edmonton protocol. Diabetes. 50:710–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaoka T: Regeneration therapy of
pancreatic beta cells: towards a cure for diabetes? Biochem Biophys
Res Commun. 296:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sekiya I, Vuoristo JT, Larson BL and
Prockop DJ: In vitro cartilage formation by human adult stem cells
from bone marrow stroma defines the sequence of cellular and
molecular events during chondrogenesis. In: Proc Natl Acad Sci USA.
99. pp. 4397–4402. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Nicola M, Carlo-Stella C, Magni M, et
al: Human bone marrow stromal cells suppress T-lymphocyte
proliferation induced by cellular or nonspecific mitogenic stimuli.
Blood. 99:3838–3843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma RR, Pollock K, Hubel A, et al:
Mesenchymal stem or stromal cells: a review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domínguez-Bendala J, Lanzoni G, Inverardi
L and Ricordi C: Concise review: mesenchymal stem cells for
diabetes. Stem Cells Transl Med. 1:59–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianco P and Robey PG: Stem cells in
tissue engineering. Nature. 414:118–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strauer BE, Brehm M, Zeus T, et al:
Regeneration of human infarcted heart muscle by intracoronary
autologous bone marrow cell transplantation in chronic coronary
artery disease: The IACT Study. J Am Coll Cardiol. 46:1651–1658.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schächinger V, Erbs S, Elsässer A, et al:
REPAIR-AMI Investigators: Intracoronary bone marrow-derived
progenitor cells in acute myocardial infarction. N Engl J Med.
355:1210–1221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chopp M and Li Y: Treatment of neural
injury with marrow stromal cells. Lancet Neurol. 1:92–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JH, Kim DY, Sung IY, et al: Long-term
results of spinal cord injury therapy using mesenchymal stem cells
derived from bone marrow in humans. Neurosurgery. 70:1238–1247.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang ZX, Guan LX, Zhang K, et al: A
combined procedure to deliver autologous mesenchymal stromal cells
to patients with traumatic brain injury. Cytotherapy. 10:134–139.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun L, Akiyama K, Zhang H, et al:
Mesenchymal stem cell transplantation reverses multiorgan
dysfunction in systemic lupus erythematosus mice and humans. Stem
Cells. 27:1421–1432. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang J, Zhang H, Hua B, et al: Allogenic
mesenchymal stem cells transplantation in refractory systemic lupus
erythematosus: a pilot clinical study. Ann Rheum Dis. 69:1423–1429.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Wang D, Liang J, et al: Umbilical
cord mesenchymal stem cell transplantation in severe and refractory
systemic lupus erythematosus. Arthritis Rheum. 62:2467–2475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng L, Xie DY, Lin BL, et al: Autologous
bone marrow mesenchymal stem cell transplantation in liver failure
patients caused by hepatitis B: short-term and long-term outcomes.
Hepatology. 54:820–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Urbán VS, Kiss J, Kovács J, et al:
Mesenchymal stem cells cooperate with bone marrow cells in therapy
of diabetes. Stem Cells. 26:244–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong QY, Chen L, Gao GQ, et al: Allogeneic
diabetic mesenchymal stem cells transplantation in
streptozotocin-induced diabetic rat. Clin Invest Med. 31:E328–E337.
2008.PubMed/NCBI
|
|
24
|
Le Blanc K and Ringdén O: Immunomodulation
by mesenchymal stem cells and clinical experience. J Intern Med.
262:509–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le Blanc K, Frassoni F, Ball L, et al:
Developmental Committee of the European Group for Blood and Marrow
Transplantation: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: a phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carlin R, Davis D, Weiss M, et al:
Expression of early transcription factors Oct-4, Sox-2 and Nanog by
porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol.
4:82006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Torrente Y and Polli E: Mesenchymal stem
cell transplantation for neurodegenerative diseases. Cell
Transplant. 17:1103–1113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton's jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yagi H, Soto-Gutierrez A, Parekkadan B, et
al: Mesenchymal stem cells: Mechanisms of immunomodulation and
homing. Cell Transplant. 19:667–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joyce N, Annett G, Wirthlin L, et al:
Mesenchymal stem cells for the treatment of neurodegenerative
disease. Regen Med. 5:933–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao YM: Medical ethics: unavoidable issue
in medical research. Zhonghua Yi Xue Za Zhi. 85:424–426.
2005.PubMed/NCBI
|
|
32
|
Moniri MR, Sun XY, Rayat J, et al:
TRAIL-engineered pancreas-derived mesenchymal stem cells:
characterization and cytotoxic effects on pancreatic cancer cells.
Cancer Gene Ther. 19:652–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haffner SM, Stern MP, Hazuda HP, et al:
Hyperinsulinemia in a population at high risk for
non-insulin-dependent diabetes mellitus. N Engl J Med. 315:220–224.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Majore I, Moretti P, Stahl F, et al:
Growth and differentiation properties of mesenchymal stromal cell
populations derived from whole human umbilical cord. Stem Cell Rev.
7:17–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tong CK, Vellasamy S, Tan BC, et al:
Generation of mesenchymal stem cell from human umbilical cord
tissue using a combination enzymatic and mechanical disassociation
method. Cell Biol Int. 35:221–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ley SH, Hamdy O, Mohan V and Hu FB:
Prevention and management of type 2 diabetes: dietary components
and nutritional strategies. Lancet. 383:1999–2007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Halban PA, Polonsky KS, Bowden DW, et al:
β-cell failure in type 2 diabetes: Postulated mechanisms and
prospects for prevention and treatment. Diabetes Care.
37:1751–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harrison LB, Adams-Huet B, Raskin P and
Lingvay I: β-cell function preservation after 3.5 years of
intensive diabetes therapy. Diabetes Care. 35:1406–1412. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hess D, Li L, Martin M, et al: Bone
marrow-derived stem cells initiate pancreatic regeneration. Nat
Biotechnol. 21:763–770. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nir T and Dor Y: How to make pancreatic
beta cells-prospects for cell therapy in diabetes. Curr Opin
Biotechnol. 16:524–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahlqvist E, Ahluwalia TS and Groop L:
Genetics of type 2 diabetes. Clin Chem. 57:241–254. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Voltarelli JC, Couri CE, Stracieri AB, et
al: Autologous hematopoietic stem cell transplantation for type 1
diabetes. Ann N Y Acad Sci. 1150:220–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang DQ, Cao LZ, Burkhardt BR, et al: In
vivo and in vitro characterization of insulin-producing cells
obtained from murine bone marrow. Diabetes. 53:1721–1732. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oh SH, Muzzonigro TM, Bae SH, et al: Adult
bone marrow-derived cells trans-differentiating into
insulin-producing cells for the treatment of type I diabetes. Lab
Invest. 84:607–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banerjee M, Kumar A and Bhonde RR:
Reversal of experimental diabetes by multiple bone marrow
transplantation. Biochem Biophys Res Commun. 328:318–325. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee RH, Seo MJ, Reger RL, et al:
Multipotent stromal cells from human marrow home to and promote
repair of pancreatic islets and renal glomeruli in diabetic
NOD/scid mice. In: Proc Natl Acad Sci USA. 103. pp. 17438–17443.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsai PJ, Wang HS, Shyr YM, et al:
Transplantation of insulin-producing cells from umbilical cord
mesenchymal stem cells for the treatment of streptozotocin-induced
diabetic rats. J Biomed Sci. 19:472012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trivedi HL, Vanikar AV, Thakker U, et al:
Human adipose tissue-derived mesenchymal stem cells combined with
hematopoietic stem cell transplantation synthesize insulin.
Transplant Proc. 40:1135–1139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moore KA and Lemischka IR: Stem cells and
their niches. Science. 311:1880–1885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cutler AJ, Limbani V, Girdlestone J and
Navarrete CV: Umbilical cord-derived mesenchymal stromal cells
modulate monocyte function to suppress T cell proliferation. J
Immunol. 185:6617–6623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang J, Gu F, Wang H, et al: Mesenchymal
stem cell transplantation for diffuse alveolar hemorrhage in SLE.
Nat Rev Rheumatol. 6:486–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shi M, Zhang Z, Xu R, et al: Human
mesenchymal stem cell transfusion is safe and improves liver
function in acute-on-chronic liver failure patients. Stem Cells
Transl Med. 1:725–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lv YT, Zhang Y, Liu M, et al:
Transplantation of human cord blood mononuclear cells and umbilical
cord-derived mesenchymal stem cells in autism. J Transl Med.
11:1962013. View Article : Google Scholar : PubMed/NCBI
|