Introduction
Percutaneous coronary intervention (PCI) with
coronary stent implantation is one of the revolutionary
advancements made in the treatment of coronary heart diseases over
the past decades. Bare metal stents (BMSs) and drug-eluting stents
(DESs) are the two main types of stents used for coronary lesions.
Although the incidence of in-stent restenosis (ISR) following stent
implantation has been reduced to <10% following the transition
from BMSs to DESs, its occurrence is not negligible due to the
large number of patients treated with BMSs or DESs, particularly
patients at high-risk of ISR (1,2);
therefore, the optimal treatment for ISR remains undefined. Several
randomized trials that compared DES use to conventional therapy for
restenosis indicated that DESs exhibit superior clinical and
angiographic results (3–5); however, the treatment of ISR with a new
type of DES is strongly dependent on long-term dual-antiplatelet
therapy, which could prove troublesome for patients who are at high
risk of bleeding (6). Furthermore,
DESs reduce the flexibility of the vessel and limit the
repeatability of the procedure.
Paclitaxel-coated balloon (PCB) technology has
emerged as a potential therapeutic alternative in PCI. The PCB is a
semi-compliant angioplasty balloon, covered with an antirestenotic
drug that is rapidly released locally into the vessel wall as the
balloon is inflated (7). The
significant advantages of this device include the homogeneous
transfer of the drug to the entire vessel wall and the absence of
polymer, which reduces the chance of late thrombosis (7). A number of registry data support the
safety and feasibility of the use of PCBs in ISR. In particular,
the use of PCBs in the treatment of BMS-ISR has achieved notable
outcomes (8); however, the majority
of randomized clinical trials are limited due to small sample sizes
and different follow-up durations. Furthermore, compared with the
widespread successful application of DESs, it is uncertain whether
additional benefits could be provided by PCBs. The present
meta-analysis was therefore conducted with the objective of
systematically reviewing the current randomized evidence regarding
the clinical and angiographic outcomes of PCBs for patients with
ISR.
Materials and methods
Identification of studies
The MEDLINE, Embase and Cochrane Central Register of
Controlled Trials databases were searched in April 2014 in order to
identify eligible clinical controlled trials comparing PCBs with
uncoated balloons (UCBs) or DESs in patients with ISR (BMS-ISR or
DES-ISR). No restrictions were imposed on the patients, sample
size, language, publication status, lesion type or follow-up
duration. The keywords included (drug-coated balloon or drug
eluting balloon) and (paclitaxel-coated balloon or
paclitaxel-eluting balloon), for the theme ‘clinical trial’.
Relevant reviews and reference lists of retrieved records were also
screened. Two authors selected the studies independently, and
disagreements were resolved by discussion.
Selection criteria
All published and/or ongoing clinical controlled
trials that focused on the comparison between PCBs and UCBs/DESs
for ISR lesions were considered eligible. The clinical and
angiographic outcomes were followed up for ≥6 months. Duplicate
studies and studies that failed to make adjustments for potential
confounders or to provide sufficient statistical analysis were
excluded.
Clinical outcomes and definitions
Data for baseline variables and clinical and
angiographic outcomes were collected. Outcomes of interest included
total mortality (TM), myocardial infarction (MI; defined according
to each study protocol), major adverse cardiac events (MACEs),
target lesion revascularization (TLR; defined as re-intervention on
the index treated lesion), stent thrombosis [ST; defined by the
Academic Research Consortium as ‘definite’ or ‘probable’ ST
(9)], binary restenosis (BR; defined
as ≥50% luminal diameter stenosis by quantitative coronary
angiography) and in-lesion late lumen loss (LLL). LLL was defined
as the difference between the postprocedural and follow-up in-stent
or in-segment minimal lumen diameter, as evaluated by quantitative
coronary angiography.
Statistical analysis
Dichotomous data were calculated as odds ratios
(ORs) and 95% confidence intervals (95% CI). Continuous variables
are presented as weighted mean difference (WMD) with a 95% CI.
P<0.05 was considered to indicate a statistically significant
difference. In the presence of inter-study heterogeneity a
random-effects model was used, whereas a fixed-effects model was
applied in the absence of heterogeneity. Following data pooling,
statistical heterogeneity was identified and evaluated by means of
the I2 statistic, which represents the proportion of the
total variability across studies (I2<25%, trivial
heterogeneity; I2<50%, moderate heterogeneity;
I2>50%, substantial and important heterogeneity).
Analysis was performed using Review Manager Software version 5.1
(The Nordic Cochrane Collaboration, Copenhagen, Denmark).
Results
Characteristics of the studies
Results from the literature search are depicted in
Fig. 1. In total, 171 articles were
reviewed, and 9 studies (10–18) [8
randomized controlled trials (RCTs) (10–17) and
1 non-RCT) (18)] with a total of
1,488 patients (1,608 lesions) met the criteria for inclusion in
the present meta-analysis. Among those patients, PCBs were used in
733 patients (779 lesions), while the control treatments included
UCBs (317 patients, 348 lesions) and DESs (438 patients, 481
lesions). One of the studies, the ISAR-DESIRE 3 trial (15), was a three-arm trial comparing PCB,
UCB and DES groups; this study was therefore treated as 2 separate
trials. Ultimately, 5 studies comparing PCBs with UCBs and 5
comparing PCBs with DESs were selected. All 9 trials had a clinical
primary endpoint, with a follow-up duration ranging from 6 to 9
months, and 8 studies had an angiographic primary endpoint, with a
follow-up duration ranging from 6 to 12 months. The studies were
multi-center in 7 cases and single center in 2. Among the 8 RCTs, 1
study was double-blind, 3 were single-blind and 4 were unblinded.
The demographic and clinical characteristics of the patients are
summarized in Tables I and II. No significant difference was found
between the PCB and control groups in the baseline characteristics
of the patients.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
|
|
|
|
|
|
| Follow-up |
|---|
|
|
|
|
|
|
|
|
|---|
| Study name/first
author (ref.) | Year | Design | Device | No. of patients | Restenotic stent
type | Angiographic | Clinical |
|---|
| PACCOCATH ISR I/II
(11) | 2008 | DB, MC | Paccocath vs.
UCB | 108 | BMS or DES | LLL, BR at 6
months | TLR/MI/MACEs at 12
months |
| PEPCAD-DES (10) | 2012 | SB, MC | SeQuent Please vs.
UCB | 110 | DES | LLL, BR at 6
months | TLR/MI/MACEs/ST at 12
months |
| Habara (12) | 2011 | SB, SC | SeQuent Please vs.
UCB | 50 | DES | LLL, BR at 6
months | TLR/MI/MACEs/ST at 6
months |
| PEPCAD China ISR
(13) | 2014 | SB, MC | SeQuent Please vs.
DES (TAXUS Liberté) | 215 | DES | LLL, BR at 9
months | TLR/MI/MACEs at 12
months |
| Unverdorben (14) | 2009 | NB, MC | SeQuent Please vs.
DES (TAXUS Liberté) | 131 | BMS | LLL, BR at 6
months | TLR/MI/MACEs/ST at 12
months |
| ISAR-DESIRE 3
(15) | 2013 | NB, MC | SeQuent Please vs.
DES (TAXUS Liberté) vs. UCB | 402 | DES | LLL, BR at 6–8
months | TLR/MI/MACEs/ST at 12
months |
| Habara (16) | 2013 | NB, MC | SeQuent Please vs.
UCB | 208 | DES or BMS | LLL, BR at 6
months | TLR/MI/MACEs/ST at 6
months |
| RIBS V (17) | 2014 | NB, MC | SeQuent Please vs.
DES (Xience Prime) | 189 | BMS | LLL, BR at 9
months | TLR/MI/MACEs/ST at
12 months |
| Almalla (18) | 2013 | NRCT, SC | PCB vs. DES | 86 | DES | NR | TLR/MI/MACEs/ST at
12 months |
 | Table II.Baseline characteristics of the
patients. |
Table II.
Baseline characteristics of the
patients.
|
|
|
|
|
| Before procedure
(mm) | Follow-up (n) |
|---|
|
|
|
|
|
|
|
|
|---|
| Study name/first
author (ref.) | Group | No. of
patients | Mean age
(years) | DM (%) | MLD | Lesion length | Angiographic | Clinical |
|---|
| PACCOCATH ISR I/II
(10) | PCB | 54 | 65.4±10.3 | 17 | 0.63±0.29 | 18.3±9.7 | 48 | 54 |
|
| UCB | 54 | 66.3±9.8 | 41 | 0.70±0.35 | 18.6±8.3 | 49 | 54 |
| PEPCAD-DES
(9) | PCB | 72 | 69.8±10.8 | 36.1 | 0.66±0.40 | 11.2±6.5 | 64 | 72 |
|
| UCB | 38 | 64.0±11.3 | 34.2 | 0.62±0.44 | 12.2±8.2 | 31 | 38 |
| Habara (11) | PCB | 25 | 69.9±11.0 | 56 | 0.99±0.32 | 12.7±5.3 | 23 | 25 |
|
| UCB | 25 | 68.9±9.9 | 68 | 0.92±0.51 | 13.2±5.5 | 24 | 25 |
| PEPCAD China ISR
(12) | PCB | 109 | 61.8±9.3 | 40.4 | 0.85±0.38 | 12.52±6.55 | 97a | 109 |
|
| PES | 106 | 62.1±9.3 | 33.0 | 0.86±0.41 | 13.08±7.13 | 84a | 106 |
| Unverdorben
(13) | PCB | 66 | 64.6±9.7 | 33.3 | 0.74±0.27 | 15.7±6.6 | 57 | 66 |
|
| PES | 65 | 65.1±8.7 | 26.2 | 0.77±0.30 | 15.4±6.6 | 59 | 65 |
| ISAR-DESIRE 3
(14) | PCB | 137 | 67.7±10.4 | 41 | 0.97±0.48 | N/A | 147a | 136 |
|
| PES | 131 | 68.8±10.0 | 47 | 0.93±0.50 | N/A | 142a | 127 |
|
| UCB | 134 | 67.1±9.3 | 37 | 0.88±0.49 | N/A | 127a | 129 |
| Habara (15) | PCB | 137 | 68.3±10.3 | 46 | 0.86±0.32 | 12.8±6.5 | 139a | 136 |
|
| UCB | 71 | 70.4±10.2 | 42 | 0.84±0.34 | 13.7±5.8 | 69a | 71 |
| RIBS V (16) | PCB | 95 | 67±11 | 32 | 1.02±0.4 | 13.7±7 | 84 | 95 |
|
| EES | 94 | 64±12 | 20 | 0.93±0.4 | 13.8±6 | 86 | 94 |
| Almalla (17) | PCB | 46 | 69.6±9.6 | 39.1 | 0.57±0.30 | 9.0±5.2 | N/A | 46 |
|
| EES | 40 | 67.7±10.8 | 35 | 0.51±0.41 | 12.3±11.0 | N/A | 40 |
Angiographic outcomes
LLL
At a follow-up period of 6–9 months, data on LLL
were recorded in 5 PCB versus UCB studies and 4 PCB versus DES
studies. A statistical heterogeneity was observed in subgroup PCB
versus DES (I2=66%; P=0.03); therefore the
random-effects model was used. Comparable results were found in the
two subgroups. Patients treated with PCBs exhibited a significant
reduction in LLL compared with patients treated with UCBs (WMD,
−0.46; 95% CI, (−0.59)-(−0.34); P<0.00001). The PCB versus DES
subgroup analysis showed a similar trend towards lower LLL in the
PCB group, although the difference was not significant (WMD, −0.04;
95% CI, −0.18–0.10; P=0.57) (Fig.
2).
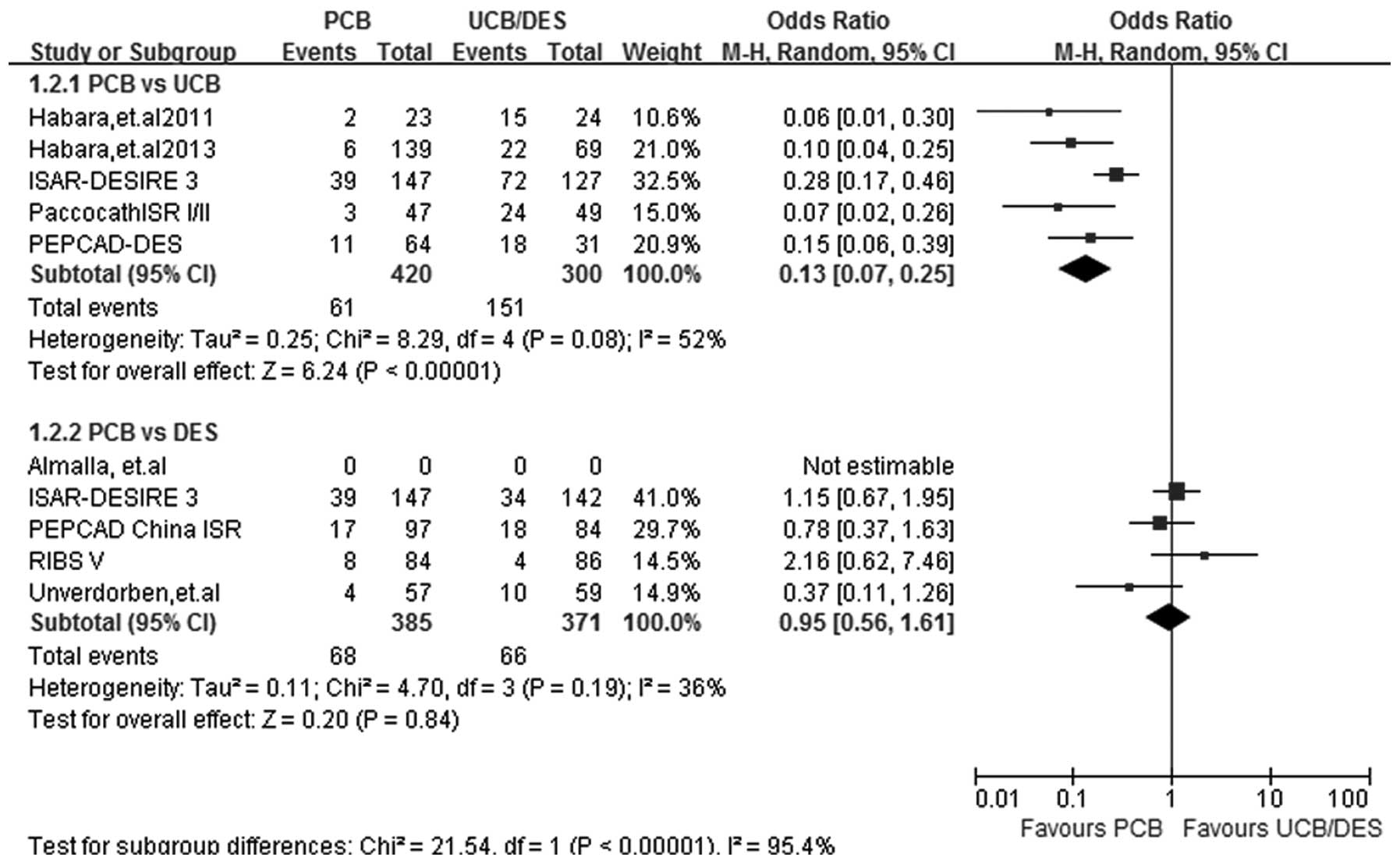
BR
At a follow-up period of 6–9 months, the rate of BR
recorded in the 5 PCB versus UCB and 4 PCB versus DES studies was
analyzed by the random-effects model, since a significant study
heterogeneity was observed (I2=52%; P=0.08) in subgroup
PCB versus UCB. A statistically significant effect favoring PCB was
detected in the PCB versus UCB subgroup analysis (OR, 0.13; 95% CI,
0.07–0.25; P<0.00001). PCB versus DES subgroup analysis showed a
benefit associated with DES use, although the difference between
PCB and DES use was not significant (OR, 0.95; 95% CI, 0.56–1.61;
P=0.84) (Fig. 3).
Clinical outcomes
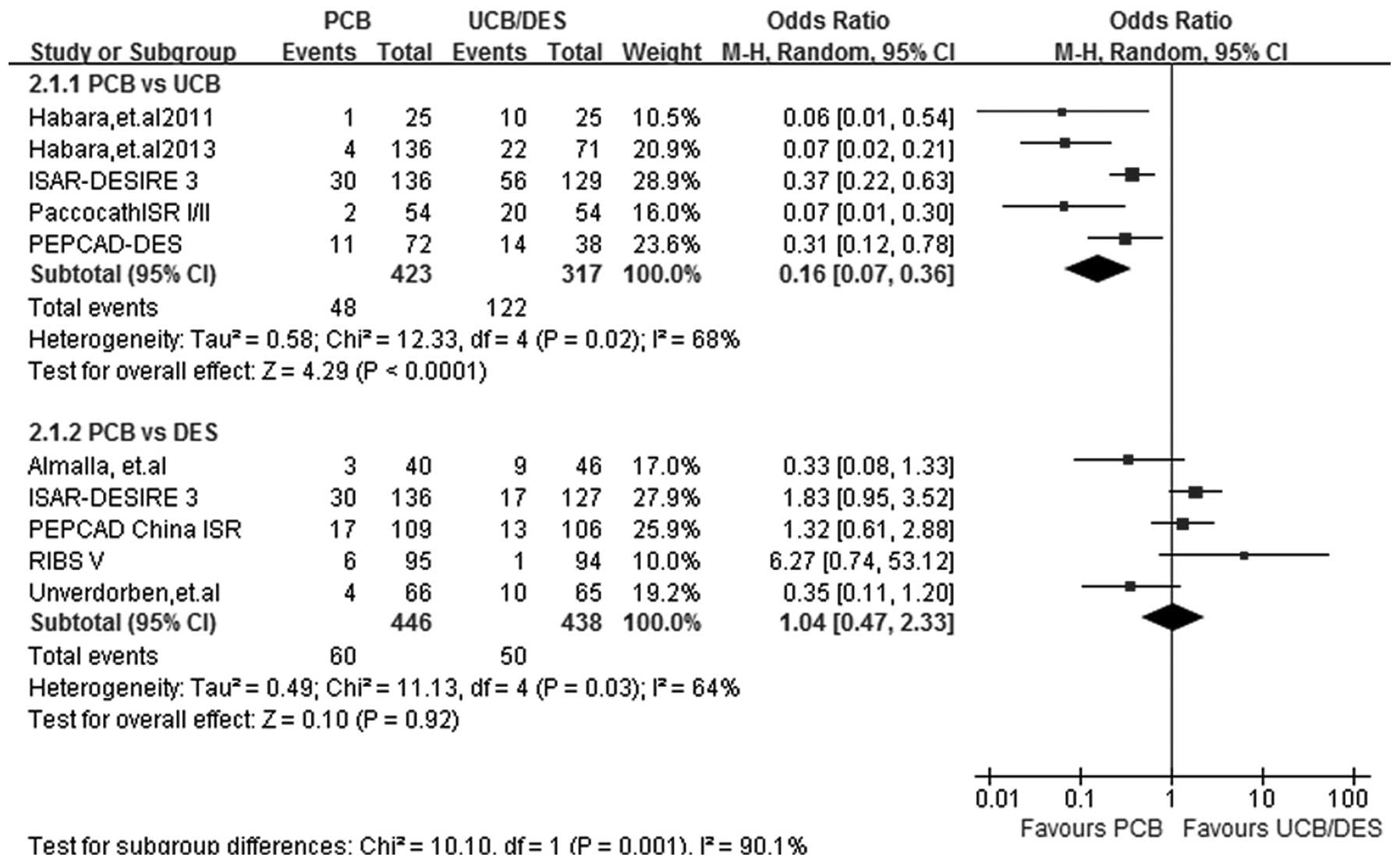
TLR
At a follow-up period of 6–12 months, TLR data were
acquired from all 9 studies. Statistical heterogeneity was noted in
the two subgroups (I2=68%; P=0.02 for PCB versus UCB and
I2=64%; P=0.03 for PCB versus DES) and therefore the
random-effects model was used. Five out of the 9 studies were
included in the PCB versus UCB subgroup analysis. The risk of TLR
was significantly lower in patients treated with PCBs (OR, 0.16;
95% CI, 0.07–0.36; P<0.00001). No significant difference was
found in the incidence rate of TLR between the PCB and DES groups
(OR, 1.04; 95% CI, 0.47–2.33; P=0.92) (Fig. 4).
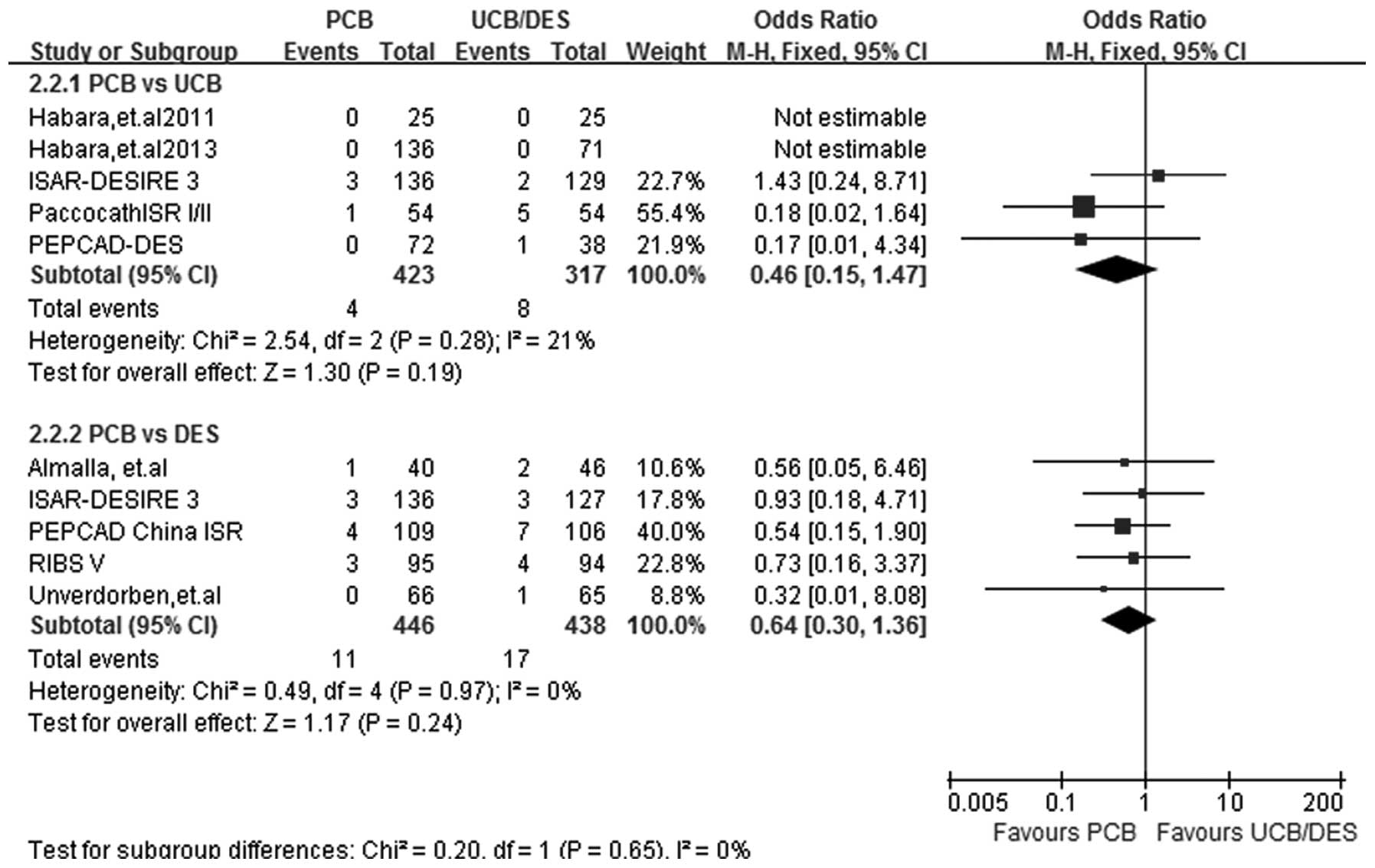
MI
At a follow-up period of 6–12 months, MI data were
acquired from all 9 studies. A fixed-effect model was selected and
the test for heterogeneity showed that the subgroup differences
were all non-significant (I2=21%; P=0.28 for PCB versus
UCB and I2=0%; P=0.97 for PCB versus DES). No
significant difference between the effect of PCBs and UCBs on the
incidence of MI was identified in the subgroup analysis (OR, 0.46;
95% CI, 0.15–1.47; P=0.19). Similarly, the incidence rate of MI was
comparable following PCB and DES implantation (OR, 0.64; 95% CI,
0.30–1.36; P=0.24) (Fig. 5).
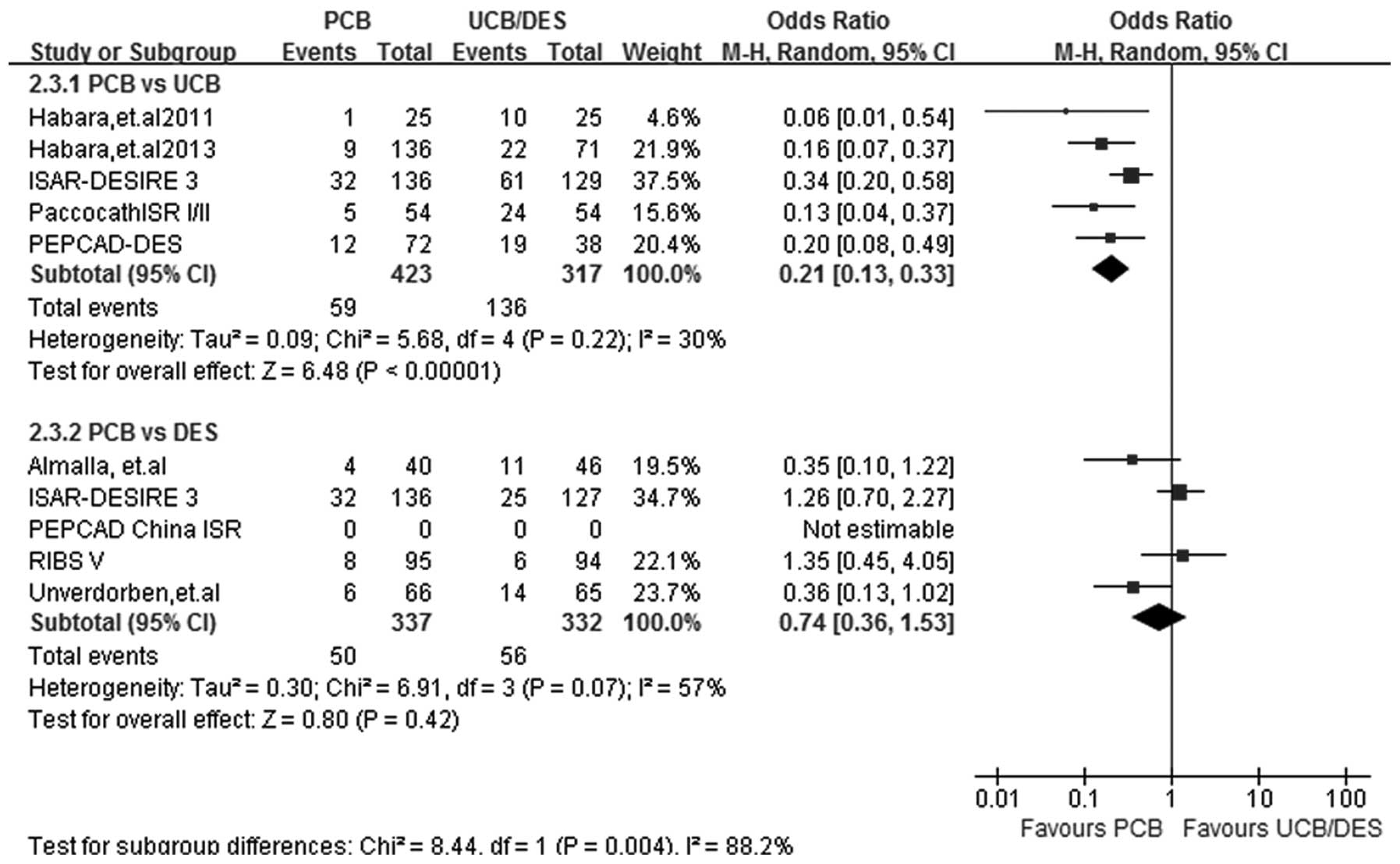
MACEs
At a follow-up period of 6–12 months, MACE results
were obtained from 8 out of the 9 studies. Heterogeneity was
observed in subgroup PCB versus DES (I2=57%; P=0.07) and
therefore a random-effects model was selected. Subgroup analysis
showed that PCB treatment had an advantage over UCB treatment in
reducing the incidence of MACEs (OR, 0.21; 95% CI, 0.13–0.33;
P<0.00001). Furthermore, PCB treatment appeared to be equally
efficacious to DES treatment in reducing the incidence rate of
MACEs (OR, 0.74; 95% CI, 0.36–1.53; P=0.42) (Fig. 6).
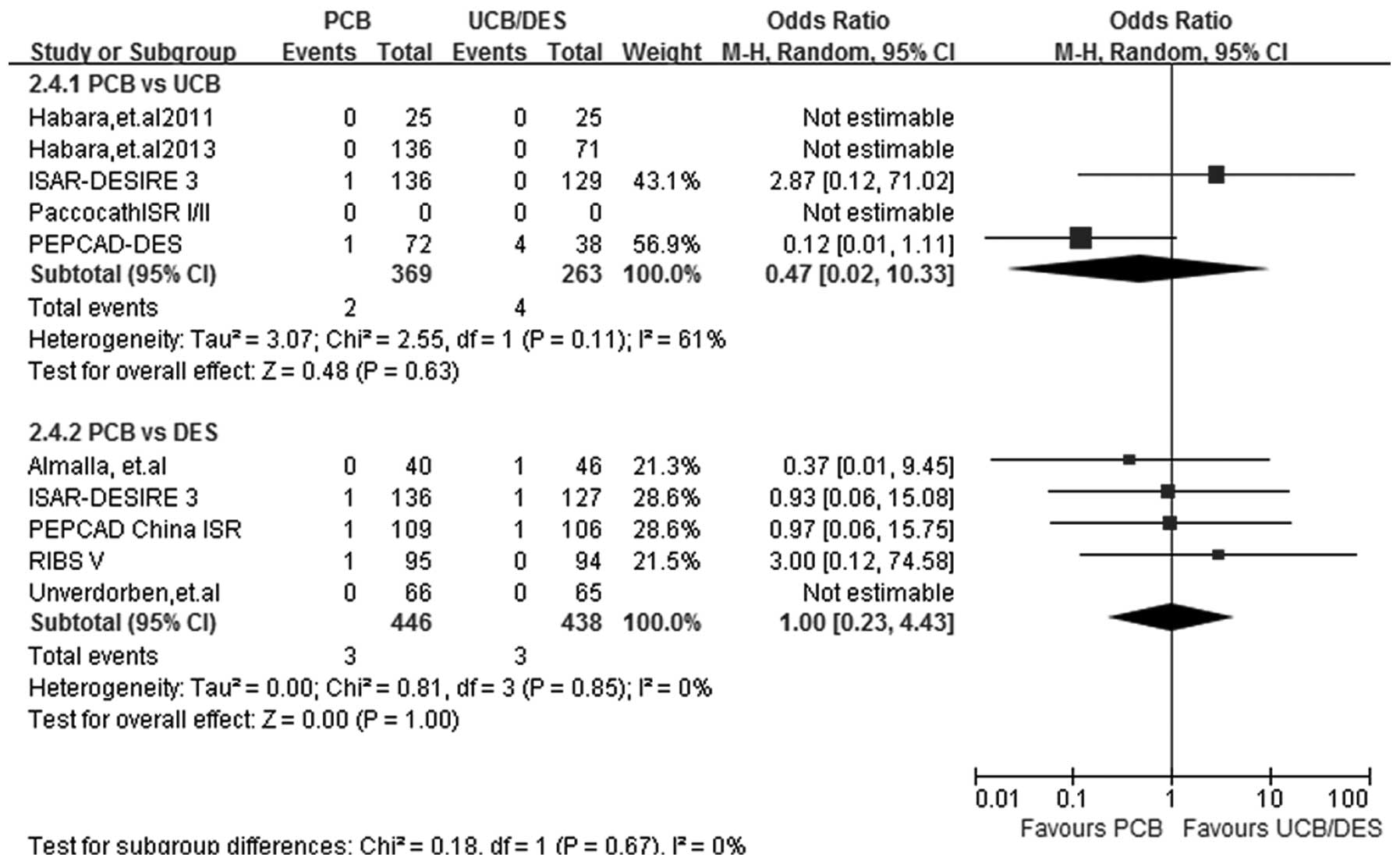
ST
At a follow-up period of 6–12 months, ST results
were obtained from 8 out of the 9 studies. Heterogeneity was
observed in the PCB versus UCB subgroup analysis
(I2=61%; P=0.11) and therefore a random-effects model
was used. The PCB versus UCB subgroup analysis showed that the two
treatments had a similar effect in reducing the incidence of ST
(OR, 0.47; 95% CI, 0.02–10.33; P=0.63), and similar results were
also found in the PCB versus DES subgroup analysis (OR, 1.00; 95%
CI, 0.23–4.43; P>0.99) (Fig.
7)
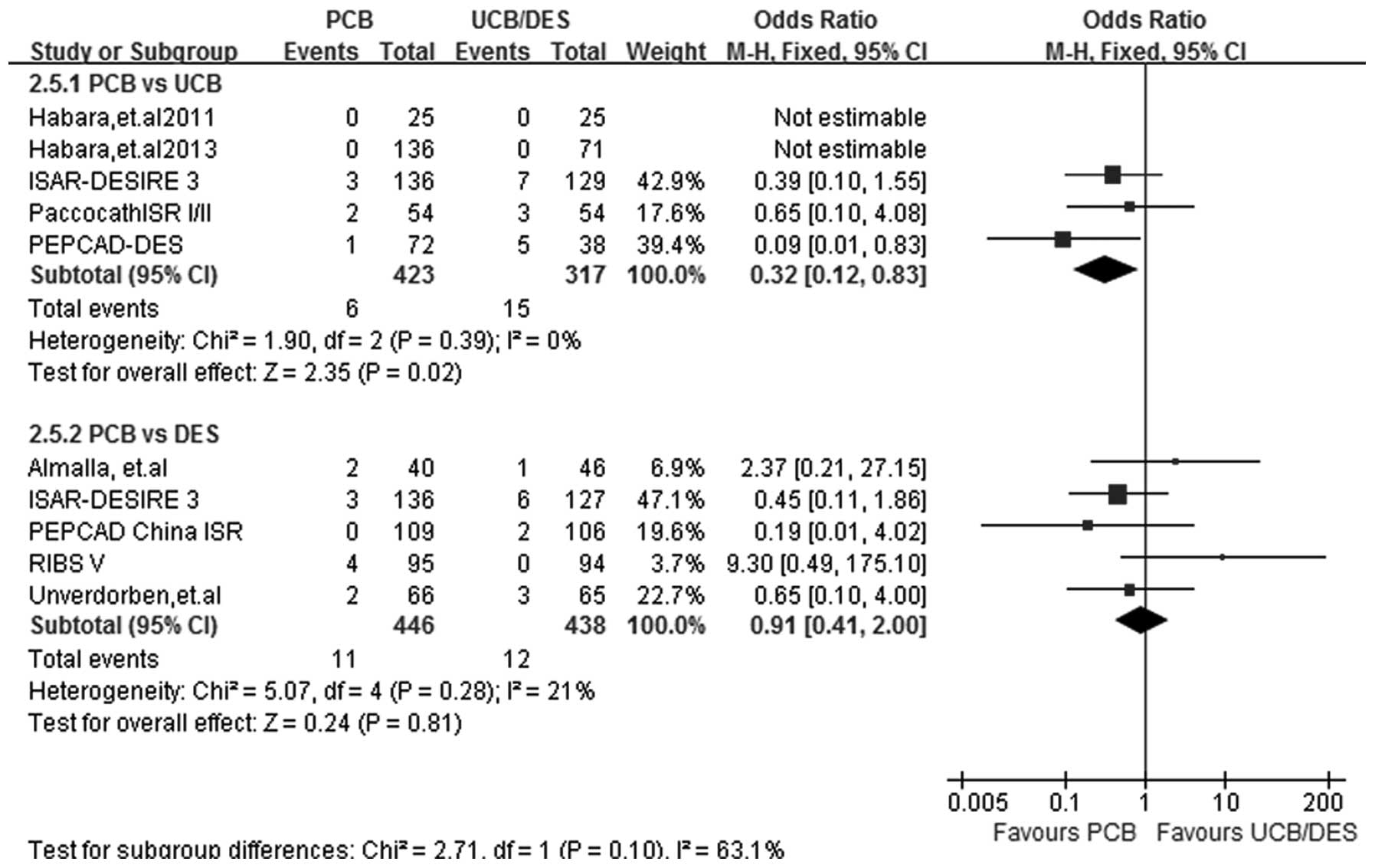
TM
At a follow-up period of 6–12 months, TM data were
acquired from all 9 studies. The incidence of TM was significantly
lower in the PCB-treated patients than in the UCB-treated patients
(OR, 0.32; 95% CI, 0.12–0.83; P=0.02). No significant difference
was detected in the incidence of TM between the PCB and DES groups
(OR, 0.91; 95% CI, 0.41–2.00; P=0.81). A fixed-effect model was
selected due to the absence of heterogeneity in the two subgroups
(I2=0%, P=0.39 for PCB versus UCB; I2=21%,
P=0.28 for PCB versus DES) (Fig.
8).
Sensitivity analysis
Since the test for heterogeneity showed significant
differences in the LLL, BR, TLR, MACE and TM results between the
PCB and control groups, subgroup analysis was performed according
to the different control groups, giving PCB versus UCB and PCB
versus DES subgroups. By removing one study at a time, a
sensitivity analysis was performed. This analysis did not detect
any effect of any single study on the results, with the exception
of the TM results in the PCB versus UCB subgroup analysis, which
indicated that these results were statistically reliable; however,
following the removal of the PEPCAD-DES study (10) from the PCB versus UCB subgroup
analysis, the significant difference was lost. We therefore
hypothesized that this may have been due to the small sample size
of the study and the heterogeneity across the studies included in
the subgroup.
Discussion
Even in the DES era, the treatment of ISR remains a
challenge for interventional cardiology. Currently, the placement
of a second (drug-eluting) stent appears to be the most effective
treatment method (19); however, it
is still controversial whether or not DES implantation could have a
positive outcome in high-risk patients with diabetes mellitus or
minor or diffuse vessel lesion. This controversy prompted the
development of PCB devices as a therapeutic alternative for the
constantly increasing number of patients suffering from ISR. The
first-in-human study to explore PCB use in patients with ISR was
the PACCOCATH-ISR trial (20), which
demonstrated the superiority of the angiographic and clinical
outcomes of PCB treatment as compared with those of UCB treatment
after a 6- to 12-month follow-up. Several small-scale studies were
subsequently conducted, designed as randomized or non-randomized,
single or multicenter, blind or unblinded, and compared with
different control groups. None of those studies, however, came to a
final conclusion regarding the real effect of PCB in the treatment
of ISR. A previous meta-analysis (21) showed that the results of PCB were
superior to those of a normal UCB in the treatment of coronary
artery disease, and another meta-analysis (22) had similar results when comparing PCB
use with traditional treatment methods, including UCBs and DESs.
Despite these results, however, both analyses had major
limitations: The former meta-analysis did not divide the patients
with ISR or de novo lesions into different subgroups and the
latter did not divide the control treatments into different
subgroups (UCB or DES).
In the present meta-analysis, the pooled results
comparing PCB implantation versus UCB and DES implantation in
patients with ISR were reported. The principal advantage of this
meta-analysis is that it divided 9 clinical studies (1488 patients,
1,608 lesions) into two subgroups (PCB versus UCB and PCB versus
DES). In the PCB versus UCB subgroup analysis, the results of the
6- to 9-month angiographic follow-up showed that PCB treatment had
a significant advantage over UCB treatment in reducing the
incidence rate of LLL and BR in ISR therapy. In addition, the
pooled estimates demonstrated a clear superiority of PCB in the 6-
to 12-month follow-up clinical results (TLR, MACEs and TM), and
PCBs proved more effective than UCBs in reducing the incidence of
MI (4/423 vs. 8/317) and ST (2/369 vs. 4/263); however the
difference was non-significant. We hypothesized that this lack of
significance may have been due to the small sample size and the
lack of ST data in the PACCOCATH-ISR I/II trial (11). In the other subgroup, 5 trials (4
RCTs and 1 NRCT) that compared PCBs and DESs in the treatment of
ISR were included. Notably, the pooled data showed that PCBs
appeared to be equally effective as DESs in the treatment of ISR,
based on angiographic and clinical findings, although moderate to
substantial statistical heterogeneity was observed across the
studies. We conjectured that the heterogeneity may have occurred
due to the fact that the 1 NRCT [Almalla, et al (18)], which was designed as an
observational trial and only reported the clinical outcomes at the
follow-up period of 12 months, was included in the study. The NRCT
was subsequently omitted for further analysis; however, no
significant differences were observed in the clinical outcomes
(TLR, MI, MACE, ST and TM) between the PCB and DES treatment
groups. This clinical evidence supported the hypothesis that PCBs
are comparable to DESs in the treatment of coronary ISR with regard
to efficacy and safety, obviating the necessity of implanting an
additional metal layer.
Previous studies have indicated that there are
significant differences between the occurrence of ISR following BMS
and DES implantion (23,24). These differences manifest themselves
in various ways, such as time of presentation, morphological
patterns, underlying mechanisms, tissue composition and response to
implantation (25). The angiographic
pattern of ISR is different following BMS implantation from that
subsequent to DES implantation. The angiographic pattern of DES-ISR
is predominantly focal and is associated with better prognosis,
whereas diffuse or proliferative patterns are rare. On the
contrary, a non-focal pattern frequently occurs following BMS
implantation and is associated with a high incidence of restenosis
(2). It is apparent that
head-to-head comparisons between the use of PCBs for the treatment
of BMS-ISR and DES-ISR are lacking. The SeQuentPlease World Wide
Registry (26) was a large-scale,
prospective registry study in which the TLR rate was significantly
lower in patients who underwent a PCB angiography for BMS-ISR
compared with that in patients who underwent a PCB angiography for
DES-ISR (3.8 vs. 9.6%, P<0.001). In 5 studies (10,12,13,15,18) in
the present meta-analysis, the restenosis type being treated was
DES-ISR, and a comparison between PCB and control (UCB or DES)
treatments was made. The clinical and angiographic results reported
for the PCB treatment were considered to be comparable to those for
the control treatments; therefore, we consider PCB to be an
attractive optimal treatment strategy for DES and BMS restenosis,
despite the effect of PCBs on DES restenosis being shown to be
relatively inferior to that on BMS restenosis (26).
Several limitations were noted in this
meta-analysis. Firstly, since one of the included studies was an
observational registry study, it was more likely to have been
biased in terms of selection, performance, attrition and detection.
Secondly, the definition of angiographic outcomes (LLL and BR)
differed in each study due to the angiographic measurements being
performed at the target lesion either over the entire length of the
study device (in-stent) or within 5 mm proximal and distal to the
target lesion (in-segment). Thirdly, the short follow-up period
(6–12 months) in all studies was inadequate for detecting late
adverse events, such as very late thrombosis. In addition, despite
the fact that the control groups were split into UCB and DES
subgroups and analyzed using a random-effects model or for
sensitivity to minimize heterogeneity, a potential heterogeneity in
the methods, patients, sample size and baseline characteristics of
the patients still existed; therefore caution should be taken when
interpreting the results.
In conclusion, this meta-analysis, based on the
currently available angiographic and clinical data from clinical
evidence, shows a significant superiority of PCB over UCB in the
treatment of ISR. Furthermore, the findings support the conclusion
that PCB implantation is at least as effective and tolerable as DES
implantation. These results cannot, however, replace the results of
those multicenter, prospective RCTs with a long follow-up duration
in critically evaluating the more reliable ‘real-world’ clinical
evidence found in PCB-treated patients with ISR.
References
|
1
|
Moses JW, Leon MB, Popma JJ, et al: SIRIUS
Investigators: Sirolimus-eluting stents versus standard stents in
patients with stenosis in a native coronary artery. N Engl J Med.
349:1315–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dangas GD, Claessen BE, Caixeta A, Sanidas
EA, Mintz GS and Mehran R: In-stent restenosis in the drug-eluting
stent era. J Am Coll Cardiol. 56:1897–1907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim YH, Lee BK, Park DW, et al: Comparison
with conventional therapies of repeated sirolimus-eluting stent
implantation for the treatment of drug-eluting coronary stent
restenosis. Am J Cardiol. 98:1451–1454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mishkel GJ, Moore AL, Markwell S, Shelton
MC and Shelton ME: Long-term outcomes after management of
restenosis or thrombosis of drug-eluting stents. J Am Coll Cardiol.
49:181–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone GW, Ellis SG, O'Shaughnessy CD, et
al: TAXUS V ISR Investigators: Paclitaxel-eluting stents vs
vascular brachytherapy for in-stent restenosis within bare-metal
stents: The TAXUS V ISR randomized trial. JAMA. 295:1253–1263.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatt DL: Intensifying platelet inhibition
- navigating between Scylla and Charybdis. N Engl J Med.
357:2078–2081. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loh JP and Waksman R: Paclitaxel
drug-coated balloons: A review of current status and emerging
applications in native coronary artery de novo lesions. JACC
Cardiovasc Interv. 5:1001–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gray WA and Granada JF: Drug-coated
balloons for the prevention of vascular restenosis. Circulation.
121:2672–2680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cutlip DE, Windecker S, Mehran R, et al:
Academic Research Consortium: Clinical end points in coronary stent
trials: A case for standardized definitions. Circulation.
115:2344–2351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rittger H, Brachmann J, Sinha AM, et al: A
randomized, multicenter, single-blinded trial comparing
paclitaxel-coated balloon angioplasty with plain balloon
angioplasty in drug-eluting stent restenosis: The PEPCAD-DES study.
J Am Coll Cardiol. 59:1377–1382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheller B, Hehrlein C, Bocksch W, et al:
Two year follow-up after treatment of coronary in-stent restenosis
with a paclitaxel-coated balloon catheter. Clin Res Cardiol.
97:773–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Habara S, Mitsudo K, Kadota K, et al:
Effectiveness of paclitaxel-eluting balloon catheter in patients
with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv.
4:149–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B, Gao R, Wang J, et al: PEPCAD China
ISR Trial Investigators: A prospective, multicenter, randomized
trial of paclitaxel-coated balloon versus paclitaxel-eluting stent
for the treatment of drug-eluting stent in-stent restenosis:
Results from the PEPCAD China ISR trial. JACC Cardiovasc Interv.
7:204–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Unverdorben M, Vallbracht C, Cremers B, et
al: Paclitaxel-coated balloon catheter versus paclitaxel-coated
stent for the treatment of coronary in-stent restenosis.
Circulation. 119:2986–2994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrne RA, Neumann FJ, Mehilli J, et al:
ISAR-DESIRE 3 investigators: Paclitaxel-eluting balloons,
paclitaxel-eluting stents, and balloon angioplasty in patients with
restenosis after implantation of a drug-eluting stent (ISAR-DESIRE
3): A randomised, open-label trial. Lancet. 381:461–467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Habara S, Iwabuchi M, Inoue N, et al: A
multicenter randomized comparison of paclitaxel-coated balloon
catheter with conventional balloon angioplasty in patients with
bare-metal stent restenosis and drug-eluting stent restenosis. Am
Heart J. 166:527–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alfonso F, Perez-Vizcayno MJ, Cardenas A,
et al: RIBS V Study Investigators, under the auspices of the
Working Group on Interventional Cardiology of the Spanish Society
of Cardiology: A randomized comparison of drug-eluting balloon
versus everolimus-eluting stent in patients with bare-metal
stent-in-stent restenosis: The RIBS V Clinical Trial (Restenosis
Intra-stent of Bare Metal Stents: Paclitaxel-eluting balloon vs
everolimus-eluting stent). J Am Coll Cardiol. 63:1378–1386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almalla M, Schroder J, Pross V, Marx N and
Hoffmann R: Paclitaxel-eluting balloon versus everolimus-eluting
stent for treatment of drug-eluting stent restenosis. Catheter
Cardiovasc Interv. 83:881–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kastrati A, Mehilli J, von Beckerath N, et
al: ISAR-DESIRE Study Investigators: Sirolimus-eluting stent or
paclitaxel-eluting stent vs balloon angioplasty for prevention of
recurrences in patients with coronary in-stent restenosis: A
randomized controlled trial. JAMA. 293:165–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheller B, Hehrlein C, Bocksch W, et al:
Treatment of coronary in-stent restenosis with a paclitaxel-coated
balloon catheter. N Engl J Med. 355:2113–2124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu CM, Kwong JS and Sanderson JE:
Drug-eluting balloons for coronary artery disease: A meta-analysis
of randomized controlled trials. Int J Cardiol. 168:197–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Navarese EP, Austin D, Gurbel PA, et al:
Drug-coated balloons in treatment of in-stent restenosis: A
meta-analysis of randomised controlled trials. Clin Res Cardiol.
102:279–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinberg DH, Gaglia MA Jr, Pinto Slottow
TL, et al: Outcome differences with the use of drug-eluting stents
for the treatment of in-stent restenosis of bare-metal stents
versus drug-eluting stents. Am J Cardiol. 103:491–495. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Byrne RA, Cassese S, Windisch T, et al:
Differential relative efficacy between drug-eluting stents in
patients with bare metal and drug-eluting stent restenosis;
evidence in support of drug resistance: Insights from the
ISAR-DESIRE and ISAR-DESIRE 2 trials. Euro Intervention. 9:797–802.
2013.PubMed/NCBI
|
|
25
|
Alfonso F: Treatment of drug-eluting stent
restenosis the new pilgrimage: Quo vadis? J Am Coll Cardiol.
55:2717–2720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wohrle J, Zadura M, Mobius-Winkler S, et
al: SeQuentPlease World Wide Registry: Clinical results of SeQuent
please paclitaxel-coated balloon angioplasty in a large-scale,
prospective registry study. J Am Coll Cardiol. 60:1733–1738.
2012.PubMed/NCBI
|