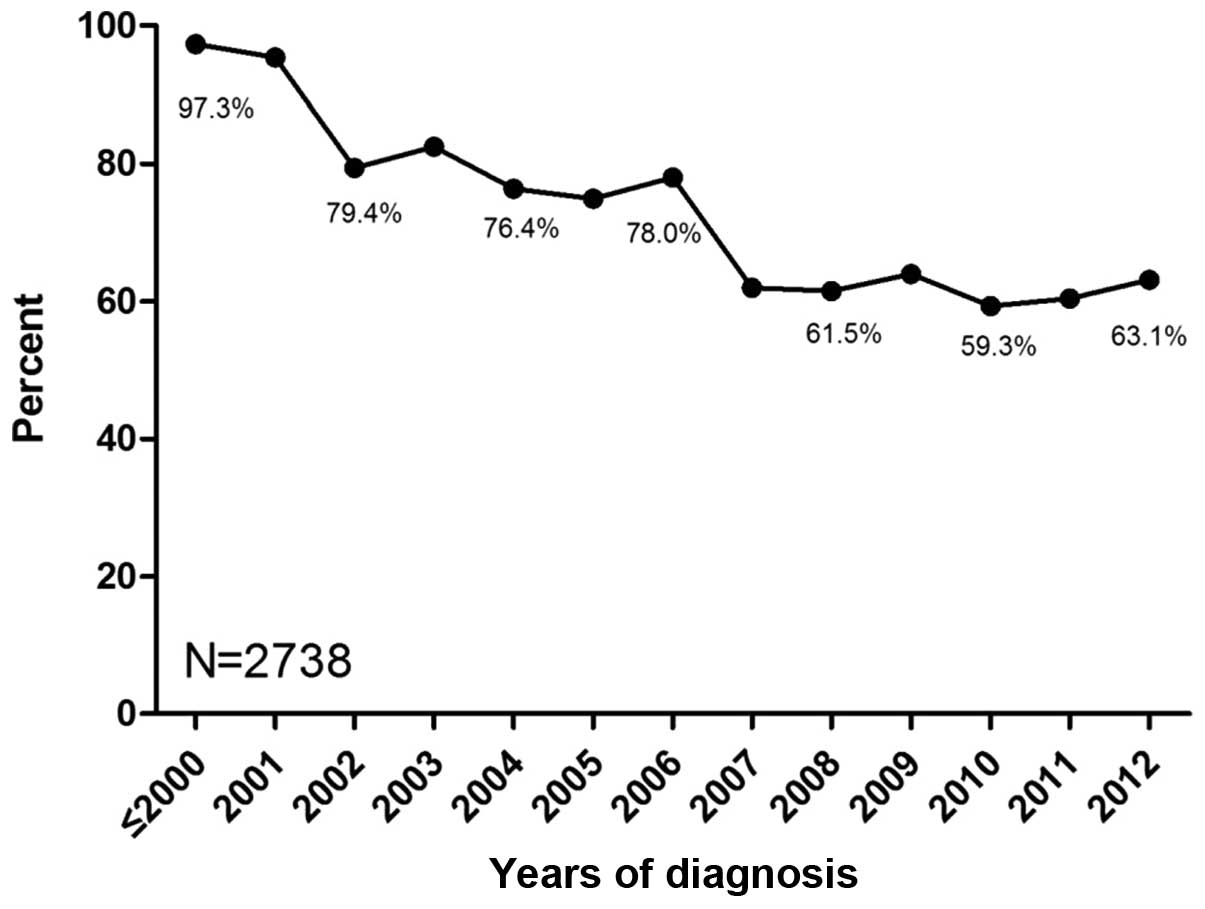
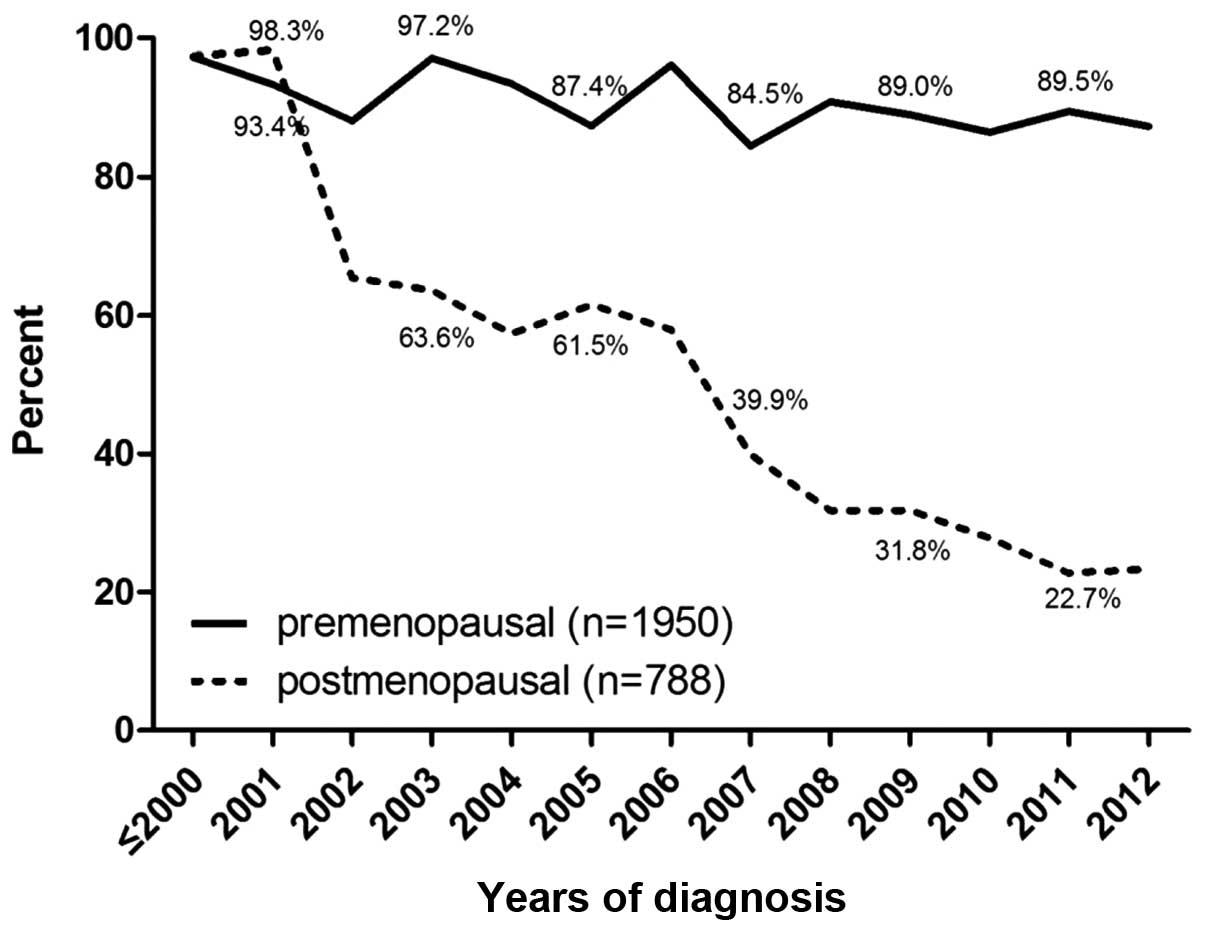
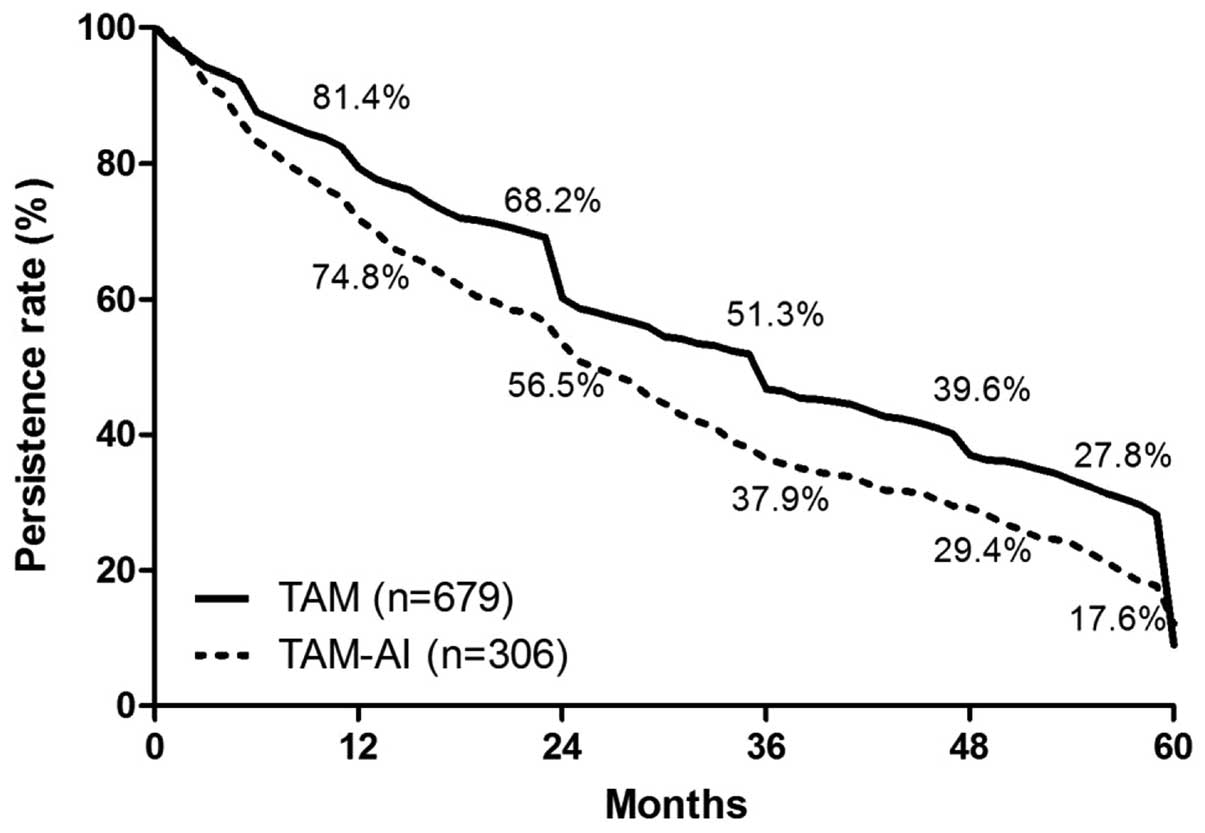
|
1
|
Althuis MD, Dozier JM, Anderson WF, Devesa
SS and Brinton LA: Global trends in breast cancer incidence and
mortality 1973–1997. Int J Epidemiol. 34:405–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000 The global picture. Eur J Cancer. 37:(Suppl
8). S4–S66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park WC and Jordan VC: Selective estrogen
receptor modulators (SERMS) and their roles in breast cancer
prevention. Trends Mol Med. 8:82–88. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen MH, Hirschfeld S, Flamm Honig S, et
al: Drug approval summaries: arsenic trioxide, tamoxifen citrate,
anastrazole, paclitaxel, bexarotene. Oncologist. 6:4–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher B, Costantino JP, Wickerham DL, et
al: Tamoxifen for prevention of breast cancer: report of the
National Surgical Adjuvant Breast and Bowel Project P-1 Study. J
Natl Cancer Inst. 90:1371–1388. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies C, Pan H, Godwin J, et al: Longer
Against Shorter (ATLAS) Collaborative Group: Long-term effects of
continuing adjuvant tamoxifen to 10 years versus stopping at 5
years after diagnosis of oestrogen receptor-positive breast cancer:
ATLAS, a randomised trial. Lancet. 381:805–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fisher B, Dignam J, Bryant J and Wolmark
N: Five versus more than five years of tamoxifen for lymph
node-negative breast cancer: updated findings from the National
Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J
Natl Cancer Inst. 93:684–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barron TI, Connolly R, Bennett K, Feely J
and Kennedy MJ: Early discontinuation of tamoxifen: a lesson for
oncologists. Cancer. 109:832–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wigertz A, Ahlgren J, Holmqvist M, et al:
Adherence and discontinuation of adjuvant hormonal therapy in
breast cancer patients: a population-based study. Breast Cancer Res
Treat. 133:367–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy CC, Bartholomew LK, Carpentier MY,
Bluethmann SM and Vernon SW: Adherence to adjuvant hormonal therapy
among breast cancer survivors in clinical practice: a systematic
review. Breast Cancer Res Treat. 134:459–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thorpe KE, Zwarenstein M, Oxman AD, et al:
A pragmatic-explanatory continuum indicator summary (PRECIS): a
tool to help trial designers. J Clin Epidemiol. 62:464–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Booth CM and Mackillop WJ: Translating new
medical therapies into societal benefit: the role of
population-based outcome studies. JAMA. 300:2177–2179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krumholz HM: Real-world imperative of
outcomes research. JAMA. 306:754–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaptchuk TJ: The double-blind, randomized,
placebo-controlled trial: gold standard or golden calf? J Clin
Epidemiol. 54:541–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hershman DL and Wright JD: Comparative
effectiveness research in oncology methodology: observational data.
J Clin Oncol. 30:4215–4222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trotter JP: Patient registries: a new gold
standard for ‘real world’ research. Ochsner J. 4:211–214.
2002.PubMed/NCBI
|
|
18
|
Parkin DM: The evolution of the
population-based cancer registry. Nat Rev Cancer. 6:603–612. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albain KS, Barlow WE, Ravdin PM, et al:
Breast Cancer Intergroup of North America: Adjuvant chemotherapy
and timing of tamoxifen in postmenopausal patients with
endocrine-responsive, node-positive breast cancer: a phase 3,
open-label, randomised controlled trial. Lancet. 374:2055–2063.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hershman DL, Shao T, Kushi LH, et al:
Early discontinuation and non-adherence to adjuvant hormonal
therapy are associated with increased mortality in women with
breast cancer. Breast Cancer Res Treat. 126:529–537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goss PE, Ingle JN, Martino S, et al: A
randomized trial of letrozole in postmenopausal women after five
years of tamoxifen therapy for early-stage breast cancer. N Engl J
Med. 349:1793–1802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coombes RC, Hall E, Gibson LJ, et al:
Intergroup Exemestane Study: A randomized trial of exemestane after
two to three years of tamoxifen therapy in postmenopausal women
with primary breast cancer. N Engl J Med. 350:1081–1092. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howell A, Cuzick J, Baum M, et al: ATAC
Trialists' Group: Results of the ATAC (Arimidex, Tamoxifen, Alone
or in Combination) trial after completion of 5 years' adjuvant
treatment for breast cancer. Lancet. 365:60–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baum M, Budzar A, Cuzick J, et al: ATAC
Trialists' Group: Anastrozole alone or in combination with
tamoxifen versus tamoxifen alone for adjuvant treatment of
postmenopausal women with early breast cancer: first results of the
ATAC randomised trial. Lancet. 359:2131–2139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldhirsch A, Wood WC, Gelber RD, et al:
Meeting highlights: updated international expert consensus on the
primary therapy of early breast cancer. J Clin Oncol. 21:3357–3365.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thürlimann B, Keshaviah A, Coates AS, et
al: Breast International Group (BIG) 1–98 Collaborative Group: A
comparison of letrozole and tamoxifen in postmenopausal women with
early breast cancer. N Engl J Med. 353:2747–2757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldhirsch A, Ingle JN, Gelber RD, et al:
Panel members: Thresholds for therapies: highlights of the St
Gallen International Expert Consensus on the primary therapy of
early breast cancer 2009. Ann Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forbes JF, Cuzick J, Buzdar A, et al:
Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists'
Group: Effect of anastrozole and tamoxifen as adjuvant treatment
for early-stage breast cancer: 100-month analysis of the ATAC
trial. Lancet Oncol. 9:45–53. 2008. View Article : Google Scholar : PubMed/NCBI
|