Introduction
Embolization with Gugliemi detachable coils (GDCs)
is considered to be the first-line treatment option for the
majority of intracranial aneurysms with small necks (1). However, endovascular treatment for
aneurysms of complex morphologies, wide necks or unfavorable
dome-to-neck ratios remains a challenge. To improve the efficacy
and durability of endovascular treatment for wide-neck aneurysms
(2), novel coil designs (including
3D coils) (3) and liquid embolic
agents (4) have been developed in
the past few years. The efficacy of bioactive coils is
controversial, and the development of self-expanding stents has
offered more options during the treatment of these aneurysms.
The Enterprise (EP; Codman & Shurtleff, Inc.,
Miami, FL, USA) is a self-expanding, closed-cell design stent with
flared ends in which each end has four radiopaque markers that
flare out when fully deployed. The EP stent can be retrieved into
the delivery catheter unless more than two-thirds of the entire
stent length has been deployed (5).
The Solitaire™ AB (ST; ev3 Neurovascular, Irvine, CA, USA) stent is
a laser-cut, self-expanding and fully retrievable split-design
nitinol device. The distinctive feature of this device is its full
retrievability until it is electrically detached from the push wire
(6).
During the last decade, these devices have been
widely used and are generally accepted as endovascular treatment
for intracranial aneurysms. Various studies have reported the
characteristics of EP and ST stents (5,7,8); however, to date, there is limited data
with regard to the direct comparative benefit of the two stents for
stent-assisted coiling (SAC). Consequently, in the present
single-center study, the feasibility, rate of procedure-related
complications and midterm angiographic follow-up outcomes of the EP
and ST stent deployments were analyzed and compared.
Materials and methods
Stent selection
Indications of the two used stents included mainly
complex unruptured or ruptured aneurysms, including fusiform, large
and/or giant, dissecting or wide-neck aneurysms. In addition, small
aneurysms, which may not be embolized by conventional coiling and
recurrences, were considered amenable to stent-assisted coiling. EP
stents were used for aneurysms arising from a parent vessel with a
diameter of 2.5–4 mm, while ST stents were recommended for
aneurysms arising from a parent vessel with a diameter of 2.5–5.5
mm. Wide-necked aneurysms were defined as having a neck of ≥4 mm or
a dome-to-neck ratio of <2 mm.
Patients
The study was approved by the Institutional Review
Board of the Zhujiang Hospital, Southern Medical University
(Guangzhou, China). Written informed consent was obtained from the
patients' families. In total, 81 patients with 90 aneurysms
underwent treatment between January 2010 and January 2012.
Initially, the aim was to use EP-assisted coiling in 43 aneurysms
(47.8%) and ST-assisted coiling in 47 aneurysms (52.2%); however,
EP-assisted coiling was not successful in four patients. These
patients subsequently underwent treatment with the ST stent and all
the aneurysms were successfully stented and coiled (EP, n=39,
43.3%; ST, n=51, 56.7%). The following information was recorded:
Patient characteristics, aneurysm demographics, technical and
procedure-related complications. Follow-up angiograms, typically
obtained at six and 18 months, were reviewed for data on stent
patency and aneurysm recurrence. Stent-related complications,
including delayed stent migration and in-stent stenosis, were also
recorded.
Antiplatelet regimen
All the subjects with unruptured or non-acute
ruptured aneurysms had been treated with dual antiplatelet therapy
(75 mg clopidogrel and 100 mg aspirin per day) for five days prior
to SAC. In the acute phase of the ruptured aneurysms, heparin was
injected at the beginning and was maintained for 48 h. Four
clopidogrel pills (300 mg) were crushed and injected into the
nasogastric tube 2 h prior to surgery. The adequacy of the systemic
anticoagulation therapy was monitored by frequent measurements of
the activated clotting time (ACT). A baseline ACT was obtained
prior to the bolus infusion of 5,000 IU heparin, and hourly
thereafter. Clopidogrel (75 mg/day) was administered for six months
post-surgery and daily administration of 100 mg aspirin was
required for one year.
Endovascular procedure
A biplane flat panel digital subtraction unit
(Neurostar or Axiom Artis; Siemens Healthcare, Erlangen, Germany)
was used to performed the endovascular procedure. After a 6-French
sheath (Terumo, Fujinomiya, Japan) was successfully inserted into
the right common femoral artery, the 6-F guide catheter (Envoy;
Codman Neurovascular, Miami Lakes, FL, USA) was inserted into the
parent vessel. Over a 0.014 inch Transend or Synchro microguidewire
(Boston Scientific, Fremont, CA, USA), a microcatheter was
delivered into the normal distal artery beyond the aneurysm by 1 to
2 cm. The aneurysm was then embolized once the coiling catheter was
navigated within the aneurysm sac. Following the coiling procedure,
the stent was pushed through the microcatheter and aligned directly
across the neck of the aneurysm. When the appropriate position was
achieved, the microcatheter was gently pulled back to unsheathe the
stent. The stent would not be fully deployed until the distal
markers were completely open. Typically, after full deployment, the
position was confirmed by routine diagnostic cerebral angiography.
Two stents could be inserted under a necessary position, while for
an imperative reposition, repeating the aforementioned processes
was required. Microplex coils (MicroVention, Inc., Aliso Viejo, CA,
USA), GDCs (Boston Scientific) or Axium coils (ev3 Neurovascular)
were delivered via the second catheter into the aneurysm, as
reported previously (9). Following
an ideal coiling embolization, the stent was detached from the push
wire subsequent to routine diagnostic cerebral angiography using a
high-resolution biplane angiographic unit.
Packing density
Packing density, also known as the volume
embolization ratio, was calculated as the ratio of the volume of
the deployed coils to the aneurysm volume. The coil volume was
calculated by summing the individual coil volumes, as indicated by
the manufacturers. Aneurysm dimensions were measured by 3D images
derived from rotational angiography. Subsequently, the aneurysm
volume and packing density were calculated using Angiocalc software
(available at http://www.angiocalc.com).
Follow-up examination
Angiographic follow-up examinations were performed
with conventional angiography at six and 12 months post-surgery,
and every year annually thereafter. For follow-up imaging, the
patients underwent conventional digital subtraction angiography
(DSA) or magnetic resonance angiography, or both. DSA was used for
sole analysis whenever available. All the treated aneurysms were
graded independently by two interventional neuroradiologists, using
several views for each treated aneurysm, including 3D angiography.
The angiographic results were classified according to the
Raymond-Roy Occlusion Classification (10): Complete occlusion (class 1), neck
remnant (class 2) and residual aneurysm (class 3).
Changes in the angiographic outcome were classified
as follows: Stable (no change in coil configuration, obliteration
grade or contrast filling), improved (progressive occlusion or
involution of the neck remnant or contrast filling in the aneurysm)
and recanalized (aneurysm recurrence evident due to neck growth,
coil compaction, coil extrusion by aneurysm degradation or new sac
formation). Additionally, newly visualized or increased contrast
filling inside an aneurysm was regarded as recanalization.
Statistical analysis
Data were analyzed using the SPSS 13.0 statistical
package (SPSS, Inc., Chicago, IL, USA), and are presented as the
mean ± standard deviation. Statistical analyses were performed
using the Student's t-test, Fisher's exact test, χ2 test
and analysis of variance, as appropriate, where P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the treated
aneurysms
In total, 81 patients with 90 aneurysms underwent
treatment using EP (n=43) or ST (n=47) SAC at the Department of
Neurosurgery, Zhujiang Hospital, between January 2010 and January
2012. The characteristics of all the subjects are shown in Tables I and II. No statistically significant
differences were observed with regard to age, gender, aneurysm size
and location between the two groups. All the aneurysms (EP, n=39;
ST, n=51) were successfully stented and coiled. Of these aneurysms,
74 (82.2%) were located in the anterior circulation, while 16
(17.8%) were located in the posterior circulation (Table III).
 | Table I.Baseline demographics of the patients
with aneurysms treated by stent-assisted coiling using the EP or ST
stent. |
Table I.
Baseline demographics of the patients
with aneurysms treated by stent-assisted coiling using the EP or ST
stent.
| Variable | Total | EP | ST | P-value |
|---|
| Age,
yearsa | 53±13.1 | 52±14.6 | 53±11.3 | 0.712 |
| Female gender, n
(%) | 45 (55.6) | 19 (52.8) | 26 (57.8) | 0.661 |
| Aneurysm size,
mma | 9.5±6.0 | 9.3±6.3 | 9.7±5.3 | 0.962 |
| Ruptured aneurysms, n
(%) | 44 (48.9) | 20 (51.3) | 24 (47.1) | 0.832 |
| Packing
densitya | 34.3±14.4 | 33.9±16.3 | 35.6±14.5 | 0.338 |
 | Table II.Patient presentations. |
Table II.
Patient presentations.
| Symptom | Total (n=90) | EP (n=39) | ST (n=51) | P-value |
|---|
| Subarachnoid
hemorrhage, n (%) | 40 (44.4) | 18 (46.2) | 22 (43.1) | 0.280 |
| Cranial nerve palsy,
n (%) | 14 (15.6) | 5 (12.7) | 9 (17.6) | 1.000 |
| Ischemic attack, n
(%) | 7 (7.8) | 4 (10.3) | 3 (5.9) | 0.698 |
| Headache, n (%) | 12 (13.3) | 4 (10.3) | 8 (15.8) | 0.533 |
| Asymptomatic, n
(%) | 17 (18.9) | 8 (20.5) | 9 (17.6) | 0.243 |
 | Table III.Stented aneurysm locations. |
Table III.
Stented aneurysm locations.
| Location | Total (n=90) | EP (n=39) | ST (n=51) | P-value |
|---|
| Anterior
circulation, n (%) | 74 (82.2) | 32 (82.1) | 42 (82.4) | 1.000 |
| ICA
cavernous | 10 (11.1) | 4 (10.3) | 6 (11.8) | 1.000 |
| ICA
ophthalmic | 10 (11.1) | 3 (7.7) | 7 (13.7) | 0.505 |
| ICA
supraclinoid | 18 (20.0) | 7 (17.9) | 11 (21.6) | 0.793 |
|
MCA | 5 (5.6) | 2 (5.1) | 3 (5.9) | 1.000 |
|
AcomA | 4 (4.4) | 2 (5.1) | 2 (3.9) | 1.000 |
|
PcomA | 27 (30.0) | 14 (35.9) | 13 (25.5) | 0.355 |
| Posterior
circulation, n (%) | 16 (17.8) | 7 (17.9) | 9 (17.6) | 1.000 |
| Basilar
tip | 7 (7.8) | 2 (5.1) | 5 (7.8) | 0.694 |
|
PCA | 2 (2.2) | 2 (5.1) | 0 (0) | 0.185 |
|
Vertebral artery | 6 (6.7) | 2 (5.1) | 4 (5.9) | 0.694 |
|
PICA | 1 (1.1) | 1 (2.6) | 0 (0) | 0.433 |
Procedural feasibility of the
stents
Inability to position the delivery system occurred
with greater frequency in the EP group when compared with the ST
group (P=0.022). In addition, the rate of technical complications
in the EP group was significantly higher compared with that in the
ST group (14.0 and 0%, respectively). Thus, the ST stent was more
feasible to deploy (100%) compared with the EP stent (90.7%;
Table IV).
 | Table IV.Procedural feasibility and
procedure-related morbidity in patients treated with stent-assisted
coiling. |
Table IV.
Procedural feasibility and
procedure-related morbidity in patients treated with stent-assisted
coiling.
| Complications | Total | EP | ST | P-value |
|---|
| Technical, n
(%) | 6/90 (6.7) | 6/43 (14.0) | 0/47 (0) | 0.022a |
| Failed to
deploy | 4 (4.4) | 4 (9.3) | 0 (0) | 0.048a |
| Stent
migration | 2 (2.2) | 2 (4.7) | 0 (0) | 0.478 |
| Procedure-related,
n (%) | 6/90 (6.7) | 1/39 (2.6) | 5/51 (9.8) | 0.228 |
| Thromboembolic
events | 2 (2.2) | 1 (2.6) | 1 (2.0) | 1.000 |
| Intraprocedure
rupture | 1 (1.1) | 0 (0) | 1 (2.0) | 1.000 |
| Post-procedure
rupture | 3 (3.3) | 0 (0) | 3 (5.9) | 0.255 |
Of the 43 aneurysms intended to be treated by SAC
using EP stents, 39 (90.7%) were successfully stented and coiled.
Two stent migrations occurred in the EP group following final
deployment; in both patients this was managed by implantation of a
second ST stent. In the remaining four cases where the EP stent was
not navigable, the ST stent was successfully navigated without
complications during subsequent coiling. In one of the four
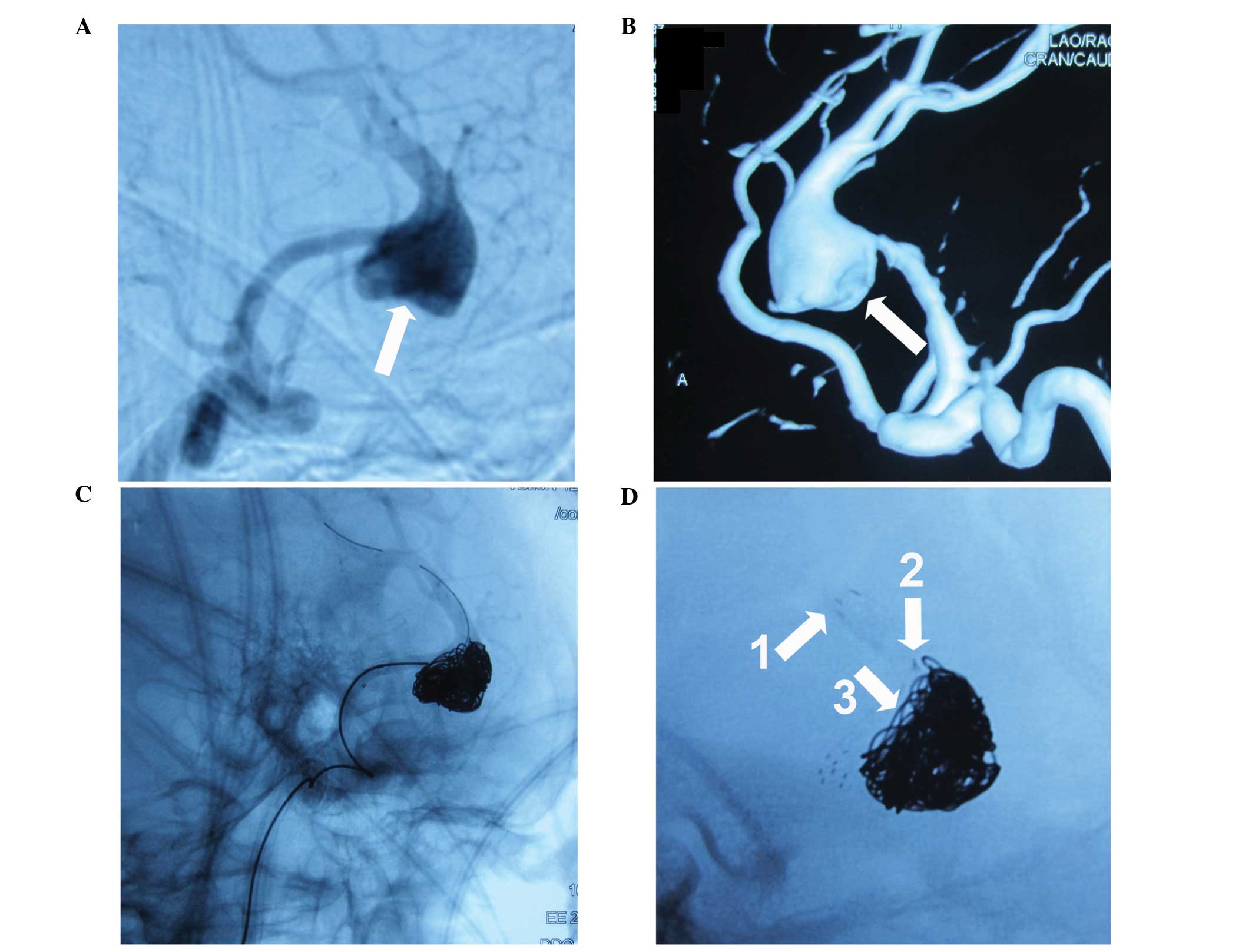
patients, the EP stent was unable to be expanded as desired, and
the body of the stent experienced deformation towards the aneurysm
sac subsequent to the stent being detached from the push wire
(Fig. 1).
Of the 47 aneurysms intended to be treated with the
ST stent, all were successfully deployed with subsequent coiling.
The total success rate of ST-assisted coiling was 100%. However, in
two patients, the stent required retrieval and repositioning
subsequent to the initial full deployment. No patients experienced
stent migration following the final placement in the ST group.
Procedure-related morbidity and
mortality rates
No statistically significant differences were
observed in the procedure-related complication rates (P=0.228) and
procedure-related mortality rates (P=1.000) between the EP and ST
groups (Table IV). In the EP group,
there was one case of a procedure-related complication
(thromboembolic event), which occurred in the cavernous segment of
the internal carotid artery and resulted in lower limb fatigue
thereafter. In response, thrombolytic therapy was administered
immediately following identification intraoperatively with
urokinase. However, the patient exhibited little neurological
improvement after six weeks of hospitalization.
In the ST group, five patients (9.8%) had aneurysms
that were associated with procedure-related complications. The
events included one thromboembolic event, one intraprocedure
rupture, which was possibly caused by mechanical irritations during
stent deployment, and three hemorrhages following SAC within one
month, which were possibly associated with antiplatelet therapy and
high blood pressure. Of these five patients, the patient who
suffered the intraprocedure rupture succumbed, two patients with
early hemorrhage events succumbed following surgery for
non-endovascular events, while the other two patients (one
thromboembolic event and one hemorrhage) survived.
Immediate angiographic results
Immediate angiographic results on the occlusion
grade following the SAC were analyzed and the results are
summarized in Table V. Complete
occlusion was obtained in 19/39 (48.7%) of the EP group and 26/51
(51.0%) of the ST group. Neck remnants were present in 13/39
(33.3%) of the EP group and 15/51 (29.4%) of the ST group. In
addition, a residual aneurysm was present in 7/39 (17.9%) of the EP
group and 10/51 (19.6%) of the ST group. No statistically
significant differences were identified between the two groups
(P>0.05). Furthermore, the two groups achieved a high packing
density, and no statistically significant difference was observed
between the EP and ST groups (P=0.338).
 | Table V.Immediate angiographic results. |
Table V.
Immediate angiographic results.
| Occlusion
gradea | Total (n=90) | EP (n=39) | ST (n=51) | P-value |
|---|
| Class 1, n (%) | 45 (50.0) | 19 (48.7) | 26 (51.0) | 1.000 |
| Class 2, n (%) | 28 (31.1) | 13 (33.3) | 15 (29.4) | 0.819 |
| Class 3, n (%) | 17 (18.9) | 7 (17.9) | 10 (19.6) | 1.000 |
Follow-up angiographic results
At the most recent angiographic follow-up
post-embolization, 29/39 (74.4%) of the EP group and 36/51 (70.6%)
patients in the ST group underwent cerebral angiography. The mean
follow-up times in the EP and ST groups were 15.9±11.4 and 14.0±6.8
months, respectively. Changes in the angiographic outcomes in the
EP group were as follows: Stable in 16/29 patients (55.2%),
progressive occlusion in 11/29 patients (37.9%) and recurrence in
2/29 patients (6.9%). Changes in the angiographic outcomes in the
ST group were as follows: Stable in 21/36 patients (58.3%),
progressive occlusion in 13/36 patients (36.1%) and recurrence in
2/36 patients (5.6%). In total, 24/65 (36.9%) aneurysms that were
followed-up underwent progressive occlusion (Table VI). In the four cases that
demonstrated an aneurysm sac, recanalization was observed without
further treatment since the recanalization was minor. Each group
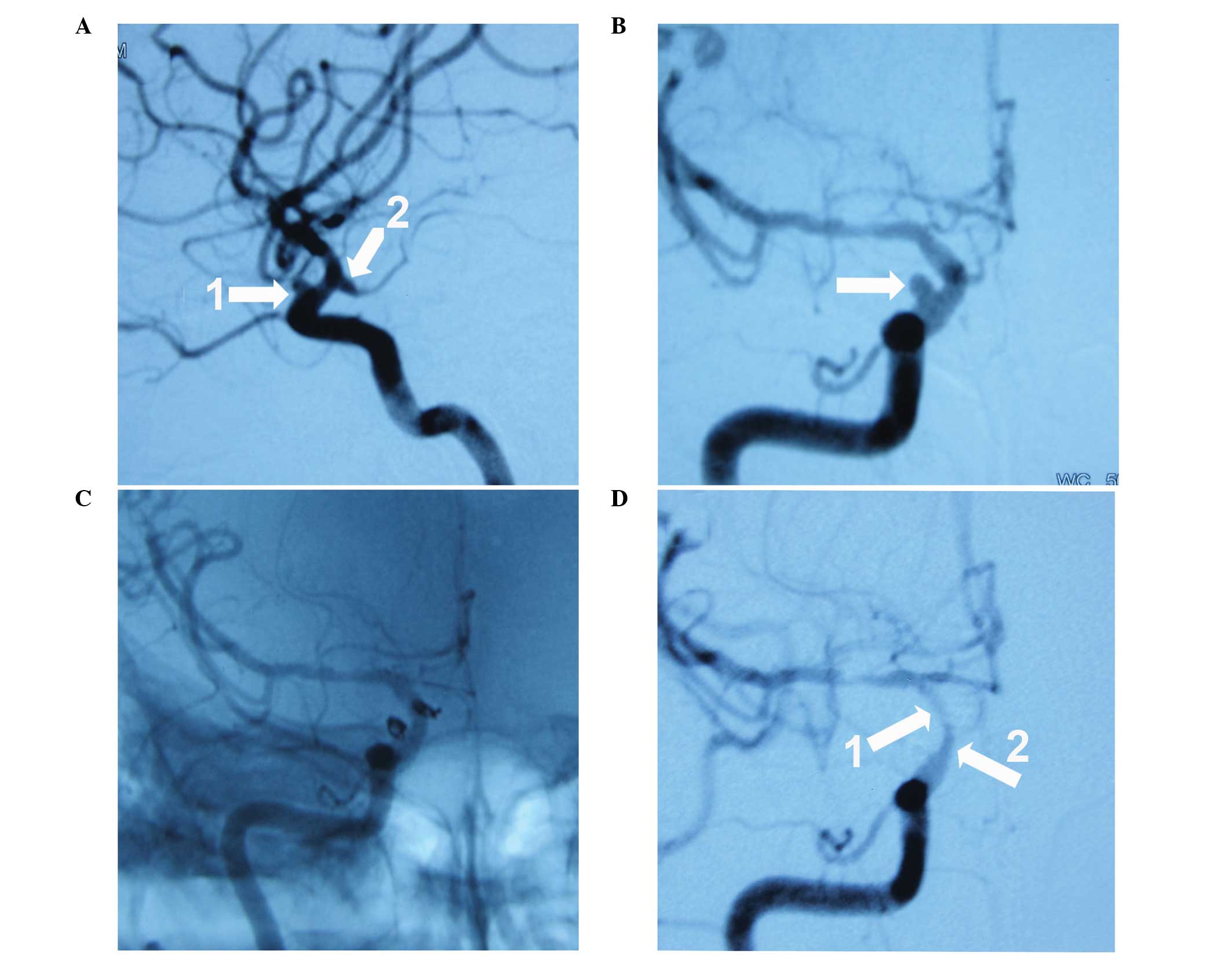
had one patient that presented with an in-stent stenosis at the
follow-up (EP, Fig. 2; ST, Fig. 3). The conditions of the two patients
were observed during the first 12 months of the follow-up period
and were asymptomatic; thus, further intervention was not
required.
 | Table VI.Follow-up angiographic results. |
Table VI.
Follow-up angiographic results.
| Parameter | Total (n=65) | EP (n=29) | ST (n=36) | P-value |
|---|
| Mean follow-up,
months | 15.1±9.8 | 15.9±11.4 | 14.0±6.8 | 0.418 |
| Stable, n (%) | 37 (56.9) | 16 (55.2) | 21 (58.3) | 0.807 |
| Progressive
occlusion, n (%) | 24 (36.9) | 11 (37.9) | 13 (36.1) | 1.000 |
| Recurrence, n
(%) | 4 (6.2) | 2 (6.9) | 2 (5.6) | 1.000 |
| In-stent stenosis,
n (%) | 2 (3.1) | 1 (3.4) | 1 (2.8) | 1.000 |
Discussion
Previous studies have analyzed the procedural
feasibility, initial grade of occlusion and complication rates
associated with each stent individually. A wide range of results
have been reported for each stent with regard to the stenting
success rates, with a range of 93.9–100% for the EP stent (11,12), and
93.3–100% for the ST stent (13,14).
However, there are limited data directly comparing these two
stents. In the present study, success rates of 90.7 and 100% were
achieved in the EP and ST groups, respectively. Notably, in the
four cases where EP placement had failed, the ST stent was
successfully deployed without complications during subsequent
coiling. The results indicated that the ST stent is extremely
flexible and technically easy to deploy, and can be easily and
safely manoeuvred through severe tortuous vessels.
Certain studies have reported their initial
experiences of stent migration in SAC using EP and ST stents
(8,15). Heller and Malek (16) hypothesized that the possible reason
was the lack of complete stent apposition to the wall, which may
contribute to this phenomenon by decreasing the surface in contact
with the vessel wall or increasing the exposure of stent elements.
In the present study, two stent migrations occurred in the EP group
and required a second stent, while no stent migration was observed
in the ST group. This may be associated with the following reasons.
Firstly, the ST stent is laser-cut from a nitinol plate into a
folded honeycomb pattern that allows the stent to have a higher
apposition to the wall of the vessel in large or small vessels.
Secondly, the EP stent has two different diameters along its
length, and the flared ends have a 4.5-mm diameter to anchor within
the parent artery, whereas the stent itself has a 4-mm diameter. In
small vessels, the EP stent can anchor tightly with small diameters
within the vessels by its flared ends without migration. However,
in larger vessels, a decreased contact area and higher central flow
velocities may induce a possible stent migration, which may explain
the relative increase in delayed migration of the EP stent.
A number of studies have reported that SAC
procedures are associated with higher rates of procedure-related
complications compared with coiling procedures that are performed
without the use of a stent (7,17–20).
However, the observations of the present study indicated that there
were no statistically significant differences in the overall
procedure-related complication rate and procedure-related mortality
rate between the EP and ST groups. This phenomenon may be explained
by the relatively unfavorable anatomical access, the subarachnoid
hemorrhage-related hypercoagulable state, vasospasm and the long
duration of the procedure. Furthermore, there is also a potential
selection bias in selecting the ST stent versus the EP stent for a
particular aneurysm.
Although the two groups achieved a high packing
density and complete occlusion, there was no statistically
significant difference in the packing density or occlusion grade
between the two stents in the present study. The observations
regarding the packing density and immediate aneurysm occlusion
grade in the present study were similar to results of previous
studies with a larger series of aneurysms treated with stent
assistance (7,8,11). The
closed-cell design of these two stents is hypothesized to play a
role in these two aspects.
The use of a stent may contribute to the progression
of thrombosis of aneurysms. Izar et al (19) reported 14 months of angiographic
follow-up data in 61 aneurysms treated with SAC embolization. The
study demonstrated an improvement in progressive occlusion in 36%
of the followed-up aneurysms. In a series of 104 aneurysms treated
with ST stents, Clajus et al (8) reported that 39.2% of the followed-up
aneurysms exhibited an improvement in progressive occlusion
following 13.6 months of angiographic follow-up.
The mechanisms underlying progressive occlusion and
the low recurrence rate of aneurysms treated with SAC may be due to
the technical and physiological properties of the two stents.
Primarily, stents provide a mechanical scaffold for coils within an
aneurysm, particularly in wide-necked or giant aneurysms, allowing
for increased packing density, improved neck coverage and the
prevention of coil protrusion into the parent artery (21). Furthermore, the stent has
flow-diverting properties, resulting in the modifications of the
intra-aneurysmal hemodynamics. Subsequently, the flow within the
aneurysm becomes disordered, which may lead to spontaneous and
delayed aneurysm thrombosis, reduced coil compaction in the region
of the inflow zone and a decrease in the wall shear stress and
subsequent growth of the aneurysm that remains untreated (22,23).
Finally, the stent may provide a structural basis for neointimal
proliferation at the aneurysm neck, resulting in permanent
separation of the aneurysm from the parent vessel lumen (24).
In the present study, the in-stent stenosis rate was
relatively low, with only one patient from each group presenting
with a stenosis during the follow-up period. The two patients were
asymptomatic; thus, no further intervention was required. Certain
studies have reported that the in-stent stenosis rates remain
relatively low following EP and ST SAC, ranging between 6.9 and 22%
(25,26). The deployment of a stent inevitably
causes endothelial injury over the treated vascular segment. The
proliferation and activation of regional smooth muscle cells occur,
resulting in stenosis within the stent (27). The degree of neointimal hyperplasia
and in-stent stenosis following SAC may be associated with the
severity of endothelial injury during the stent deployment, as well
as further manipulations affecting the stent stability during the
initial procedure. These results indicate that the two devices can
be well-positioned at the aneurysm neck without inducing major
endothelial injuries.
On the basis of previous observations and
experiences, the EP stent was preferentially selected when the
diameter of the parent artery was <4 mm. In particular, the EP
stent was selected for small arteries, including the A2 segment, M2
segment and posterior inferior cerebellar artery, where the ST
stent was relied on as an easy-to-deliver backup for SAC. By
contrast, the ST stent was preferable when the diameter of the
parent artery was >4 mm.
There are a number of limitations that require
addressing with regard to the present study. Firstly, the study was
a retrospective analysis; thus, was subject to the inherent biases
of the study design. Secondly, the patients were recruited from a
single institution (patient-selection bias), and the sample size
involved in the present study was relatively small, therefore
further studies are required. Therefore, definitive conclusions are
unable to be drawn.
In conclusion, the results of the present study
demonstrated that the two stents are relatively safe and effective
for the treatment of intracranial aneurysms. The two stents
achieved high packing densities, complete occlusion, high
progressive occlusion and low recurrence rates. However, there were
more procedure-related complications in the ST group when compared
with the EP group. Therefore, due to the higher stenting success
rate of the ST stent, this device was demonstrated to be more
flexible and feasible compared with the EP stent. However, further
studies are required, particularly with a longer follow-up period
and a larger study size population.
References
|
1
|
Lubicz B, Balériaux D, Lefranc F, et al:
Endovascular treatment of intracranial aneurysms as the first
therapeutic option. J Neuroradiol. 34:250–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moret J, Cognard C, Weill A, Castaings L
and Rey A: Reconstruction technic in the treatment of wide-neck
intracranial aneurysms. Long-term angiographic and clinical
results. Apropos of 56 cases. J Neuroradiol. 24:30–44. 1997.(In
French). PubMed/NCBI
|
|
3
|
Cloft HJ, Joseph GJ, Tong FC, Goldstein JH
and Dion JE: Use of three-dimensional Guglielmi detachable coils in
the treatment of wide-necked cerebral aneurysms. AJNR Am J
Neuroradiol. 21:1312–1314. 2000.PubMed/NCBI
|
|
4
|
Molyneux AJ, Cekirge S, Saatci I and Gál
G: Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial:
results of a prospective observational study in 20 European
centers. AJNR Am J Neuroradiol. 25:39–51. 2004.PubMed/NCBI
|
|
5
|
Mocco J, Snyder KV, Albuquerque FC, et al:
Treatment of intracranial aneurysms with the Enterprise stent: a
multicenter registry. J Neurosurg. 110:35–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liebig T, Henkes H, Reinartz J,
Miloslavski E and Kühne D: A novel self-expanding fully retrievable
intracranial stent (SOLO): experience in nine procedures of
stent-assisted aneurysm coil occlusion. Neuroradiology. 48:471–478.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piotin M, Blanc R, Spelle L, et al:
Stent-assisted coiling of intracranial aneurysms: clinical and
angiographic results in 216 consecutive aneurysms. Stroke.
41:110–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clajus C, Sychra V, Strasilla C and Klisch
J: Stent-assisted coil embolization of intracranial aneurysms using
the Solitaire™ AB Neurovascular Remodeling Device: initial and
midterm follow-up results. Neuroradiology. 55:629–638. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Long XA, Luo B, Karuna T and Duan
CZ: Factors responsible for poor outcome after intraprocedural
rerupture of ruptured intracranial aneurysms: identification of
risk factors, prevention and management on 18 cases. Eur J Radiol.
81:e77–e85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raymond J, Guilbert F, Weill A, et al:
Long-term angiographic recurrences after selective endovascular
treatment of aneurysms with detachable coils. Stroke. 34:1398–1403.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kadkhodayan Y, Rhodes N, Blackburn S, et
al: Comparison of Enterprise with Neuroform stent-assisted coiling
of intracranial aneurysms. AJR Am J Roentgenol. 200:872–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Xu D, Xiang Y, et al: Endovascular
treatment for wide-necked intracranial aneurysms with the
Enterprise stent. Neurol India. 59:548–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lubicz B, Collignon L, Raphaeli G, et al:
Solitaire stent for endovascular treatment of intracranial
aneurysms: immediate and mid-term results in 15 patients with 17
aneurysms. J Neuroradiol. 37:83–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao KJ, Zhang YW, Xu Y, et al:
Reconstruction of saccular and dissected intracranial aneurysms
using Solitaire™ AB stents. PLoS One. 8:e572532013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao B and Malek AM: Possible mechanisms
for delayed migration of the closed cell - designed enterprise
stent when used in the adjunctive treatment of a basilar artery
aneurysm. AJNR Am J Neuroradiol. 31:E85–E86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heller RS and Malek AM: Delivery technique
plays an important role in determining vessel wall apposition of
the Enterprise self-expanding intracranial stent. J Neurointerv
Surg. 3:340–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krischek O, Miloslavski E, Fischer S,
Shrivastava S and Henkes H: A comparison of functional and physical
properties of self-expanding intracranial stents [Neuroform3,
Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg.
54:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yahia AM, Gordon V, Whapham J, et al:
Complications of Neuroform stent in endovascular treatment of
intracranial aneurysms. Neurocrit Care. 8:19–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Izar B, Rai A, Raghuram K, Rotruck J and
Carpenter J: Comparison of devices used for stent-assisted coiling
of intracranial aneurysms. PLoS One. 6:e248752011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv X, Li Y, Xinjian Y, Jiang C and Wu Z:
Results of endovascular treatment for intracranial wide-necked
saccular and dissecting aneurysms using the Enterprise stent: a
single center experience. Eur J Radiol. 81:1179–1183. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colby GP, Paul AR, Radvany MG, et al: A
single center comparison of coiling versus stent assisted coiling
in 90 consecutive paraophthalmic region aneurysms. J Neurointerv
Surg. 4:116–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu DQ, Zhang X, Luo B, Long XA and Duan
CZ: The effect of Neuroform stent-assisted coil embolization of
wide-necked intracranial aneurysms and clinical factors on
progressive aneurysm occlusion on angiographic follow-up. J Clin
Neurosci. 20:244–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mangiafico S, Guarnieri G, Consoli A,
Ambrosanio G and Muto M: Endovascular strategy for unruptured
cerebral aneurysms. Eur J Radiol. 82:1638–1645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pereira VM, Brina O, Gonzalez AM, et al:
Biology and hemodynamics of aneurismal vasculopathies. Eur J
Radiol. 82:1606–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lubicz B, Bandeira A, Bruneau M, et al:
Stenting is improving and stabilizing anatomical results of coiled
intracranial aneurysms. Neuroradiology. 51:419–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weber W, Bendszus M, Kis B, et al: A new
self-expanding nitinol stent (Enterprise) for the treatment of
wide-necked intracranial aneurysms: initial clinical and
angiographic results in 31 aneurysms. Neuroradiology. 49:555–561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JI, Ko JK, Choi BK and Choi CH:
In-stent stenosis of stent-assisted coil embolization of the
supraclinoid internal carotid artery aneurysm. J Korean Neurosurg
Soc. 51:370–373. 2012. View Article : Google Scholar : PubMed/NCBI
|