Introduction
Non-small cell lung cancer (NSCLC) remains a
refractory disease, even in the era of development of
molecular-targeting agents. Mutated epidermal growth factor
receptor (EGFR) is a favorable prognostic factor; it is observed in
20–30% of cases, and the incidence is high in the Asian population
(1). EGFR-tyrosine kinase inhibitors
(TKIs) have been recognized as key drugs for NSCLC; the majority of
studies have reported their effectiveness in the treatment of
EGFR-mutated NSCLC (2). The present
study reports a case of NSCLC with EFGR mutation in which the
administration of gefitinib was reduced to once every 3 days.
Case report
A 65-year-old woman with no medical history
presented with the incidental detection of a nodule 34 mm in
diameter on chest radiography at the Mito Medical Center of the
University of Tsukuba (Mito, Japan). The patient was asymptomatic
and had been in good health. The physical examination was
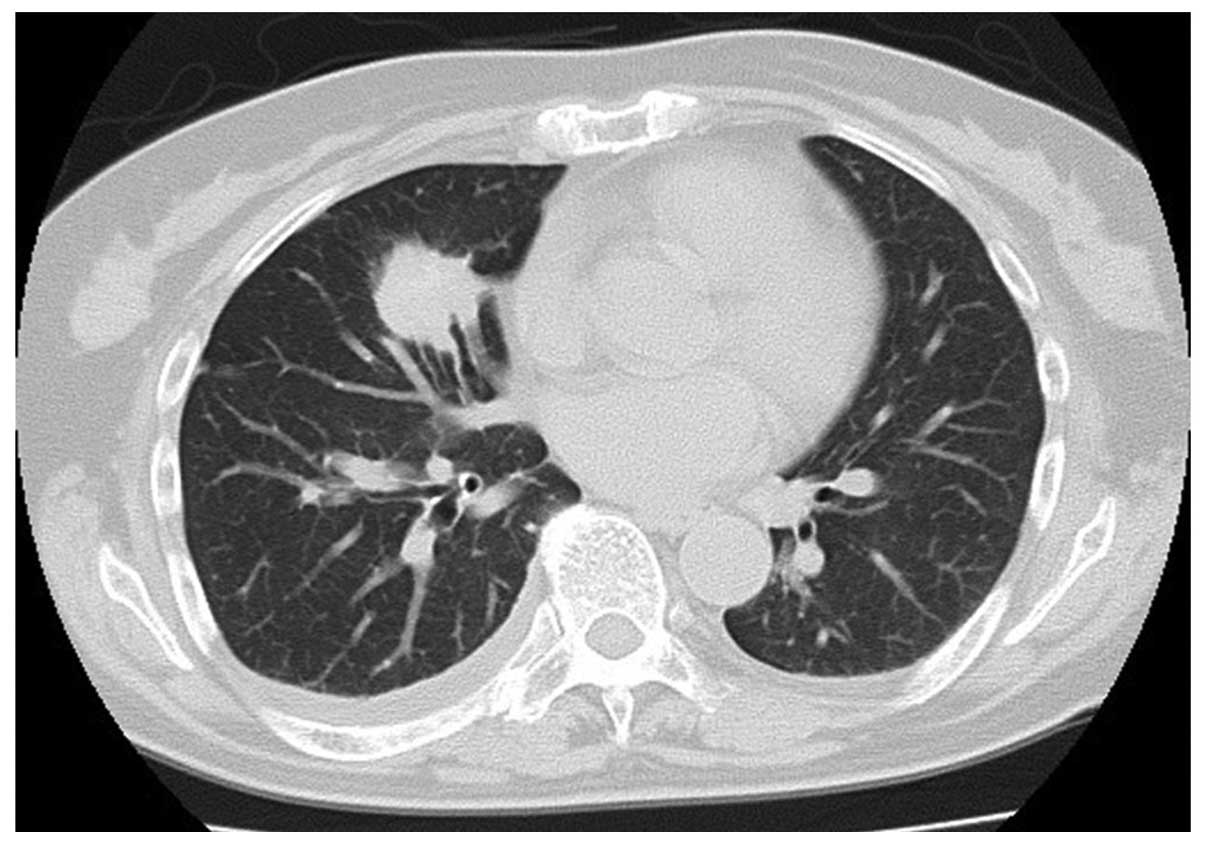
unremarkable. The chest computed tomography (CT) scan revealed a
well-circumscribed mass in the middle lobe of the lung that
measured 34×30×24 mm with specular appearance (Fig 1). The routine laboratory tests were
normal, as were tumor markers including carcinoembryonic antigen.
The patient was diagnosed with adenocarcinoma on the basis of
cytological examination of transbrochial biopsy specimens. Distant
metastasis was not detected. The patient underwent lobectomy of the
middle of the lung and mediastinal lymph node dissection. The
patient's pleural fluid was malignant and the final pathological
diagnosis was lung adenocarcinoma staged as pT4bN2M1a, stage IV. An
EGFR exon 19 deletion was identified. Soon after the surgical
resection, the patient received five courses of chemotherapy with
carboplatin and paclitaxel. In addition, the patient was treated
with uracil and tegafur (UFT; Taiho Pharmaceutical Co., Ltd.,
Tokyo, Japan) for 6 months. However, 24 months after the surgery,
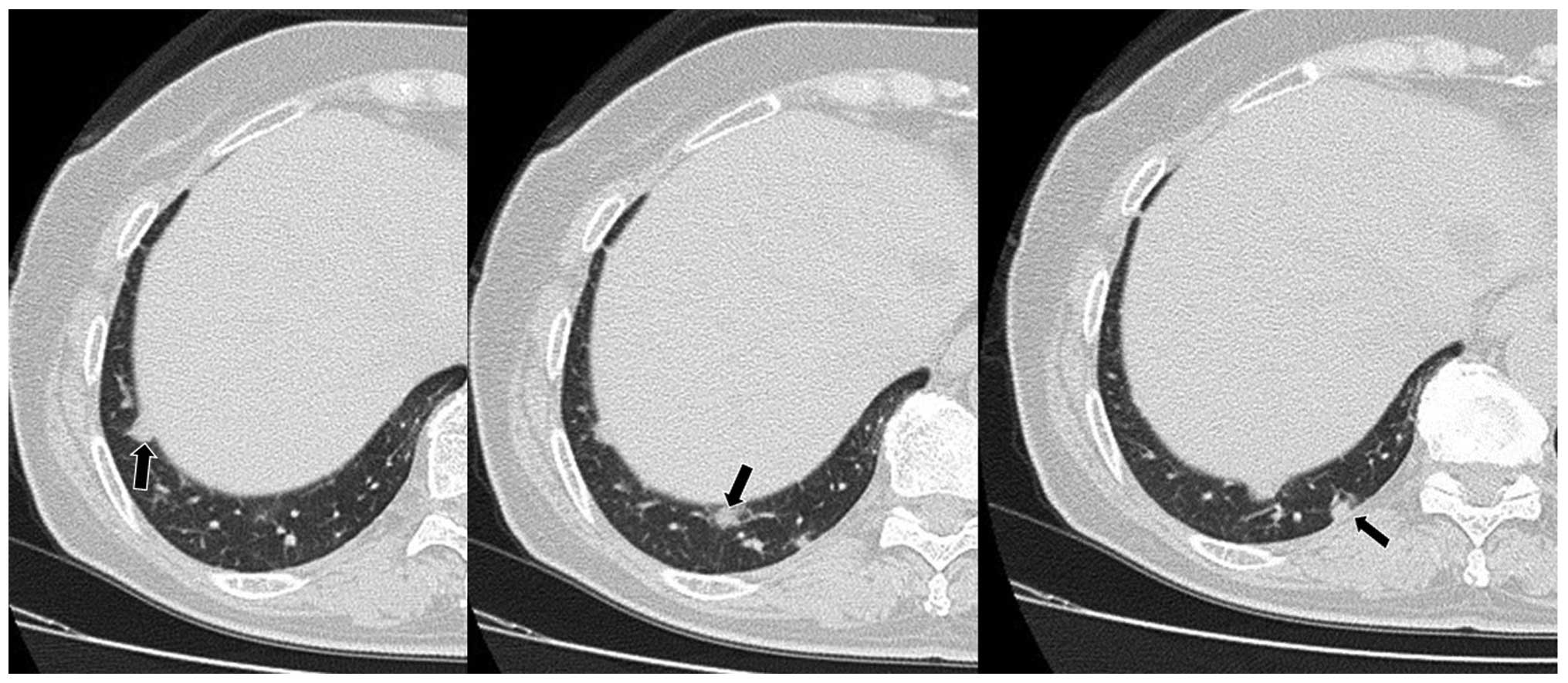
four metastatic lesions ≤15 mm in diameter were identified in the
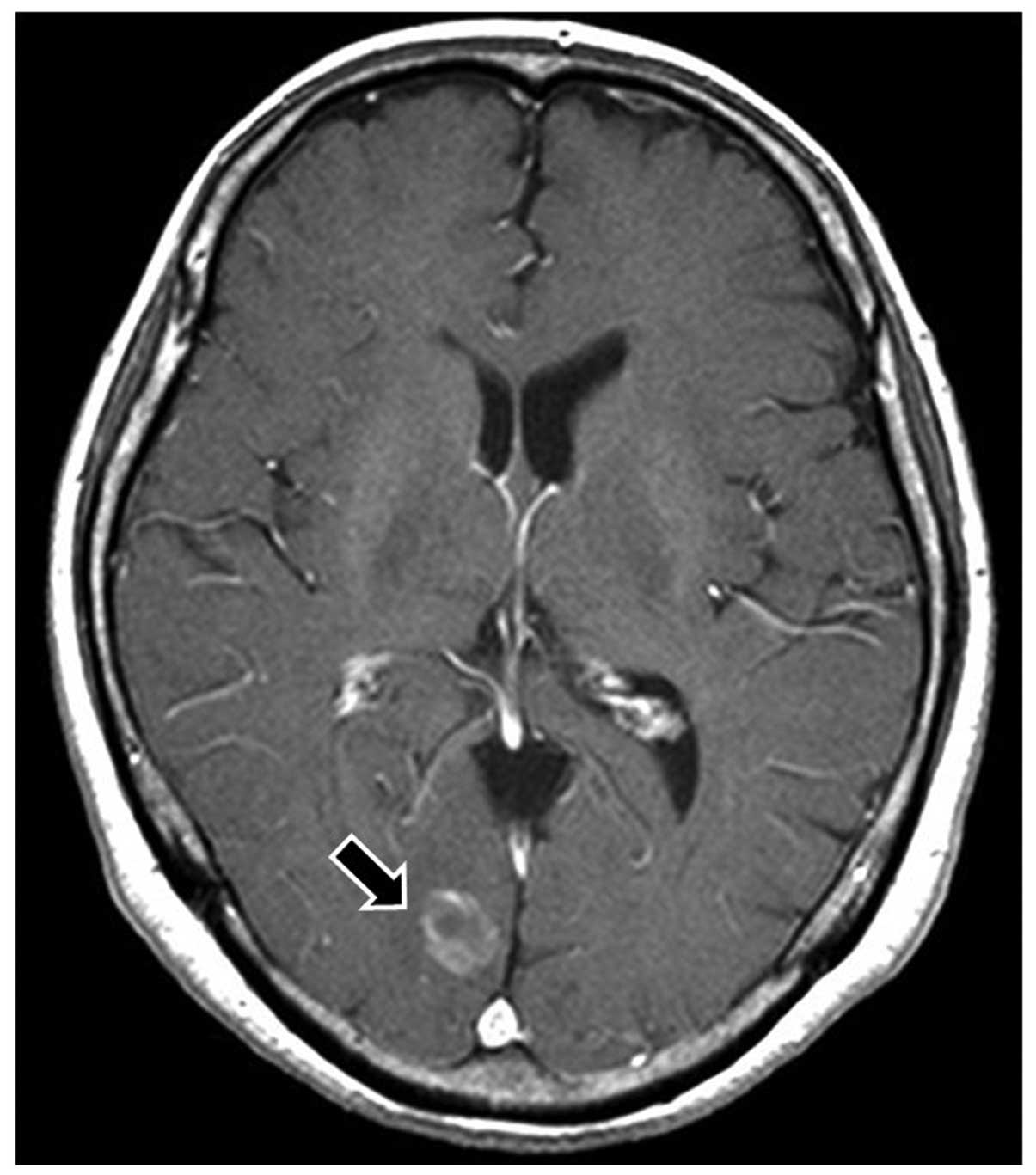
right lung (Fig 2). A cerebellar
metastasis with a diameter of 16 mm was also observed in brain
magnetic resonance imaging (MRI) (Fig
3), which was treated with gamma knife radiotherapy.
Thereafter, treatment with 250 mg gefitinib once daily was
initiated. A chest CT scan after 1 month of gefitinib
administration revealed that the pulmonary metastatic tumors in the
right lower lobe of the lung had disappeared. A grade 3 skin
adverse effect (AE) according to the National Cancer Institute
Common Toxicity Criteria (version 4.0) was present. Due to the AE,
the administration of gefitinib was changed to every other day
following daily administration for 2 months. However, the skin AE
continued and the patient requested further dose reduction. After
discontinuation of gefitinib for 1 week, treatment was restored at
a frequency of once every 3 days. Treatment with gefitinib was
continued in the outpatient clinic without tumor recurrence,
including in the lung as well as in the brain, for 27 months from
the initiation of the reduction in frequency of gefitinib treatment
to once every 3 days. Informed consent was obtained from the
patient prior to treatment.
Discussion
Gefitinib has been reported to be the preferred
choice of treatment for NSCLC with EGFR mutation (2). Three previous case reports of
successful treatment with a low dose of gefitinib exist, and they
are restricted to elderly patients and patients with severe AEs
(3–5). Hanaoka et al reported a case of
postoperative recurrent lung cancer in an octogenarian patient that
responded to treatment with radiation and gefitinib. The authors
considered that a useful treatment option for octogenarians with
postoperative recurrent lung cancer could be the administration of
gefitinib every other day following daily administration for two
weeks (3). Tomisaki et al
reported the successful treatment of a patient with advanced lung
cancer and brain metastasis after gamma knife radiotherapy and
chemotherapy, when gefitinib was administered every other day. The
frequency of administration of gefitinib was reduced to every other
day because of grade 3 skin reaction and rash (4). The reduction was initiated following
the discontinuation of treatment after 1 month of daily
administration of gefitinib (4).
Kitamura et al reported a case who remained in good
condition over 5 years with the administration of gefitinib every
other day (5). Very recently, we
also evaluated the dose reduction of TKIs in the treatment of NSCLC
(6). In the patients tested, not
only patients with AEs but also elderly patients and patients with
low body surface area underwent dose reduction of TKIs (6). Notably, the progression-free survival
of patients who underwent dose reduction was not shorter than that
of the patients who did not undergo dose reduction (6).
Currently, the present case has been in remission
from lung adenocarcinoma for 27 months with once every 3 day
administration of 250 mg gefitinib, which, to the best of our
knowledge, is the lowest dose to be reported in a successfully
treated case of EGFR-mutated NSCLC. In this case, dose reduction
was performed due to skin AE. The patient was treated with
gefitinib daily for 2 months, every other day for 1 month and then
once every 3 days. From the results of previous studies (3–6) and the
present one, it is speculated that dose reduction would be
indicated in patients who respond well to the daily administration
of gefitinib for >1 month, if required.
In the present case, measurements of serum gefitinib
levels were not taken. There have been certain reports showing that
the serum levels of gefitinib have an association with the effect
of the drug (7,8). By contrast, other studies did not
confirm the association (9–11). At present, therefore, it remains
beyond our knowledge to whom exactly the reduction of gefitinib
dosage should be permitted without any loss of clinical
efficacy.
In summary, the present study describes a case in
which dose-reduced gefitinib was effective and a 27-month disease
control was achieved. However, such an irregular administration of
gefitinib is not recommended. Dose reduction may be an alternative
treatment option to be considered for patients who respond well to
gefitinib, in cases where there is no other satisfactory choice of
treatment.
References
|
1
|
Yang CH: EGFR tyrosine kinase inhibitors
for the treatment of NSCLC in East Asia: Present and future. Lung
Cancer. 60:(Suppl 2). S23–S30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanford M and Scott LJ: Gefitinib: A
review of its use in the treatment of locally advanced/metastatic
non-small cell lung cancer. Drugs. 69:2303–2328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanaoka T, Sone S, Yamaguchi S, Okada M
and Hayano T: An octogenarian case of postoperative recurrent lung
cancer responding to treatments with radiation plus gefitinib. Gan
To Kagaku Ryoh. 34:1701–1703. 2007.(In Japanese).
|
|
4
|
Tomisaki S, Takenaka T, Morizono G, Tanaka
T, Momosaki N and Inoue F: Successful gefitinib every other day
administration in an advanced lung cancer patient with brain
metastasis after gamma knife radiotherapy and chemotherapy. Gan To
Kagaku Ryoho. 36:2607–2610. 2009.(In Japanese). PubMed/NCBI
|
|
5
|
Kitamura Y, Sakakura N, Uchida T and
Suyama M: Limitation of apoptosis induced by gefitinib in a
long-surviving postoperative recurrent lung cancer case. Gan To
Kagaku Ryoho. 38:275–277. 2011.(In Japanese). PubMed/NCBI
|
|
6
|
Sato S, Kurishima K, Miyazaki K, Kodama T,
Ishikawa H, Kagohashi K, Tamura T, Homma S, Satoh H and Hizawa N:
Efficacy of tyrosine kinase inhibitors in non-small-cell lung
cancer patients undergoing dose reduction and those with a low body
surface area. Mol Clin Oncol. 2:604–608. 2014.PubMed/NCBI
|
|
7
|
McKillop D, Partridge EA, Kemp JV, Spence
MP, Kendrew J, Barnett S, Wood PG, Giles PB, Patterson AB, Bichat
F, Guilbaud N and Stephens TC: Tumor penetration of gefitinib
(Iressa), an epidermal growth factor receptor tyrosine kinase
inhibitor. Mol Cancer Ther. 4:641–649. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Zhou Q and Gallo JM: Demonstration
of the equivalent pharmacokinetic/pharmacodynamic dosing strategy
in a multiple-dose study of gefitinib. Mol Cancer Ther.
8:1438–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranson M, Hammond LA, Ferry D, Kris M,
Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C,
Feyereislova A, Swaisland H and Rowinsky EK: ZD1839, a selective
oral epidermal growth factor receptor-tyrosine kinase inhibitor, is
well tolerated and active in patients with solid, malignant tumors:
Results of a phase I trial. J Clin Oncol. 20:2240–2250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa K, Tamura T, Negoro S, Kudoh S,
Yamamoto N, Yamamoto N, Takeda K, Swaisland H, Nakatani I, Hirose
M, Dong RP and Fukuoka M: Phase I pharmacokinetic trial of the
selective oral epidermal growth factor receptor tyrosine kinase
inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with
solid malignant tumors. Ann Oncol. 14:922–930. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolf M, Swaisland H and Averbuch S:
Development of the novel biologically targeted anticancer agent
gefitinib: Determining the optimum dose for clinical efficacy. Clin
Cancer Res. 10:4607–4613. 2004. View Article : Google Scholar : PubMed/NCBI
|