Introduction
Adult stem cells are a class of undifferentiated
cells, exhibiting long-term self-renewal proliferative and
multi-lineage differentiation potential (1). These cells can be obtained from
multiple types of tissue, including bone marrow, tendon and
adipose. Due to these characteristics, the application of such
cells for tissue repair and regeneration has attracted the
attention of many researchers. As a member of the adult stem cell
family, adipose-derived stem cells (ADSCs) are accessible and
possess strong self-renewal capacity; thus, they may serve as the
ideal seeding cells for tissue regeneration (2).
Platelet-rich plasma (PRP) is a fraction of the
whole blood, with the platelet concentration above the baseline
level (3). PRP can be extracted by
the centrifugation of whole blood and contains a high concentration
of platelets, fibrin and white blood cells. The concept that PRP
could promote tissue regeneration and cell differentiation is based
on the role of platelets (4,5). When activated, a variety of growth
factors, including transforming growth factor-β (TGF-β), platelet
derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1),
vascular endothelial growth factor (VEGF), basic fibroblastic
growth factor (bFGF) and epidermal growth factor (EGF) are secreted
by the platelets (5–7). The growth factors present in PRP play a
critical role in tissue repair and regeneration (8).
The repair of articular cartilage defects has long
been an obstacle for orthopedic studies (9,10).
Tissue engineering is a promising option for cartilage regeneration
(11,12) when an appropriate class of seeding
cells is selected, particularly adult stem cells with the advantage
of strong proliferative and differentiative activity. Thus, ADSCs
as an accessible source of seeding cells for cartilage
regeneration, when induced towards the chondrogenic lineage, are a
promising prospect for the creation of tissue-engineered
cartilage.
The present study primarily isolated and
characterized ADSCs from rabbits, and then explored the ability of
PRP obtained by laboratory centrifugation to induce ADSCs towards
the chondrogenic lineage, with the aim of providing a new approach
for cartilage regeneration.
Materials and methods
Animal treatment
All experimental procedures involving animals
conformed with the National Institutes of Health (NIH) Guide for
the Care and Use of Laboratory Animals and were approved by the
Administration Committee of Experimental Animals, Jiangsu,
China.
Isolation and culture of ADSCs
Ten 4-month-old New Zealand white rabbits (2.8–3.5
kg; Jiangsu Academy of Agricultural Sciences, Nanjing, China) were
used. Under sterile conditions, adipose tissues (∼10 g) were
carefully taken from the rabbits following anesthesia. Anesthesia
was induced via an intravenous injection of ketamine hydrochloride
(60 mg/kg) and xylazine (6 mg/kg) (Shanghai Tongwei Biological
Technology Co., Ltd., Shanghai, China). These samples were minced
and then digested for 1 h at 37°C with type I collagenase (2.5
mg/ml; Sigma-Aldrich, St Louis, MO, USA). During the digestion
time, these tissues were swiftly shaken every 20 min. The digested
samples were passed through a 70-μm cell strainer (Becton
Dickinson, Franklin Lakes, NJ, USA) to yield a single-cell
suspension. These released cells were washed with
phosphate-buffered saline (PBS) and re-suspended in complete
culture medium containing low-glucose Dulbecco's modified Eagle's
medium (LG-DMEM; Gibco Life Technologies, Carlsbad, CA, USA), 10%
fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml
streptomycin and 2 mM L-glutamine (all from Invitrogen Life
Technologies, Carlsbad, CA, USA) at a density of 500
cells/cm2. The resuspended cells were cultured in a
humidified atmosphere containing 5% CO2 at 37°C. At day
3 after initial plating, the cells were washed twice with PBS to
remove the non-adherent cells. At day seven to ten, these colonies
were trypsinized and mixed together as passage 0.
Multidifferentiation potential
The osteogenic, adipogenic and chondrogenic
differentiation potential of adipose-derived stem cells at passage
3 was investigated according to the method of Rui et al
(13) with certain modifications, as
described below.
Adipogenic differentiation assays
ADSCs were plated at 4×103
cells/cm2 in a six-well plate and cultured in complete
culture medium until the cells reached confluence. The medium was
replaced with complete medium or adipogenic medium, which was
complete culture medium and adipogenic reagents. The adipogenic
reagents included dexamethasone (500 nM), isobutylmethylxanthine
(0.5 mM), indomethacin (50 mM) and insulin (10 mg/ml) (all from
Sigma-Aldrich). The culture medium was replaced every 3–4 days.
After 21 days of incubation, Oil Red O staining was performed to
confirm the formation of oil droplets. For Oil Red O staining, the
cells were fixed in 70% ethanol for 10 min and stained with 0.3%
fresh Oil Red O solution (Sigma-Aldrich) for 2 h.
Osteogenic differentiation assays
ADSCs were plated at the same density as in the
previously described adipogenic assays. Then, these cells were
cultured with complete medium or osteogenic medium, which were
complete culture medium and osteogenic reagents. The osteogenic
reagents included dexamethasone (1 nM), ascorbic acid (50 mM) and
β-glycerolphosphate (20 mM) (all from Sigma-Aldrich). The culture
medium was replaced every 3–4 days. After 28 days of incubation,
0.5% alizarin red (pH 4.1; Sigma-Aldrich) was utilized to stain the
cells for 30 min, subsequent to the cells being fixed in 70%
ethanol for 10 min.
Chondrogenic differentiation
assays
A pellet culture system was used for chondrogenic
differentiation. Approximately 8×105 ADSCs were
centrifuged at 450 × g for 10 min in a 15-ml conical polypropylene
tube to form a micromass and incubated in complete medium or a
chondrogenic medium that comprised complete culture medium and
chondrogenic reagents. The chondrogenic reagents comprised LG-DMEM,
supplemented with 10 ng/ml TGF-β3 (R&D Systems, Minneapolis,
MN, USA), 500 ng/ml bone morphogenetic protein-2 (BMP-2; R&D
Systems), 10−7 M dexamethasone, 50 μg/ml
ascorbate-2-phosphate, 40 μg/ml proline, 100 μg/ml pyruvate (all
from Sigma-Aldrich) and 1:100 diluted ITS+ Premix [6.25
mg/ml insulin, 6.25 mg/ml transferrin, 6.25 mg/ml selenous acid,
1.25 mg/ml bovine serum albumin (BSA) and 5.35 mg/ml linoleic acid;
Becton Dickinson]. After 21 days of incubation, the pellet was
fixed for histology and the immunohistochemical staining of
collagen type II (Col II).
Immunohistochemical staining of Col
II
The immunohistochemical staining was performed as
follows. Briefly, paraffin-embedded sections were deparaffinized in
xylene and dehydrated through a graded series of alcohol. Then,
dewaxed slices of cell mass were incubated in 3% hydrogen peroxide
at room temperature for 20 min. Antigen retrieval was performed
with 2 mg/ml protease (Calbiochem, Bie and Berntsen, Rødovre,
Denmark) at 37°C for 30 min for Col II detection. The sections were
incubated with mouse monoclonal antibody against rabbit Col II
(cat. no. sc-52658, Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; 1:100 dilution with 5% goat serum in PBS containing 1% BSA)
overnight at 4°C after blocking with 5% goat serum for 20 min at
room temperature. The spatial localization of Col II was observed
by incubating with goat polyclonal anti-mouse IgG
tetramethylrhodamine-conjugated secondary antibody (cat. no T5393,
Sigma-Aldrich; 1:200 dilution with 5% goat serum in PBS containing
1% BSA) for 1 h at room temperature, followed by
3,3′-diaminobenzidine tetrahydrochloride (Dako, Glostrup, Denmark)
in the presence of H2O2. The slides were
gently washed with deionized water and rinsed slowly, followed by
dehydration through a graded series of ethanol and xylene, and
mounted with DPX for light microscopy (Leica DMRXA2; Leica
Microsystems, Wetzlar, Germany).
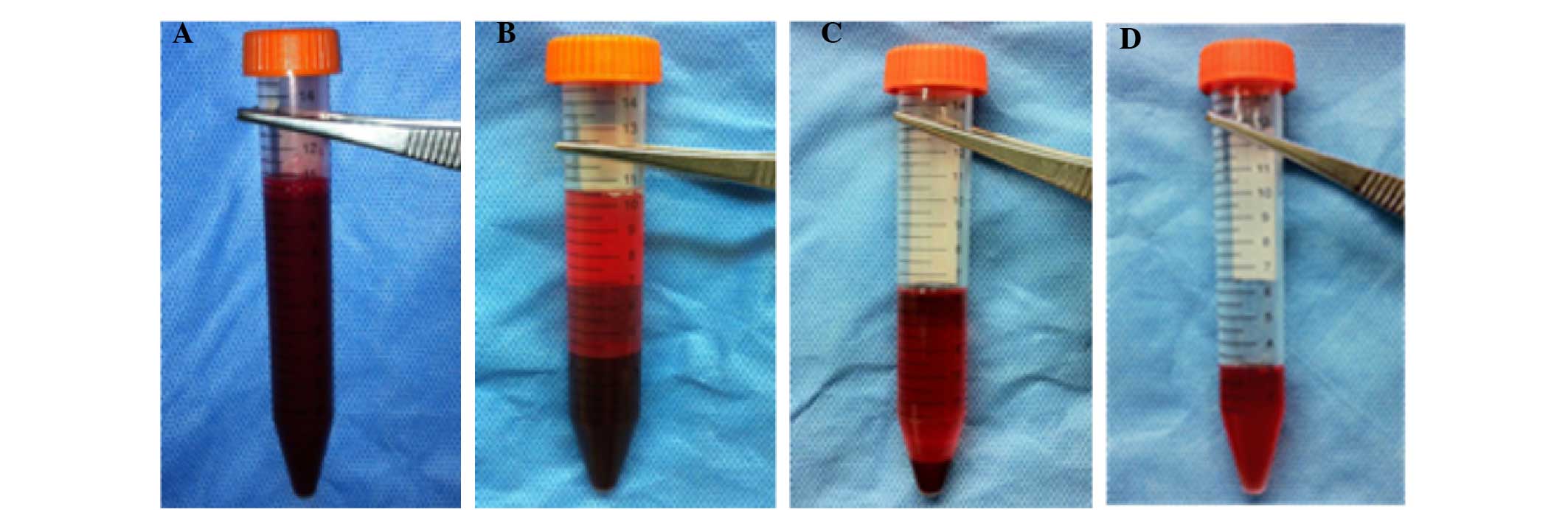
Preparation of PRP
PRP was prepared according to the method described
by Nagae et al (14) with
certain modifications. The procedures of PRP preparation were as
follows (Fig. 1). Under general
anesthesia, 10 ml fresh blood was obtained using a syringe
containing 1.0 ml acid citrate dextrose solution A as
anticoagulant. The whole blood was centrifuged using a
centrifugation apparatus (KN70; Kubota, Tokyo, Japan) at 250 × g
for 10 min. Subsequently, the single plasma fraction was collected
and further centrifuged at 1,000 × g for 10 min. The precipitated
platelets at the bottom of the centrifuge tube were collected with
3 ml of the supernatant (platelet-poor plasma) to yield PRP.
Experimental grouping
ADSCs at passage 3 were utilized, and plated at
1×108 cells/l in 12-well culture plates containing
coverslips. These wells were randomly assigned into a PRP group and
a control group, each with six wells. The ADSCs in the PRP group
were cultured with complete medium containing 10% PRP. In the
control group, the ADSCs were cultured with the complete medium.
The culture medium was replaced every 3–4 days.
Immunofluorescence staining and
toluidine blue staining
Following two weeks of culture, ADSCs in the PRP
group were fixed with methanol for 10 min, then washed with PBS for
5 min. Subsequently, PBS with Tween 20 (PBST) with 5% BSA was
utilized to fix the washed cells. Goat polyclonal anti-rabbit Coll
II antibody (cat. no. sc-52658; Santa Cruz Biotechnology, Inc.;
1:200 dilution in PBS) was used as a primary antibody for
incubation with the induced ADSCs for 1 min; the cells were then
incubated with a fluorescein isothiocyanate (FITC)
fluorescence-labeled goat anti-mouse secondary antibody (cat. no.
F0257; Sigma-Aldrich; 1:200 dilution in PBS) for a further 60 min.
Following the incubation, the slides were washed with PBS and
observed under a fluorescence microscope (EVOS® FL; Life
Technologies, Carlsbad, CA, USA). For toluidine blue staining, the
procedures performed to fix the induced ADSCs were the same as
those described for immunofluorescence staining. Then, the slides
were dyed with 1% toluidine blue for 2 h prior to washing with PBS.
Finally, the slides were mounted with neutral gum for
observation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To compare the expression of type II collagen α1
chain (COL2A1) and aggrecan (AGC) mRNA expression between the PRP
and control groups, RT-qPCR was performed. Following two weeks'
culture, ADSCs from the PRP and control groups were harvested and
subjected to RNA extraction with an RNeasy mini kit (Qiagen GmbH,
Hilden, Germany). mRNA was reverse-transcribed to complementary DNA
(cDNA) using the First Strand cDNA kit (Promega Corporation,
Madison, WI, USA). Then, 5 μl of total cDNA from each sample was
amplified in a final volume of 25 μl of reaction mixture containing
Platinum SYBR Green qPCR SuperMix-UDG ready-to-use reaction
cocktail and specific primers for COL2A1, AGC and β-actin (all from
Mergene; Table I). Cycling
conditions were denaturation at 95°C for 1 min, 45 cycles at 95°C
for 20 sec, optimal annealing temperature (as defined in Table I) for 15 sec, 72°C for 45 sec and at
60–95°C with a heating rate of 0.1°C/sec. Target gene expression
was normalized to that of β-actin. Relative gene expression was
calculated with the 2−ΔΔCt formula (15).
 | Table I.Primer sequences of COL2A1, AGC and
β-actin, product size and annealing temperature used in the reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences of COL2A1, AGC and
β-actin, product size and annealing temperature used in the reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer | Product size
(bp) | Annealing temperature
(°C) |
|---|
| COL2A1 | Forward:
5〲-TCCTAAGGGTGCCAATGGTGA-3〲 | 112 | 61 |
|
| Reverse:
5〲-AATGTCAACAATGGGAAGGGGT-3〲 |
|
|
| AGC | Forward:
5〲-TCCGCTGGTCTGATGGACAC-3〲 | 101 | 55 |
|
| Reverse:
5〲-AGGACCAACTTTGCCTTGAGGAC-3〲 |
|
|
| β-actin | Forward:
5〲-AGATGTGGATCAGCAAGCAGGAGT-〲3 | 133 | 60 |
|
| Reverse:
5〲-TCTCGTTTCTGCGCCGTTAGGTTT-〲3 |
|
|
Statistical analysis
Comparisons between groups were performed using a
paired t-test. All data analysis was conducted using SPSS
statistical software (version 17.0; SPSS Inc, Chicago, IL, USA).
P≥0.01 was considered to indicate a statistically significant
result.
Results
Isolation and culture of ADSCs
It was shown in the present study that the ADSCs
isolated from rabbit adipose tissue were adherent to the plastic
culture flask after being cultured for 24 h. Following four days of
culture, long spindle cells were observed and the number of cells
was significantly increased. Passage 3 ADSCs retained the long
spindle morphology and proliferative potential.
Multi-lineage differentiation
potential of ADSCs
Following the various multi-lineage induction
processes, ADSCs were successfully induced toward adipogenic,
osteogenic and chondrogenic lineages. After 21 days of adipogenic
induction, lipid droplets were formed and confirmed by Oil Red O
staining (Fig. 2A). This was not
observed in the group cultured with complete medium only (Fig. 2B). The osteogenic differentiation
potential of the isolated ADSCs was determined in vitro.
Alizarin-red staining was positive as calcium nodules were observed
after 21 days of induction (Fig.
2C). In the group cultured with complete medium only, alizarin
red staining was negative (Fig. 2D).
The chondrogenic differentiation potential of the ADSCs was
determined in vitro by pellet culture. Following 21 days of
chondrogenic induction, the diameter of the pellet was ∼1 mm
(Fig. 2E). The induced ADSC pellet
was rich in Col II, as indicated by immunohistochemical staining
(Fig. 2F).
Chondrogenic differentiation of ADSCs
induced by PRP
Following two weeks of induction, ADSCs in the PRP
group produced Col II, as indicated by immunofluorescence staining
(Fig. 3A). No green fluorescent
cells were seen in the control group (Fig. 3B), indicating that there was no
significant expression of Col II. Toluidine blue staining indicated
that PRP was a potent reagent for inducing ADSCs to produce
aggrecan. Toluidine blue staining was positive in the PRP group
(Fig. 3C) but negative in the
control group (Fig. 3D). RT-qPCR
confirmed that CoL2A1 and AGC mRNA expression was significantly
upregulated in the PRP group compared with that in the control
group (P<0.01; Table II).
 | Table II.Analysis of COL2A1 and AGC mRNA
expression by ADSCs in the PRP and control groups. |
Table II.
Analysis of COL2A1 and AGC mRNA
expression by ADSCs in the PRP and control groups.
| Gene | PRP group ΔCt | Control group
ΔCt | |ΔΔCt| | t-value | P-value |
|---|
| COL2A1 |
4.28±0.06 |
8.94±0.46 |
4.66±0.49 | -30.902 | 0.001 |
| AGC1 |
4.17±0.22 |
7.30±1.43 |
3.13±1.29 | -12.237 | 0.047 |
Discussion
Repair and regeneration of articular cartilage
defects has been a subject of heated debate and of focused study in
orthopedic research. Traditional therapeutic strategies for
cartilage vary in outcome and long-term results are not
satisfactory (16). In recent years,
the development of tissue engineering, including bioactive growth
factors, seeding cells and good bio-compatible scaffolds, has
provided a wider choice for cartilage regeneration (10,11). As
an important factor, the choice of the seeding cells is critical
for its proliferative and differentiation potential in maintaining
the extracellular matrix and the function of the cartilage
(17). Adult stem cells have
multi-lineage differentiation potential, which could be applied in
multiple tissue-engineering strategies (18). Adult stem cells are potentially
useful in promoting bone healing (19), tendon injury (20), intervertebral disc degeneration
(21) and cartilage injury (22), and hold promising prospects in
cartilage regeneration. Among adult stem cells, bone marrow-derived
stem cells (BMSCs) and ADSCs as mesoderm-derived cells have been
widely used in tissue-engineering strategies for tissue
regeneration. Compared with BMSCs, ADSCs are superior in the range
of sources, ease of obtaining and proliferative potential, and hold
a broad future in cartilage regeneration (23,24).
Currently, collagenase digestion is a commonly used
method to obtain ADSCs, as ADSCs have very low density in adipose
tissues which mainly include Col I (25). The isolated passage 0 ADSCs in the
present study became adherent to the plastic plates within 24 h.
The morphology of the passage 0 ADSCs was similar to that of
fibroblasts with long spindle shapes. Then, following several
passages, the ADSCs continued to maintain a fibroblastic morphology
and proliferative vitality. The ADSCs were successfully isolated
and their multi-lineage potential was confirmed by their induction
towards adipogenic, osteogenic and chondrogenic lineages in
vitro.
Finding suitable bioactive substances to induce
seeding cells toward chondrogenic differentiation is a research
focus of cartilage tissue engineering (26). PRP, when activated, can release
multiple growth factors, including BMP-2, connective tissue growth
factor (CTGF), fibroblastic growth factor-2 (FGF-2) and TGF-β2
(5–7). BMP-2 is able to promote the
proliferation and chondrogenic extracellular matrix production of
adult mesenchymal stem cells (27).
CTGF has the ability to stimulate mesenchymal cell proliferation,
migration and aggregation (28).
FGF-2 is able to upregulate proteoglycan synthesis and enhance cell
proliferation (29,30). TGF-β2, as a common component of
chondrogenic agent, is a potent biological active substance for
enhancing chondrogenesis (31,32).
Thus, to induce ADSCs toward chondrogenic differentiation and
produce cartilage related extracellular matrix, PRP was utilized.
The multiple growth factors released from PRP may play a
synergistic role in the differentiation of ADSCs and maintenance of
cell proliferation and chondrocyte phenotype.
According to recent studies, PRP has great potential
in cartilage regeneration (33,34). The
study by Mardani et al (33)
indicated the promise of PRP, as it revealed that PRP could
effectively induce human ADSCs towards chondrogenic lineage.
However, currently, there is no exact definition of an ideal method
of producing PRP. Different methods of isolation and activation may
result in PRP of varying quality (35), for example, the presence or absence
of specific factors that are essential for MSC proliferation and
differentiation. In the present study, the common laboratory
centrifugation method was utilized to yield PRP because it is
simple and easy to perform. By reference to a study on PRP by Nagae
et al (14), it was
anticipated that the centrifugation steps used in that study would
be helpful in the present research. Therefore, the same centrifugal
force and centrifugation time were used in the present study to
prepare PRP for investigation of its potency in inducing the
chondrogenic differentiation of rabbit ADSCs.
In the present study, the immunofluorescence
staining of Col II was positive in the PRP group but negative in
the control group. Toluidine blue staining was also positive in the
PRP group. The mRNA expression levels of the chondrocyte-specific
markers Col2A1 and AGC were significantly upregulated. The results
suggest that PRP obtained by the laboratory centrifugation
procedure has potential in the chondrogenesis of ADSCs, which
offers a new application in cartilage tissue engineering.
The bio-activity of PRP depends on the synergistic
effects of multiple growth factors. The evaluation of the
concentration of a single growth factor within PRP is not
sufficient to indicate the potency of PRP. In addition to TGF-β,
other growth factors exhibited in PRP, such as BMP-4, BMP-7 and
GDF-5, are also important bio-activators for chondrogenic
differentiation (36). In the
present study, the concentrations of multiple growth factors and
their synergistic effects were not tested; instead, it was
anticipated that the growth factors released from PRP as a whole
could be effective in inducing the chondrogenesis of rabbit
ADSCs.
Laboratory centrifugation is a convenient method for
obtaining PRP. However, there is no consensus on the ideal
centrifugal force and time. In the present study, PRP prepared by
centrifugation was confirmed to be an effective bioactive substance
for inducing ADSCs towards chondrogenic lineage. Also, in this
study, PRP was not activated by bovine thrombin as described in
previous research (33). This was to
allow the platelets in the culture medium to slowly burst and
release the growth factors in a gradual way. In the future, the
focus of PRP research should be to confirm the presence or absence
of certain growth factors particularly associated with
chondrogenesis and investigate how the synergistic effects differ
according to different methods of preparation.
In summary, a common laboratory centrifugation
method was used to prepare PRP, and its efficacy was confirmed when
used to induce the chondrogenesis of rabbit ADSCs. The procedure
used to obtain the PRP was based on that of a previous study.
However, future studies are required to confirm the optimal
centrifugation force and time. Also, the presence or absence of
certain growth factors associated with chondrogenesis and their
synergistic effects should be measured to provide further
information for future clinical applications.
Acknowledgements
This study was supported by Jiangsu Health Research
Program, China (No. H201258).
References
|
1
|
Lim JY, Loiselle AE, Lee JS, et al:
Optimizing the osteogenic potential of adult stem cells for
skeletal regeneration. J Orthop Res. 29:1627–1633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gimble JM and Guilak F: Adipose-derived
adult stem cells: isolation, characterization, and differentiation
potential. Cytotherapy. 5:362–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marx RE: Platelet-rich plasma (PRP): what
is PRP and what is not PRP? Implant Dent. 10:225–228. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knighton DR, Hunt TK, Thakral KK and
Goodson WH III: Role of platelets and fibrin in the healing
sequence: an in vivo study of angiogenesis and collagen synthesis.
Ann Surg. 196:379–388. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brass L: Understanding and evaluating
platelet function. Hematology Am Soc Hematol Educ Program.
2010:387–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang N, Wu YP, Qian SJ, et al: Research
progress in the mechanism of effect of PRP in bone deficiency
healing. ScientificWorld Journal. 2013:1345822013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lenza M, Ferraz Sde B, Viola DC, et al:
Platelet-rich plasma for long bone healing. Einstein (Sao Paulo).
11:122–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lubkowska A, Dolegowska B and Banfi G:
Growth factor content in PRP and their applicability in medicine. J
Biol Regul Homeost Agents. 26 (2 Suppl 1):3S–22S. 2012.PubMed/NCBI
|
|
9
|
Lee HR, Park KM, Joung YK, et al:
Platelet-rich plasma loaded in situ-formed hydrogel enhances
hyaline cartilage regeneration by CB1 upregulation. J Biomed Mater
Res A. 100:3099–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wearing SC, Hennig EM, Byrne NM, et al:
Musculoskeletal disorders associated with obesity: a biomechanical
perspective. Obes Rev. 7:239–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnstone B, Alini M, Cucchiarini M, et
al: Tissue engineering for articular cartilage repair - the state
of the art. Eur Cell Mater. 25:248–267. 2013.PubMed/NCBI
|
|
12
|
Ye K, Felimban R, Moulton SE, et al:
Bioengineering of articular cartilage: past, present and future.
Regen Med. 8:333–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rui YF, Lui PP, Lee YW and Chan KM: Higher
BMP receptor expression and BMP-2-induced osteogenic
differentiation in tendon-derived stem cells compared with
bone-marrow-derived mesenchymal stem cells. Int Orthop.
6:1099–1107. 2012. View Article : Google Scholar
|
|
14
|
Nagae M, Ikeda T, Mikami Y, et al:
Intervertebral disc regeneration using platelet-rich plasma and
biodegradable gelatin hydrogel microspheres. Tissue Eng.
13:147–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2007.
View Article : Google Scholar
|
|
16
|
Kock L, van Donkelaar CC and Ito K: Tissue
engineering of functional articular cartilage: the current status.
Cell Tissue Res. 347:613–627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnstone B and Yoo J: Mesenchymal cell
transfer for articular cartilage repair. Expert Opin Biol Ther.
1:915–921. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tollervey JR and Lunyak VV: Adult stem
cells: simply a tool for regenerative medicine or an additional
piece in the puzzle of human aging? Cell Cycle. 10:4173–4176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janicki P and Schmidmaier G: What should
be the characteristics of the ideal bone graft substitute?
Combining scaffolds with growth factors and/or stem cells. Injury.
42 (Suppl 2):77–81. 2011. View Article : Google Scholar
|
|
20
|
Lui PP, Rui YF, Ni M and Chan KM:
Tenogenic differentiation of stem cells for tendon repair - what is
the current evidence? J Tissue Eng Regen Med. 5:e144–e163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang S, Tam V, Cheung KM, et al: Stem
cell-based approaches for intervertebral disc regeneration. Curr
Stem Cell Res Ther. 6:317–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lubis AM and Lubis VK: Adult bone marrow
stem cells in cartilage therapy. Acta Med Indones. 44:62–68.
2012.PubMed/NCBI
|
|
23
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casteilla L, Planat-Benard V, Bourin P, et
al: Use of adipose tissue in regenerative medicine. Transfus Clin
Biol. 18:124–128. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Markarian CF, Frey GZ, Silveira MD, et al:
Isolation of adipose-derived stem cells: a comparison among
different methods. Biotechnol Lett. 36:693–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griffin M, Hindocha S and Khan WS:
Chondrogenic differentiation of adult MSCs. Curr Stem Cell Res
Ther. 7:260–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freyria AM, Courtes S and Mallein-Gerin F:
Differentiation of adult human mesenchymal stem cells: chondrogenic
effect of BMP-2. Pathol Biol (Paris). 56:326–333. 2008.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arnott JA, Lambi AG, Mundy C, et al: The
role of connective tissue growth factor (CTGF/CCN2) in
skeletogenesis. Crit Rev Eukaryot Gene Expr. 21:43–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ellman MB, An HS, Muddasani P and Im HJ:
Biological impact of the fibroblast growth factor family on
articular cartilage and intervertebral disc homeostasis. Gene.
420:82–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stewart AA, Byron CR, Pondenis H and
Stewart MC: Effect of fibroblast growth factor-2 on equine
mesenchymal stem cell monolayer expansion and chondrogenesis. Am J
Vet Res. 68:941–945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Osch GJ, Van Der Veen SW, Burger EH
and Verwoerd-Verhoef HL: Chondrogenic potential of in vitro
multiplied rabbit perichondrium cells cultured in alginate beads in
defined medium. Tissue Eng. 6:321–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barry F, Boynton RE, Liu B and Murphy JM:
Chondrogenic differentiation of mesenchymal stem cells from bone
marrow: differentiation-dependent gene expression of matrix
components. Exp Cell Res. 268:189–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mardani M, Kabiri A, Esfandiari E, et al:
The effect of platelet rich plasma on chondrogenic differentiation
of human adipose derived stem cells in Transwell culture. Iran J
Basic Med Sci. 16:1163–1169. 2013.PubMed/NCBI
|
|
34
|
Smyth NA, Murawski CD, Fortier LA, et al:
Platelet-rich plasma in the pathologic processes of cartilage:
review of basic science evidence. Arthroscopy. 29:1399–1409. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anitua E, Andia I, Ardanza B, et al:
Autologous platelets as a source of proteins for healing and tissue
regeneration. Thromb Haemost. 91:4–15. 2004.PubMed/NCBI
|
|
36
|
Krüger JP, Freymannx U, Vetterlein S, et
al: Bioactive factors in platelet-rich plasma obtained by
apheresis. Transfus Med Hemother. 40:432–440. 2013. View Article : Google Scholar : PubMed/NCBI
|