Introduction
Nitrogen mustards (HN-1, HN-2 and HN-3) are chemical
agents analogous to sulfur mustards, with similar toxicities.
Although nitrogen mustards were applied as chemical warfare agents
in the 1930s, they are usually used to treat vitiligo, due to their
ability to increase the number of melanosomes within melanocytes
(1); however, nitrogen mustard
exposure increases the occurrence of tumors (2,3).
Nitrogen mustards are toxic to various tissues due to the formation
of reactive molecular species. One of the major side effects of
these chemicals is severe respiratory toxicity. Symptoms of acute
toxicity include chest tightness, a hacking cough and rhinorrhea.
The disorders caused by chronic toxicity include bronchiolitis,
emphysema and lung fibrosis. The severe symptoms caused by nitrogen
mustards are critical factors determining the mortality and
long-term survival of patients (4).
Nitrogen mustards cause difunctional alkylation through the
cross-linking of DNA; therefore, nitrogen mustards can lead to DNA
damage, which results in dysfunctional cellular activities,
including apoptosis and autophagy (5,6).
Nitrogen mustards can, therefore, be lethal upon absorption into
the human body, particularly through dermal, respiratory,
gastrointestinal and ocular routes of exposure (7). There is no known antidote specifically
for toxicities caused by nitrogen mustard, and successfully treated
cases are rarely reported. In the present study, a case of nitrogen
mustard-induced acute respiratory failure and myelosuppression in a
33-year-old man is described.
Case report
A 33-year-old man was accidentally exposed to
nitrogen mustard hydrochloride in a pharmaceutical factory. The
patient experienced progressing dyspnea and was immediately sent to
an emergency room at the First Affiliated Hospital of China Medical
University (Shenyang, China). Physical examination of the patient
showed that his blood pressure was 160/90 mmHg; his respiration
rate was 30 breaths/min and his heart rate was 150 bpm. Congestion
of the bulbar conjunctiva was observed in the eyes, and moist rales
were heard in the lungs. An arterial blood gas test revealed a pH
of 7.33, a partial pressure of O2 (pO2) of 63
mmHg, a pCO2 of 51 mmHg and an elevated arterial lactate
of 1.9 mmol/l. Blood cell counts revealed significant leukocytosis
with a white blood cell count of 11.36×109/l, of which
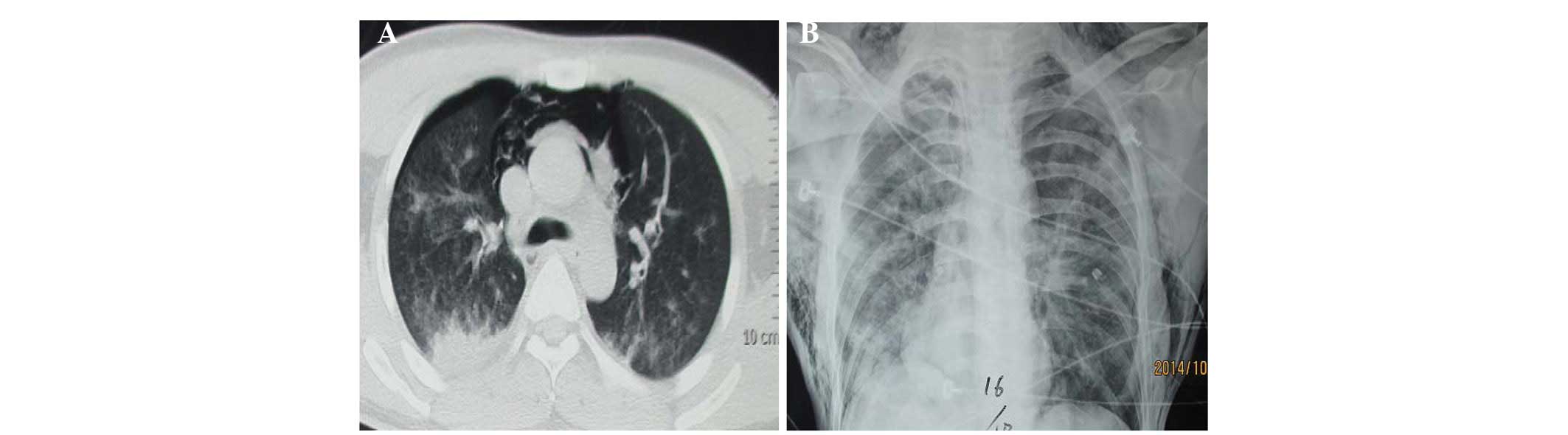
96% were neutrophils. A chest computed tomography scan showed
mediastinal emphysema and pneumothorax (Fig. 1A). The patient had a fever, with a
temperature of >39°C, and he was given an oxygen mask. The
patient's blood pressure continued to increase to 165/100 mmHg, his
heart rate increased to 140–150 bpm and oxygen saturation was 76%
in atmospheric air. Re-examination of the arterial blood gas
revealed a pH of 7.44, pO2 of 49 mmHg and
pCO2 of 72 mmHg. The patient then underwent tracheotomy
and mechanical ventilation. Due to the higher pressure of the
mechanical ventilation, the patient suffered the complication of
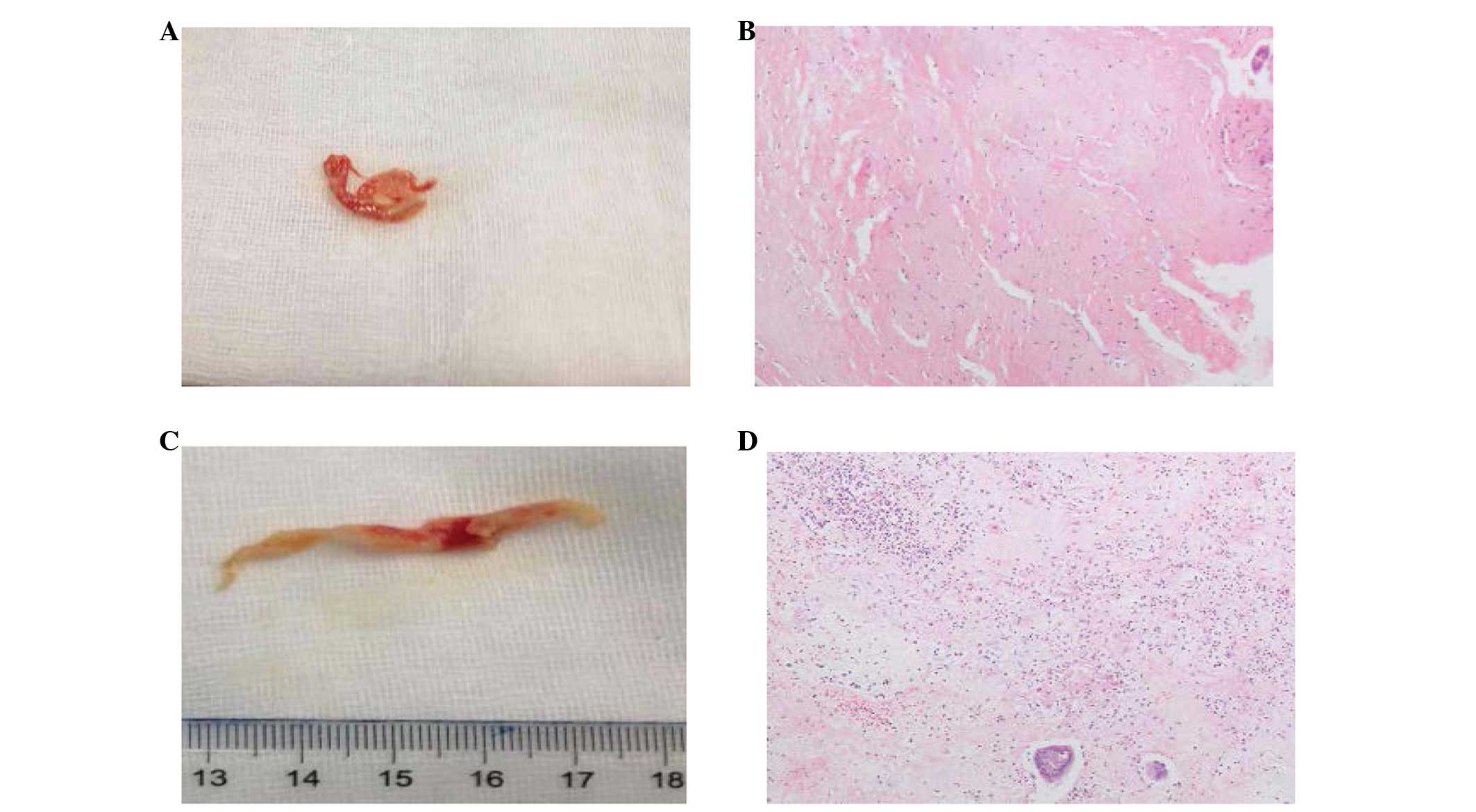
pneumothorax and subcutaneous emphysema (Fig. 1B). Bronchoscopy revealed mucosal
edema and necrosis in the airway resulting in a partial
obstruction. Mucosal pathology confirmed necrosis (Fig. 2). After 13 days of treatment, the
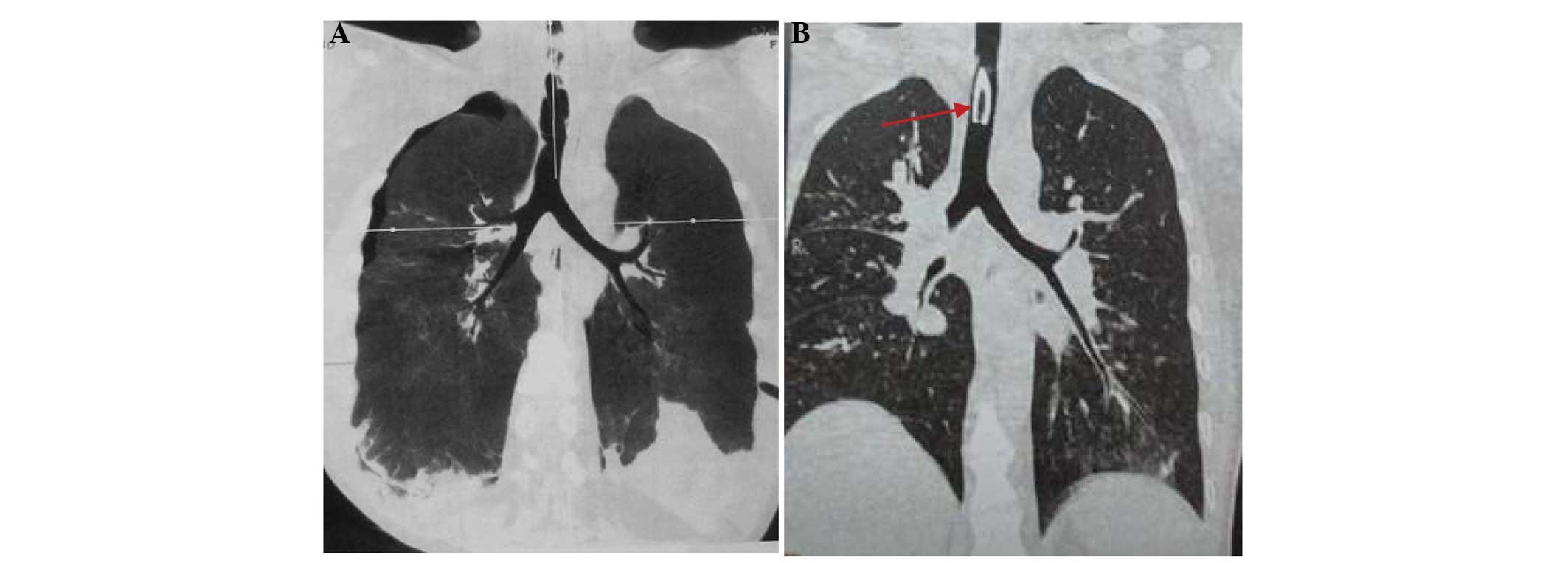
respiratory function of the patient improved markedly, although the
patient had developed airway stenosis due to the tracheotomy and
mechanical ventilation. The airway stenosis was successfully
relieved by local treatment, which included bronchoscopy for sputum
suction, and inhalation treatment with mucosalvan (60 mg/d) and
budenoside suspension (2 mg/d) (Fig.
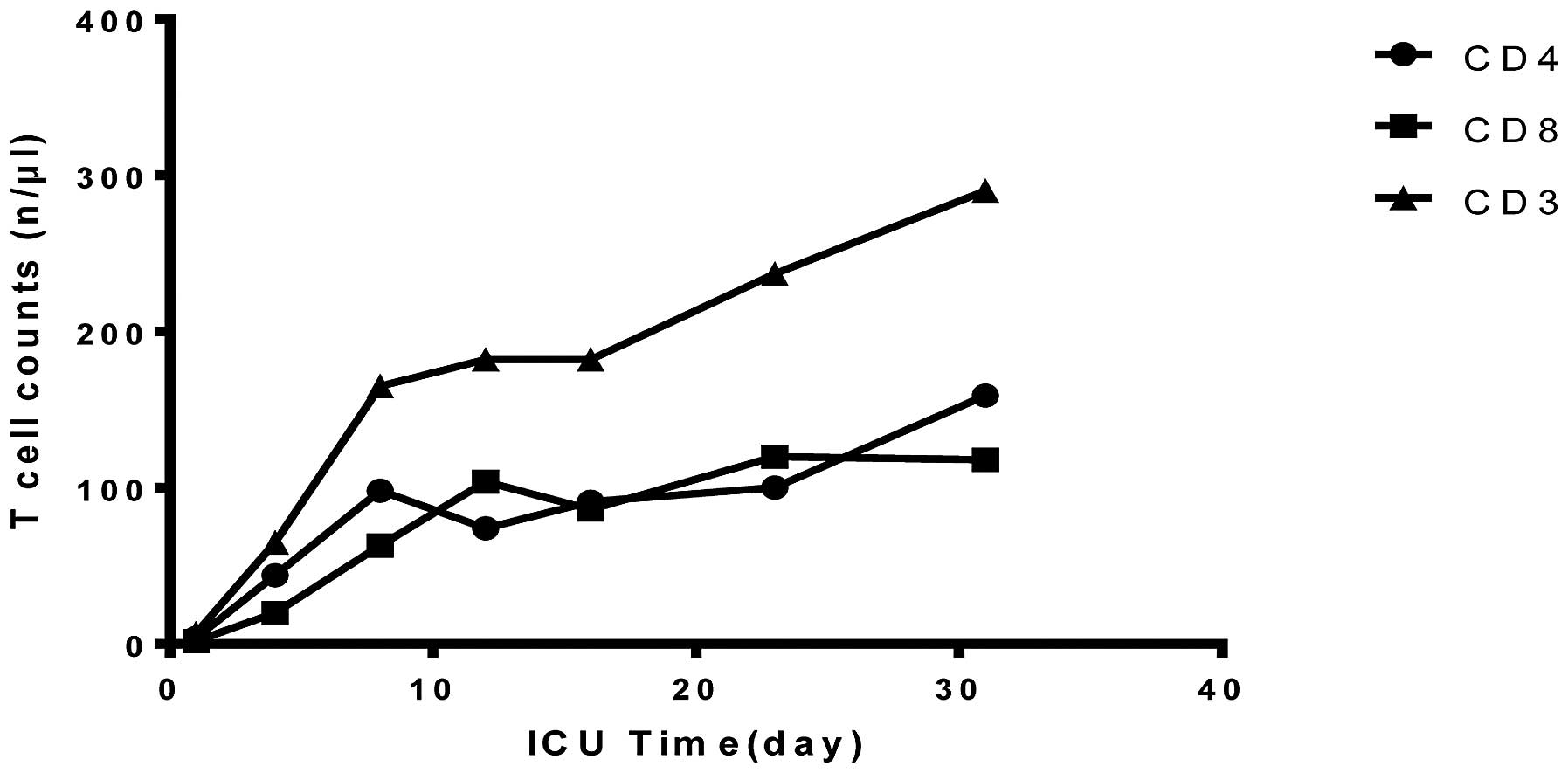
3). Despite the improved pulmonary function, the patient had a
deteriorated immune function. In the first 24 h, the levels of
cellular immune biomarkers [cluster of differentiation 4 (CD4), CD8
and CD3] decreased significantly. The patient was then given
thymopeptides (s.c., 1.6 mg/d) to restore immune function; however,
this approach was only marginally successful during the acute phase
(Fig. 4). The white blood cell count
of the patient was decreased in the first 6 days, largely resulting
from the loss of neutrophils, and the platelet count was also lower
than normal (Fig. 5). The patient
was given granulocyte-macrophage colony-stimulating factor at 200
mg/day to stimulate neutrophils. Two drops of antibiotics (whole
body piperacillin i.v. 4.5 g, levofloxacin eye drops) were
administered every 4 h to protect the eyes from nitrogen mustard
injury, and potential infection. After being treated for 32 days,
the patient fully recovered and was discharged from hospital.
Informed consent was obtained from the patient for publishing this
case and the associated images.
Discussion
One of the most severe side effects of nitrogen and
sulfur mustard is toxicity to the respiratory tract (4,8).
Transdermal injection of nitrogen mustard into rats causes alveolar
epithelial cell injury and interalveolar septal thickening
(9). These physiological changes
cause decreased lung compliance and end-tidal volume, as well as
tissue damping and elastance (10).
Nitrogen mustard impairs pulmonary function by inducing lung
inflammation and oxidative stress (8). Aminoguanidine, a nitric oxide synthase
inhibitor, has been reported to alleviate acute lung inflammation
and fibrosis caused by nitrogen mustard, suggesting that nitric
oxide pathways may play a critical role in nitrogen mustard-induced
acute lung injury (11). Nitrogen
mustard also increases levels of connective tissue growth factor
and matrix metalloproteinase-9 in rats (10). Osterlund et al (12) have shown that the activation of
extracellular signal-regulated kinase 1/2, p38 mitogen-activated
protein kinases and nuclear factor-κB are involved in nitrogen
mustard-induced injury to human lung epithelial cells in
vitro. Activation of these pathways causes subsequent
elevations of inflammatory mediators, including tumor necrosis
factor-α and intercellular adhesion molecule-1. Ucar et al
(13) reported that melatonin, an
antioxidant molecule and peroxynitrite scavenger, could reduce
nitrogen mustard-induced toxicity in the lungs by restoration of
oxidative and nitrosative stress markers.
Nitrogen mustard has a high affinity for DNA guanine
residues and forms adducts and crosslinks with DNA, RNA and
proteins; therefore, nitrogen mustard is potentially mutagenic and
carcinogenic (14). Overexpression
of the glutathione-S-transferase (GST) subfamily member GSTA2 has
been found to protect cells against nitrogen mustard-induced cell
cycle arrest and apoptosis (15).
Recently, Inturi et al (16)
studied the mechanism of DNA repair following nitrogen
mustard-induced double-strand breaks. They demonstrated that
homologous recombination repair pathways were critical in DNA
repair following nitrogen mustard toxicity, which could be useful
in developing novel therapeutic strategies against nitrogen
mustard-associated DNA damage. In addition, DNA repair following
DNA damage caused by the nitrogen mustard HN-1 depends on the
pathway affecting base excision repair, but the repair process
associated with HN-2 primarily requires the activation of a
nucleotide excision repair pathway, suggesting that HN-1 and HN-2
may induce different types of cellular damage (17).
As there is no known antidote specifically for
toxicity induced by nitrogen mustard, the most efficient treatment
to decrease tissue damage is to reduce absorption by using water or
potassium permanganate (18). Other
methods include organ support therapy, prevention of infection and
nutritional support. To the best of our knowledge, there is no case
report in recent years regarding human nitrogen mustard exposure.
In the present case, the patient presented with symptoms of severe
respiratory failure in the first 48 h, with a reduced white blood
cell count and complications that included pneumothorax and
mediastinal emphysema. Therapies including respiratory support
treatment, sputum drainage and airway pressure control played a
large role in the successful treatment in this case.
When the temperature is >70°C, nitrogen mustard
is in the gaseous state; therefore, at this temperature, nitrogen
mustard vapor can potentially be inhaled. The nitrogen mustard in
the gaseous state can cause necrosis or apoptosis of bronchial
epithelial cells (19). Once
nitrogen mustards bind to proteins in the human body, it will be
slowly released from the tissues or blood, resulting in multiple
organ injuries. The myelosuppression was severe in the present
case, and it took 7–10 days for the platelets and white blood cells
to recover. Hugel et al (20)
reported the occurrence of sulfur mustard-induced neutrophil
apoptosis. The patient in the present case had symptoms of ocular
tissue injury following exposure to nitrogen mustards. Antibiotics
were administered to protect the eyes from nitrogen mustard injury
and the potential infection; the ocular symptoms disappeared on day
16.
In conclusion, nitrogen mustards can cause severe
respiratory injury and they can also damage hematopoietic lineages
and immune cells. Timely comprehensive treatment is required to
minimize the toxicity caused by nitrogen mustards. The present
study demonstrated the importance of multidisciplinary treatments
in the intensive care unit.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301619) and
Shenyang Science and Technology plan projects (grant no.
F13-220-9-11).
References
|
1
|
Ma HJ, Zhao G, Shi F and Wang YX: Eruptive
cherry angiomas associated with vitiligo: Provoked by topical
nitrogen mustard? J Dermatol. 33:877–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abel EA, Sendagorta E and Hoppe RT:
Cutaneous malignancies and metastatic squamous cell carcinoma
following topical therapies for mycosis fungoides. J Am Acad
Dermatol. 14:1029–1038. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu LL, Zheng S, Wei H, Hong YX, Zhang L,
Zhang L, Chen HD and Gao XH: Multiple cutaneous malignancies and
cherry hemangiomas in a vitiligo patient treated with topical
nitrogen mustard. Dermatol Ther. 27:52–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinberger B, Laskin JD, Sunil VR, Sinko
PJ, Heck DE and Laskin DL: Sulfur mustard-induced pulmonary injury:
Therapeutic approaches to mitigating toxicity. Pulm Pharmacol Ther.
24:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malaviya R, Sunil VR, Cervelli J, Anderson
DR, Holmes WW, Conti ML, Gordon RE, Laskin JD and Laskin DL:
Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol
Appl Pharmacol. 248:89–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shakarjian MP, Heck DE, Gray JP, Sinko PJ,
Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL and
Laskin JD: Mechanisms mediating the vesicant actions of sulfur
mustard after cutaneous exposure. Toxicol Sci. 114:5–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang GQ and Xia ZF: Tissue injury by hot
fluid containing nitrogen mustard. Burns. 33:923–926. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sunil VR, Vayas KN, Cervelli JA, Malaviya
R, Hall L, Massa CB, Gow AJ, Laskin JD and Laskin DL:
Pentoxifylline attenuates nitrogen mustard-induced acute lung
injury, oxidative stress and inflammation. Exp Mol Pathol.
97:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macit E, Yaren H, Aydin I, Kunak ZI, Yaman
H, Onguru O, Uysal B, Korkmaz A, Turel S and Kenar L: The
protective effect of melatonin and S-methylisothiourea treatments
in nitrogen mustard induced lung toxicity in rats. Environ Toxicol
Pharmacol. 36:1283–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunil VR, Patel KJ, Shen J, Reimer D, Gow
AJ, Laskin JD and Laskin DL: Functional and inflammatory
alterations in the lung following exposure of rats to nitrogen
mustard. Toxicol Appl Pharmacol. 250:10–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malaviya R, Venosa A, Hall L, Gow AJ,
Sinko PJ, Laskin JD and Laskin DL: Attenuation of acute nitrogen
mustard-induced lung injury, inflammation and fibrogenesis by a
nitric oxide synthase inhibitor. Toxicol Appl Pharmacol.
265:279–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Osterlund C, Lilliehöök B,
Ekstrand-Hammarström B, Sandström T and Bucht A: The nitrogen
mustard melphalan activates mitogen-activated phosphorylated
kinases (MAPK), nuclear factor-kappaB and inflammatory response in
lung epithelial cells. J Appl Toxicol. 25:328–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ucar M, Korkmaz A, Reiter RJ, Yaren H,
Oter S, Kurt B and Topal T: Melatonin alleviates lung damage
induced by the chemical warfare agent nitrogen mustard. Toxicol
Lett. 173:124–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramsay DL, Meller JA and Zackheim HS:
Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol
Clin North Am. 9:1031–1056. 1995.PubMed/NCBI
|
|
15
|
Xie J, Shults K, Flye L, Jiang F, Head DR
and Briggs RC: Overexpression of GSTA2 protects against cell cycle
arrest and apoptosis induced by the DNA inter-strand crosslinking
nitrogen mustard, mechlorethamine. J Cell Biochem. 95:339–351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inturi S, TewariSingh N, Agarwal C, White
CW and Agarwal R: Activation of DNA damage repair pathways in
response to nitrogen mustard-induced DNA damage and toxicity in
skin keratinocytes. Mutat Res 763–764. 53–63. 2014. View Article : Google Scholar
|
|
17
|
DeAlencar TA, Leitão AC and Lage C:
Nitrogen mustard- and half-mustard-induced damage in Escherichia
coli requires different DNA repair pathways. Mutat Res.
582:105–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eldad A, Weinberg A, Breiterman S, Chaouat
M, Palanker D and Ben-Bassat H: Early nonsurgical removal of
chemically injured tissue enhances wound healing in partial
thickness burns. Burns. 24:166–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rappeneau S, BaezaSquiban A, Jeulin C and
Marano F: Protection from cytotoxic effects induced by the nitrogen
mustard mechlorethamine on human bronchial epithelial cells in
vitro. Toxicol Sci. 54:212–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hugel B, Weltin D, Holl V, Marchal J,
Dufour P, Freyssinet JM and Bischoff PL: Assessment of apoptosis
occurring in spleen cells from nitrogen mustard-treated or
gamma-irradiated mice. Anticancer Res. 18:3289–3294.
1998.PubMed/NCBI
|