Introduction
Saliva, which is produced by the salivary glands,
consists of mucus, various electrolytes, glycoproteins, enzymes,
and antibacterial compounds. Therefore, dysfunction or disruption
of saliva production presents a significant clinical concern. In
particular, hyposalivation, which is a characteristic of
xerostomia, may significantly reduce the quality of life of
patients (1,2). Hyposalivation is most common in
patients with Sjögren's Syndrome (3), ectodermal dysplasias (4–6), head
and neck cancer following γ-irradiation therapy (7), or as a side effect of various
medications. With regards to therapeutic strategies for the
treatment of these diseases, previous studies have adopted
regenerative medicine strategies using stem cell sources in order
to engineer artificial salivary tissue that can mitigate the
effects of xerostomia and hyposalivation (8,9). Removed
via lateral parotidectomy, in vitro isolation and
characterization of stem cells from the human parotid gland, has
previously been achieved (10).
Subsequent flow cytometric analysis demonstrated that these stem
cells were strongly positive for the classic mesenchymal stem cell
(MSC) markers: CD13, CD29, CD44, and CD90, and negative for the key
hematopoietic stem cell (HSC) markers, CD34 and CD45. MSCs are
multipotent stem cells capable of differentiating into numerous
cell lineages, including chondrocytes, adipocytes, osteoblasts,
acinar cells, and salivary epithelial cells (11–14)
Therefore, MSCs have been highlighted as powerful candidates for
experimental investigations (in vitro and in vivo)
and clinical treatment due to their anti-inflammatory effects, low
immunogenicity and potential to repair damaged tissues (11–15). In
this regard, the salivary gland-derived stem cells exhibit MSC-like
characteristics, as they can be differentiated into adipogenic,
osteogenic, and chondrogenic lineages. Therefore, MSCs may exert
useful effects for the regeneration and functional restoration of
the salivary gland.
Fluorescent transgenic (Tg) mice have been
extensively used for analyses of gene function, cellular dynamics,
and bioimaging. In addition, Tg mice have been used as in
vivo models for the study of numerous diseases (16). Fluorescent proteins (FPs) span the
entire color spectrum, and may be used to color-code cells of a
specific genotype or phenotype. For example, the behavior of one
cell type labeled with green FP (GFP) can be compared with another
cell type labeled with red FP (RFP) in vivo. Alternatively,
host and donor cells can be differentially labeled with FPs; in
this way, a Tg mouse constitutively expressing GFP can be the
recipient of transplanted cells that express RFP, in order to
visualize the interaction between the two cell types in real time.
The current FP color palette includes modified proteins based on
Aequorea GFP, as well as various FPs that have been cloned
from other marine organisms and improved for live-cell imaging
applications via genetic engineering (17,18). The
mushroom anemone Discosoma striata was the source of an RFP
known as DsRed (19); subsequently,
a much brighter dimeric RFP, called tdTomato, was generated
(20). This probe is useful for
applications that require minimal exposure to excitation
illumination to maintain cell viability. The authors of the present
study previously generated a series of fluorescent Tg mouse lines
using the pronuclear injection-based targeted transgenesis (PITT)
method, and demonstrated that these mice exhibit a high level of
transgene expression (21). In
addition, unlike those developed by random-integration-based
transgenesis, the Rosa26CAG::FP mice generated by
the PITT method exhibited stable, reproducible and uniform FP
expression in various organs, including the liver, kidney and
intestine, indicating the potential of these mice for use in
chimeric and transplantation analyses (22).
The present study established an MSC line, GManSV,
using submandibular gland (Gman) fibroblast like-cells from
Rosa26CAG::tdTomato Tg mice (tdTomato mice),
which were immortalized by transfection with a plasmid expressing
SV40 large T antigen. The aim was to evaluate the applicability of
GManSV cells for use in kinetic studies of MSC in vitro and
in vivo.
Materials and methods
Rosa26CAG::tdTomato Tg mice
(tdTomato mice)
The study was approved by the ethics committee of
the Animal Studies Committee at Iwate Medical University (nos.
21-039 and 23-075; Center for In Vivo Science, Iwate Medical
University, Yahaba, Iwate, Japan). tdTomato mice were maintained by
crossbreeding homozygous mutant mice. C57BL/6 wild-type and
tdTomato mice were maintained at the Iwate Medical University
Center for In Vivo Science under standard conditions (23°C; 12-h
light/dark cycle) with sawdust bedding and food (CE-2; CLEA Japan,
Inc., Tokyo, Japan) and water ad libitum. The tdTomato mice
were generated using the PITT method and the red fluorescence of
tdTomato was observed in all organs of the transgenic mice, as
described in a previous study (21).
Preparation and observation of tissue
sections
The tdTomato mice were sacrificed by excessive
inhalation of 90–100% CO2. Whole mice were subsequently
cryo-embedded in 5% carboxymethyl cellulose in hexane cooled by
liquid nitrogen without fixation and decalcification. The samples
were placed in a CM3050S cryostat (cutting edge angle: 7–10°, CT:
−22°C, OT: −22°C; Leica Microsystems GmbH, Wetzlar, Germany) and
10-µm frontal serial cryosections were cut using a TC-65 tungsten
carbide blade (Leica Microsystems GmbH) according to Kawamoto's
film-transfer method (23,24) with Cryofilm TYPE I-B (Leica
Microsystems GmbH). Neighboring serial sections were counterstained
with hematoxylin-eosin (Wako Pure Chemical Industries, Inc., Osaka,
Japan). The red fluorescence of tdTomato in the prepared sections
was observed using a VIOREVO BZ-9000 fluorescence microscope
(Keyence Corporation, Osaka, Japan) with a Texas Red fluorescence
filter.
Isolation of primary culture cells
from salivary glands
After the capsula covering the salivary gland of
1-week-old tdTomato mice was removed, the gland body was extracted.
The GMan and sublingual gland (GLin) were segmented as a tissue
mass using scissors. The glands were allowed to attach to the
bottom of a 35 mm plastic cell culture dish, and the cells were
cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone, GE Healthcare Life Sciences, Logan, UT,
USA) for 1 week at 37°C in a humidified atmosphere containing 5%
CO2. Adherent cells that grew out from the tissue mass were then
placed in 90 mm culture dishes and cultured in DMEM supplemented
with 10% FBS. Once the culture reached 80% confluence, the cells
were replated.
Measurement of cell migration in
salivary gland-derived primary cells
Single cell migration was evaluated by measuring the
distance moved by the cell in a time-lapse period using a VIOREVO
BZ-9000 fluorescence microscope (Keyence Corporation) with a Texas
Red fluorescence filter, in addition to an IX70 phase contrast
microscope (Olympus Corporation, Tokyo, Japan). Images were
captured from ~75 primary culture cells derived from GLin or GMan
200 times every 10 min for up to 33 h. Two-dimensional moving
distances of the cells were measured using BZ-II image analysis
software (Keyence Corporation).
Establishment of immortalized GMan
cells
The expanded cells (1.0×105) derived from
the GMan of tdTomato mice were transfected with a pBABE-puro-SV40LT
plasmid containing a puromycin resistance gene (Addgene Inc.,
Cambridge, MA, USA) using Lipofectamine LTX (Invitrogen Life
Technologies, Carlsbad, CA, USA) for 6 h at 37°C in 5% CO2,
according to the manufacturer's instructions. The cells were
exposed to DMEM supplemented with 10% FBS and 1 µg/ml puromycin
(Invitrogen Life Technologies) for 12–15 days. The surviving cells
were trypsinized (Invitrogen Life Technologies) and allowed to grow
in 90 mm culture dishes.
Detection of MSC markers by flow
cytometry
Following selection with puromycin, GMan-derived
cells (1.0×105) were suspended in phosphate-buffered
saline supplemented with 0.5% FBS and 2 mM EDTA. The cells were
incubated with fluorescein isothiocyanate (FITC)-conjugated
anti-mouse Sca-1 (1:10; 130-102-297), anti-mouse CD44 (1:10;
130-102-511), or anti-mouse CD90 (1:10; 130-102-452) antibodies for
1 h at 4°C in the dark. All FITC-conjugated antibodies were
purchased from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany).
Image acquisition was performed with an EPICS XL ADC system
(Beckman Coulter, Inc., Brea, CA, USA).
Osteogenic and adipogenic
differentiation
The in vitro differentiation method used here
was reported in our previous study (25). Briefly, to investigate osteogenic
differentiation, bone matrix mineralization was evaluated using 1%
Alizarin Red S (Sigma-Aldrich) staining. To investigate adipogenic
differentiation, lipid droplets were stained with 0.18% Oil Red O
(Sigma-Aldrich).
Statistical analysis
The experiments were repeated at least three times
and representative images or data are presented. Statistical data
are presented as the mean ± standard deviation. Differences between
samples were statistically analyzed using paired two-tailed
Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Detection of tdTomato fluorescence in
salivary glands
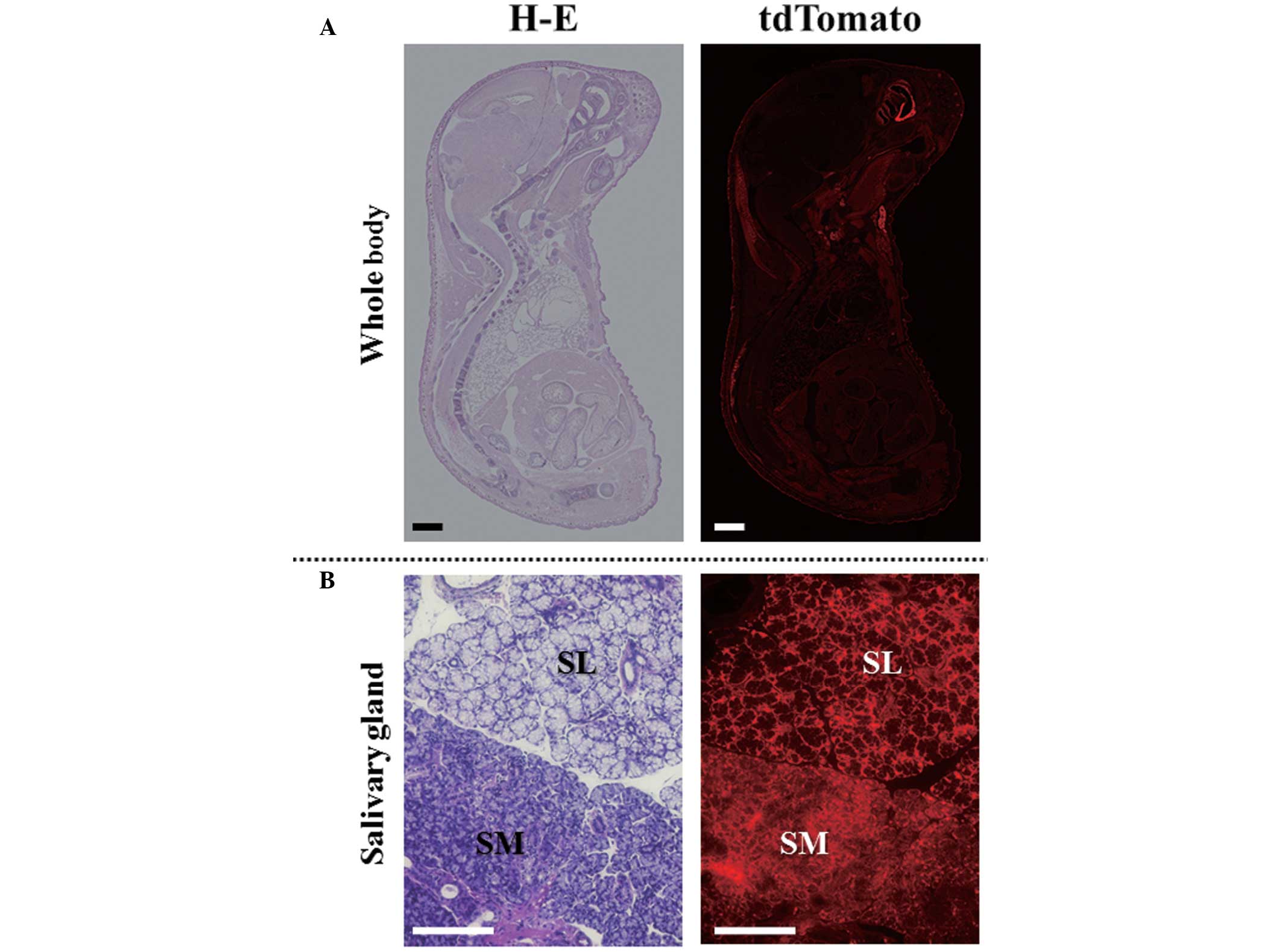
The red fluorescence of tdTomato was detected in
tissue sections prepared from tdTomato mice. As shown in Fig. 1, tdTomato fluorescence was detected
ubiquitously in all organs (Fig.
1A). In addition, fluorescence was detected in the cells that
constitute the salivary gland tissues (Fig. 1B). There was no difference in
fluorescence intensity between the GLin and GMan glands.
Evaluation of the migratory ability of
salivary gland-derived primary cells
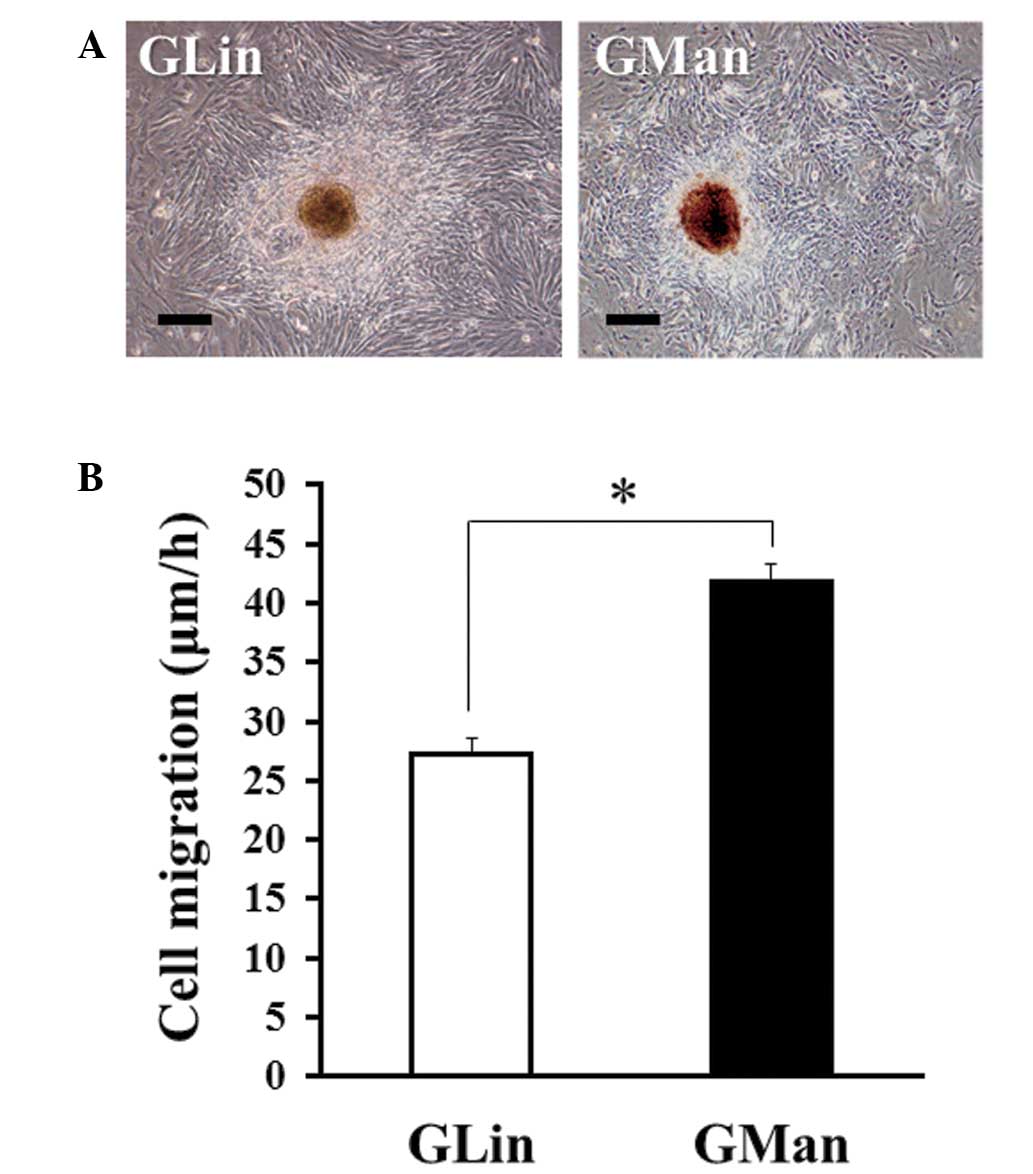
The present study subsequently isolated primary
cultured GLin and GMan cells from the tdTomato mice. As shown in
Fig. 2, the cells that grew out from
the tissue masses possessed a fibroblast-like morphology. Notably,
although the morphology of GLin and GMan cells was similar
(Fig. 2A), the migratory ability of
GMan cells was significantly higher, as compared with that of GLin
cells (Fig. 2B). Usually, MSCs
maintain a high migratory ability (26–28).
Therefore, the GMan cells were selected for the establishment of
the MSC line.
Establishment of an MSC line from GMan
cells
GMan cells were immortalized using SV40 large T
antigen (SV40LT) in order to produce GManSV cells. As shown in
Fig. 3, these cells had a
spindle-shaped fibroblastic morphology (Fig. 3A) and exhibited a strong expression
of tdTomato, as determined by fluorescence imaging (Fig. 3B). Furthermore, the expression of
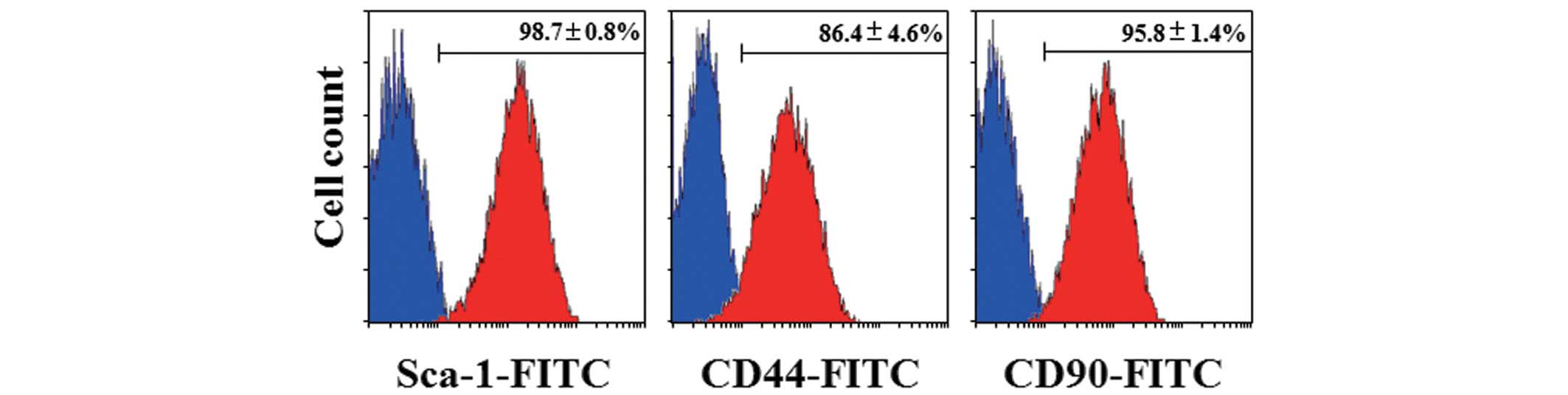
mouse MSC markers and the differentiation potential of the cell
line were determined. As shown in Fig.
4, Sca-1, one of the most functionally critical MSC markers in
mice, was strongly expressed in GManSV cells (Fig. 4A). In addition, CD90 and CD44 were
also highly expressed (Fig. 4B and
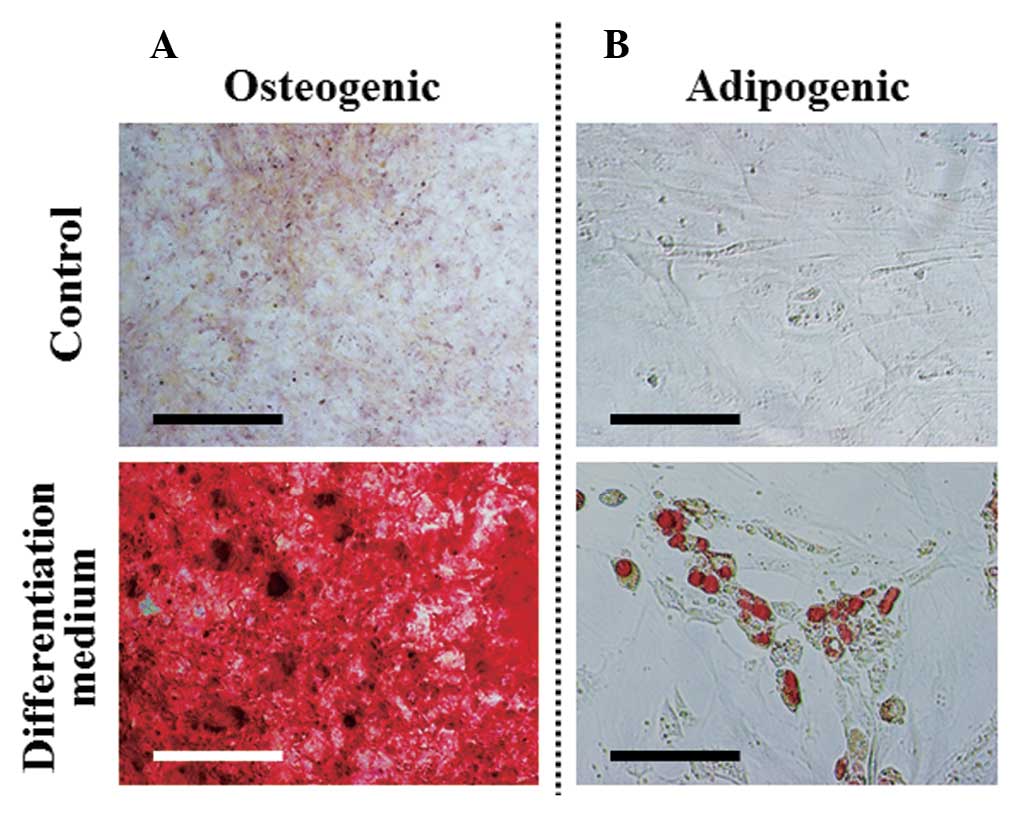
C). In order to determine whether the cell line possessed
multipotent properties, the osteogenic and adipogenic
differentiation potentials of the GManSV cells were evaluated.
Alizarin Red S staining demonstrated that GManSV cells could
differentiate into osteoblasts (Fig.
5A). In addition, Oil Red O staining demonstrated the cells
could differentiate into adipocytes (Fig. 5B). These results strongly suggest
that GManSV cells retain MSC-like multipotency.
Discussion
The present study established an MSC line from
murine GMan cells overexpressing the RFP tdTomato, by
immortalization of the cells with SV40LT. The SV40LT protein was
selected based on its role in the infection of both permissive and
non-permissive cells, leading to the production of progeny virions
and malignant transformation, respectively (29,30). In
addition, SV40LT has been used in cancer research for
immortalization and transformation of cells (31), as primary cells are non-permissive
for SV40LT, and infection with wild-type SV40LT leads to
immortalization and transformation of a small percentage of
infected cells. Such transformation approaches usually involve the
overexpression of oncogenes and/or inactivation of tumor suppressor
genes. Commonly used oncogenes include K-Ras, c-myc,
cyclin-dependent kinase 4, cyclin D1, Bmi-1, and human
papillomavirus 16 E6/E7, and frequently inactivated tumor
suppressor genes include p53, retinoblastoma, and p16INK. In
contrast to the previously mentioned oncogenes, SV40LT expression
generally leads to immortalization, but not transformation
(32–35). Therefore, SV40 is often used in order
to avoid the excessive cellular changes that are associated with
full-blown transformation.
The salivary glands of the majority of mammals
consist of three main cell types: Serous-producing acinar cells,
mucus-producing acinar cells, and myoepithelial cells (36). Serous-producing acinar cells possess
a pyramidal morphology and join together to form spheroidal shapes;
whereas mucus-producing acinar cells are cuboidal in shape and
group together to form tubules. Myoepithelial cells are located
near ductal openings and are associated with the contraction of
ducts, in order to facilitate salivary secretion (37). Furthermore, in normal salivary
glands, the mesenchymal tissue between the acinar-ductal epithelial
structures (38) may also contain
stem cells of mesenchymal origin, as detected in various other
organs, including the bone marrow (39). Since adult stem cells are generally
restricted to cell lineages of the body part of origin, previous
studies have aimed to use salivary gland-derived stem cells, in
order to reduce hyposalivation and restore natural function
(37,40,41).
Stem cells have been isolated and characterized from major salivary
glands of humans and animals (42–44). The
injection of these cells into the major salivary gland parenchyma
has been proposed as an ideal therapeutic strategy for the
restoration of irreversibly damaged gland tissue in patients with
head and neck cancer who have received radiotherapy (45). To date, numerous approaches have been
taken; some groups have harvested cells from the parotid gland
(10) and GMan (45), whereas others have harvested cells
from a combination of both glands (46), and by co-culture (47). In particular, stem cells isolated
from a combination of the human parotid gland and GMan were
demonstrated to partially restore salivary gland function in
radiation-damaged rat salivary glands in vivo (46). Furthermore, previous in vitro
studies have demonstrated that salivary gland-derived stem cells
differentiate into MSC lineages, express MSC markers (CD44, CD49f,
CD90, and CD105) in lieu of HSC markers (CD34 and CD45), and can
differentiate into amylase-expressing cells (48–50).
Successful isolation of MSCs from various organs can
be determined by a combination of criteria including morphology,
surface marker phenotype, and differentiation potential (51,52). A
defined panel of markers has been suggested in humans, however
there are currently no standard criteria that have been proposed in
mice. However, stable identification and isolation of murine MSCs
using a lineage-, and Sca-1+ phenotype has been reported (53,54).
CD90 may function as an activator of stem cell differentiation
(55), and CD44 is a marker that is
commonly detected in human MSCs (56). In the present study, the MSC line
derived from GManSV cells were shown to express Sca-1, CD44, and
CD90 on the cell surface. Furthermore, the GManSV cells retained
the multipotent characteristics of MSCs, as they could
differentiate into both osteogenic and adipogenic lineages.
Therefore, these findings suggested that GManSV cells may be used
as a GMan-derived MSC line for research focused on various
regenerative medicine strategies.
To the best of our knowledge, the present study is
the first to report a stable cell line that has been derived from
tdTomato mice. The tdTomato probe is useful for applications that
require minimal exposure to excitation illumination, in order to
maintain cell viability. By taking advantage of this attribute,
GManSV cells may be adopted as useful tools for kinetic studies of
GMan-derived MSCs for in vitro co-culture systems with other
types of salivary gland-derived cells. These cells may be well
suited for in vivo imaging studies of cell therapy and
regenerative medicine focused on salivary gland diseases.
Acknowledgements
The present study was supported in part by JSPS
KAKENHI (grant no. 25463053 to Dr Naoyuki Chosa and grant no.
23592896 to Professor Akira Ishisaki); and a Grant-in-aid for
Strategic Medical Science Research Centre from the Ministry of
Education, Culture, Sports, Science and Technology of Japan,
2010–2014.
References
|
1
|
Thomson WM, Lawrence HP, Broadbent JM and
Poulton R: The impact of xerostomia on oral-health-related quality
of life among younger adults. Health Qual Life Outcomes. 4:862006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berk L: Systemic pilocarpine for treatment
of xerostomia. Expert Opin Drug Metab Toxicol. 4:1333–1340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pillemer SR, Matteson EL, Jacobsson LT,
Martens PB, Melton LJ III, O'Fallon WM and Fox PC: Incidence of
physician-diagnosed primary Sjögren syndrome in residents of
Olmsted County, Minnesota. Mayo Clin Proc. 76:593–599. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinheiro M and Freire-Maia N: Ectodermal
dysplasias: A clinical classification and a causal review. Am J Med
Genet. 53:153–162. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordgarden H, Storhaug K, Lyngstadaas SP
and Jensen JL: Salivary gland function in persons with ectodermal
dysplasias. Eur J Oral Sci. 111:371–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clauss F, Manière MC, Obry F, Waltmann E,
Hadj-Rabia S, Bodemer C, Alembik Y, Lesot H and Schmittbuhl M:
Dento-craniofacial phenotypes and underlying molecular mechanisms
in hypohidrotic ectodermal dysplasia (HED): A review. J Dent Res.
87:1089–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jensen SB, Pedersen AM, Vissink A,
Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L,
Lopes NN, et al: Salivary Gland Hypofunction/Xerostomia Section,
Oral Care Study Group, Multinational Association of Supportive Care
in Cancer (MASCC)/International Society of Oral Oncology (ISOO): A
systematic review of salivary gland hypofunction and xerostomia
induced by cancer therapies: Prevalence, severity and impact on
quality of life. Support Care Cancer. 18:1039–1060. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelson J, Manzella K and Baker OJ: Current
cell models for bioengineering a salivary gland: A mini-review of
emerging technologies. Oral Dis. 19:236–244. 2013.PubMed/NCBI
|
|
9
|
Ogawa M, Oshima M, Imamura A, Sekin Y,
Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T and
Tsuki T: Functional salivary gland regeneration by transplantation
of a bioengineered organ germ. Nat Commun. 4:24982013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rotter N, Oder J, Schlenke P, Lindner U,
Böhrnsen F, Kramer J, Rohwedel J, Huss R, Brandau S, Wollenberg B
and Lang S: Isolation and characterization of adult stem cells from
human salivary glands. Stem Cells Dev. 17:509–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu
O, Ding G, Gao R, Zhang C, Ding Y, et al: Allogeneic mesenchymal
stem cell treatment alleviates experimental and clinical Sjögren
syndrome. Blood. 120:3142–3151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumita Y, Liu Y, Khalili S, Maria OM, Xia
D, Key S, Cotrim AP, Mezey E and Tran SD: Bone marrow-derived cells
rescue salivary gland function in mice with head and neck
irradiation. Int J Biochem Cell Biol. 43:80–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Chang FH, Chen CY, Huang CY, Hu
FC, Huang WK, Ju SS and Chen MH: Cell therapy for salivary gland
regeneration. J Dent Res. 90:341–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim
HJ, Song SU and Kim YM: Intraglandular transplantation of bone
marrow-derived clonal mesenchymal stem cells for amelioration of
post-irradiation salivary gland damage. Oral Oncol. 49:136–143.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khalili S, Liu Y, Kornete M, Roescher N,
Kodama S, Peterson A, Piccirillo CA and Tran SD: Mesenchymal
stromal cells improve salivary function and reduce lymphocytic
infiltrates in mice with Sjögren's-like disease. PLoS One.
7:e386152012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weigert R, Porat-Shliom N and
Amornphimoltham P: Imaging cell biology in live animals: Ready for
prime time. J Cell Biol. 201:969–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaner NC, Patterson GH and Davidson MW:
Advances in fluorescent protein technology. J Cell Sci.
120:4247–4260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Labas YA, Gurskaya NG, Yanushevich YG,
Fradkov AF, Lukyanov KA, Lukyanov SA and Matz MV: Diversity and
evolution of the green fluorescent protein family. Proc Natl Acad
Sci USA. 99:4256–4261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matz MV, Fradkov AF, Labas YA, Savitsky
AP, Zaraisky AG, Markelov ML and Lukyanov SA: Fluorescent proteins
from nonbioluminescent Anthozoa species. Nat Biotechnol.
17:969–973. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Day RN and Schaufele F: Fluorescent
protein tools for studying protein dynamics in living cells: A
review. J Biomed Opt. 13:0312022008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohtsuka M, Ogiwara S, Miura H, Mizutani A,
Warita T, Sato M, Imai K, Hozumi K, Sato T, Tanaka M, et al:
Pronuclear injection-based mouse targeted transgenesis for
reproducible and highly efficient transgene expression. Nucleic
Acids Res. 38:e1982010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohtsuka M, Miura H, Gurumurthy CB, Kimura
M, Inoko H, Yoshimura S and Sato M: Fluorescent transgenic mice
suitable for multi-color aggregation chimera studies. Cell Tissue
Res. 350:251–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamoto T: Light microscopic
autoradiography for study of early changes in the distribution of
water-soluble materials. J Histochem Cytochem. 38:1805–1814. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamoto T and Shimizu M: A method for
preparing 2 to 50-micron-thick fresh-frozen sections of large
samples and undecalcified hard tissues. Histochem Cell Biol.
113:331–339. 2000.PubMed/NCBI
|
|
25
|
Aomatsu E, Takahashi N, Sawada S, Okubo N,
Hasegawa T, Taira M, Miura H, Ishisaki A and Chosa N: Novel
SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic
differentiation potential in mesenchymal stem cells. Sci Rep.
4:36522014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belema Bedada, Uchida S, Martire A, Kostin
S and Braun T: Efficient homing of multipotent adult mesenchymal
stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem
Cell. 2:566–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
2013.1307632013.PubMed/NCBI
|
|
29
|
Borowiec JA, Dean FB, Bullock PA and
Hurwitz J: Binding and unwinding - how T antigen engages the SV40
origin of DNA replication. Cell. 60:181–184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prives C: The replication functions of
SV40 T antigen are regulated by phosphorylation. Cell. 61:735–738.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arlt VM, Stiborová M, vom Brocke J, Simões
ML, Lord GM, Nortier JL, Hollstein M, Phillips DH and Schmeiser HH:
Aristolochic acid mutagenesis: Molecular clues to the aetiology of
Balkan endemic nephropathy-associated urothelial cancer.
Carcinogenesis. 28:2253–2261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gee CJ and Harris H: Tumorigenicity of
cells transformed by Simian virus 40 and of hybrids between such
cells and normal diploid cells. J Cell Sci. 36:223–240.
1979.PubMed/NCBI
|
|
33
|
Howell N: Suppression of transformation
and tumorigenicity in interspecies hybrids of human
SV40-transformed and mouse 3T3 cell lines. Cytogenet Cell Genet.
34:215–229. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kahn P, Topp WC and Shin S: Tumorigenicity
of SV40-transformed human and monkey cells in immunodeficient mice.
Virology. 126:348–360. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nitta M, Katabuchi H, Ohtake H, Tashiro H,
Yamaizumi M and Okamura H: Characterization and tumorigenicity of
human ovarian surface epithelial cells immortalized by SV40 large T
antigen. Gynecol Oncol. 81:10–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holsinger FC and Bui DT: Anatomy,
function, and evaluation of the salivary glands. In: Salivary
glands disorders. Myers EN and Ferris RL: Springer-Verlag Berlin
Heidelberg; Germany: pp. 1–16. 2007
|
|
37
|
Pringle S, Van Os R and Coppes RP: Concise
review: Adult salivary gland stem cells and a potential therapy for
xerostomia. Stem Cells. 31:613–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carpenter GH and Cotroneo E: Salivary
gland regeneration. Front Oral Biol. 14:107–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coppes RP and Stokman MA: Stem cells and
the repair of radiation-induced salivary gland damage. Oral Dis.
17:143–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okumura K, Nakamura K, Hisatomi Y, Nagano
K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto
T and Endo F: Salivary gland progenitor cells induced by duct
ligation differentiate into hepatic and pancreatic lineages.
Hepatology. 38:104–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hisatomi Y, Okumura K, Nakamura K,
Matsumoto S, Satoh A, Nagano K, Yamamoto T and Endo F: Flow
cytometric isolation of endodermal progenitors from mouse salivary
gland differentiate into hepatic and pancreatic lineages.
Hepatology. 39:667–675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sato A, Okumura K, Matsumoto S, Hattori K,
Hattori S, Shinohara M and Endo F: Isolation, tissue localization,
and cellular characterization of progenitors derived from adult
human salivary glands. Cloning Stem Cells. 9:191–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scott J, Liu P and Smith PM: Morphological
and functional characteristics of acinar atrophy and recovery in
the duct-ligated parotid gland of the rat. J Dent Res.
78:1711–1719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsumoto S, Okumura K, Ogata A, Hisatomi
Y, Sato A, Hattori K, Matsumoto M, Kaji Y, Takahashi M, Yamamoto T,
et al: Isolation of tissue progenitor cells from duct-ligated
salivary glands of swine. Cloning Stem Cells. 9:176–190. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lombaert IM, Brunsting JF, Wierenga PK,
Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G and
Coppes RP: Rescue of salivary gland function after stem cell
transplantation in irradiated glands. PLoS One. 3:e20632008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong J, Baek H, Kim YJ, Choi Y, Lee H,
Lee E, Kim ES, Hah JH, Kwon TK, Choi IJ and Kwon H: Human salivary
gland stem cells ameliorate hyposalivation of radiation-damaged rat
salivary glands. Exp Mol Med. 45:e582013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kawakami M, Ishikawa H, Tachibana T,
Tanaka A and Mataga I: Functional transplantation of salivary gland
cells differentiated from mouse early ES cells in vitro. Hum Cell.
26:80–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lim JY, Yi T, Lee S, Kim J, Kim SN, Song
SU and Kim YM: Establishment and characterization of mesenchymal
stem cell-like clonal stem cells from mouse salivary glands. Tissue
Eng Part C Methods. 21:447–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park YJ, Koh J, Gauna AE, Chen S and Cha
S: Identification of regulatory factors for mesenchymal stem
cell-derived salivary epithelial cells in a co-culture system. PLoS
One. 9:e1121582014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maria OM, Maria AM, Cai Y and Tran SD:
Cell surface markers CD44 and CD166 localized specific populations
ofsalivary acinar cells. Oral Dis. 18:162–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
52
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y,
Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori
T, et al: Prospective identification, isolation, and systemic
transplantation of multipotent mesenchymal stem cells in murine
bone marrow. J Exp Med. 206:2483–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Taichman RS, Wang Z, Shiozawa Y, Jung Y,
Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, et al:
Prospective identification and skeletal localization of cells
capable of multilineage differentiation in vivo. Stem Cells Dev.
19:1557–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen XD, Qian HY, Neff L, Satomura K and
Horowitz MC: Thy-1 antigen expression by cells in the osteoblast
lineage. J Bone Miner Res. 14:362–375. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|