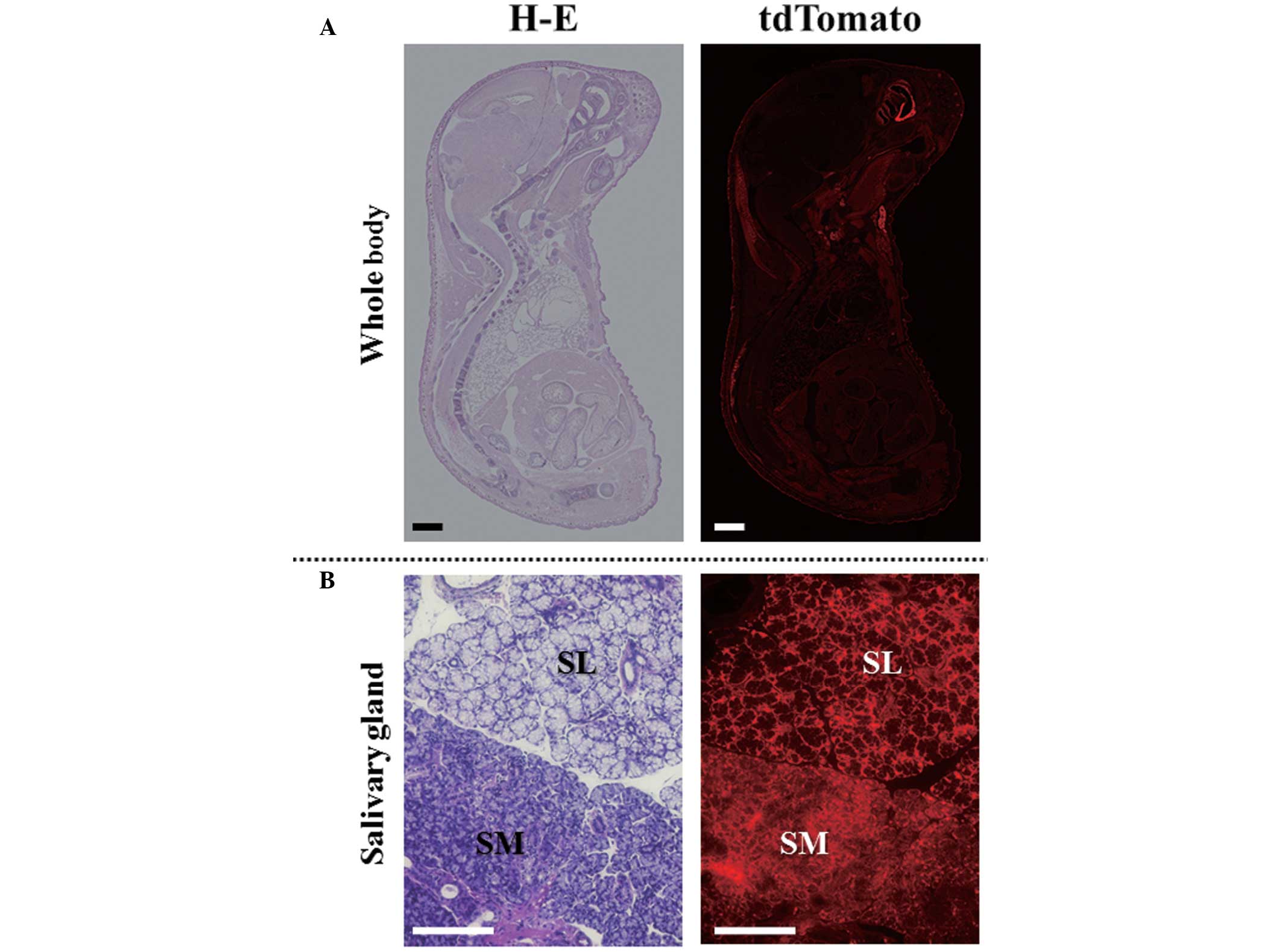
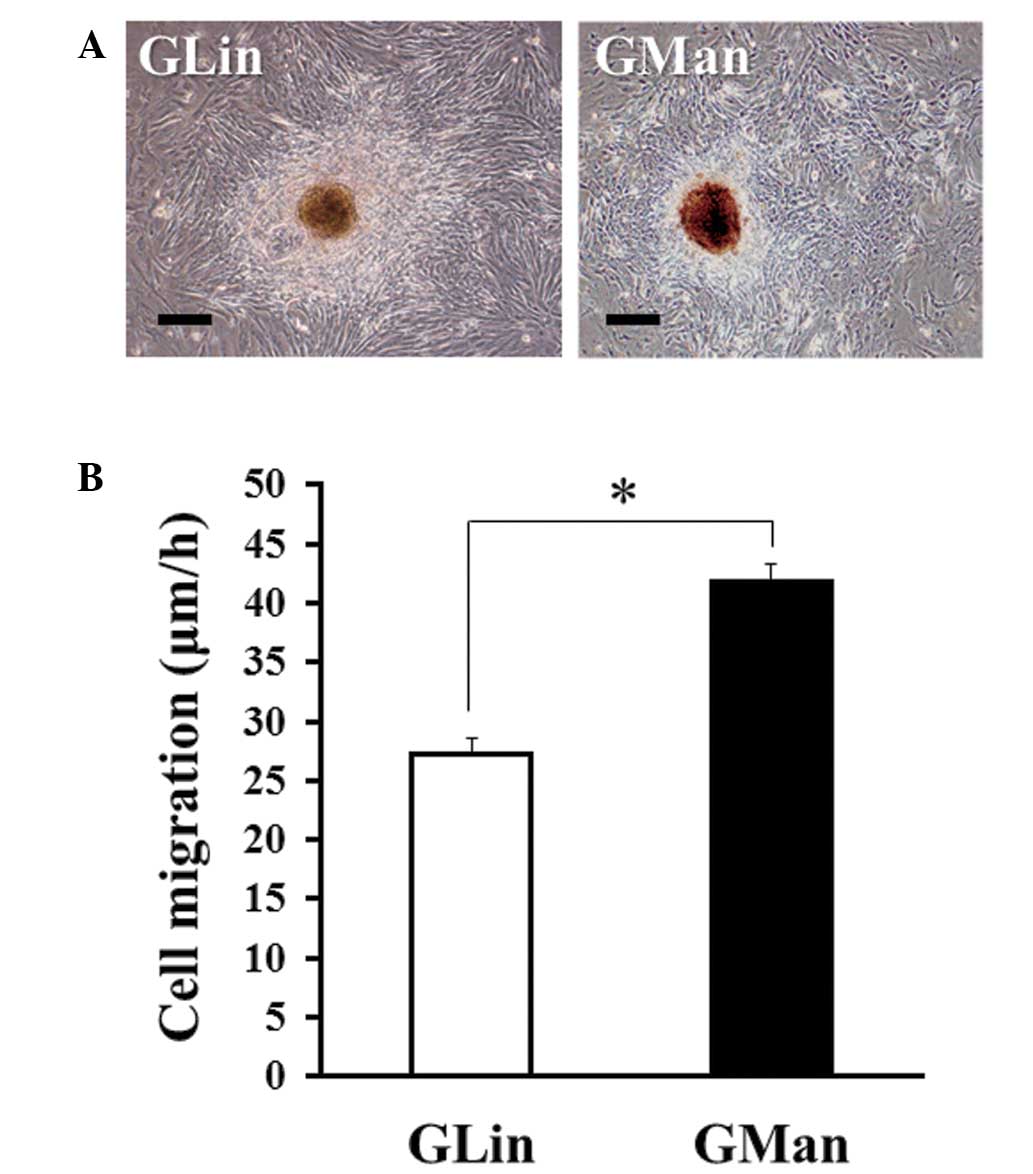
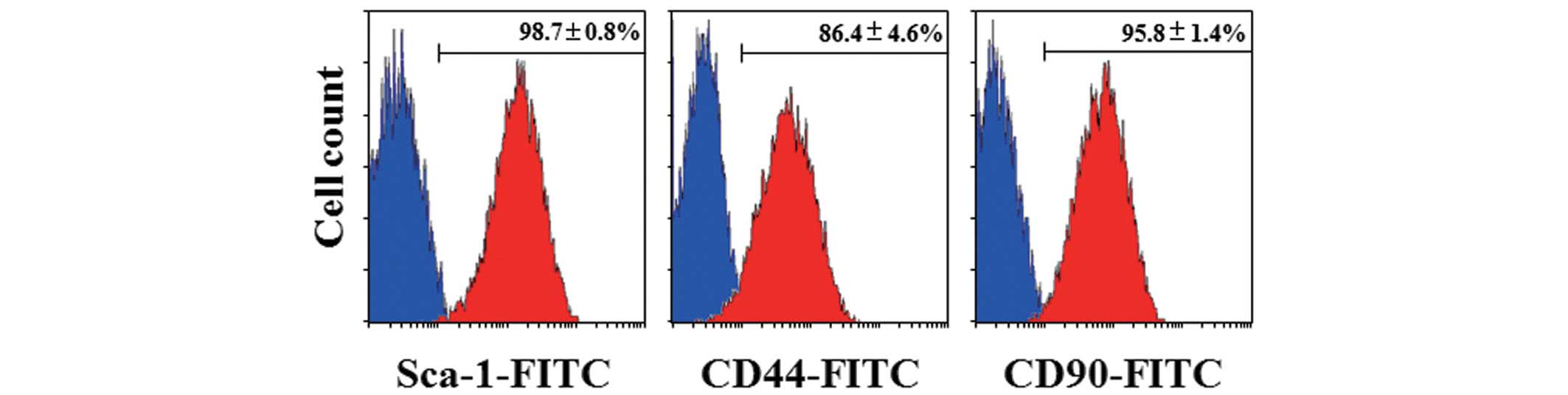
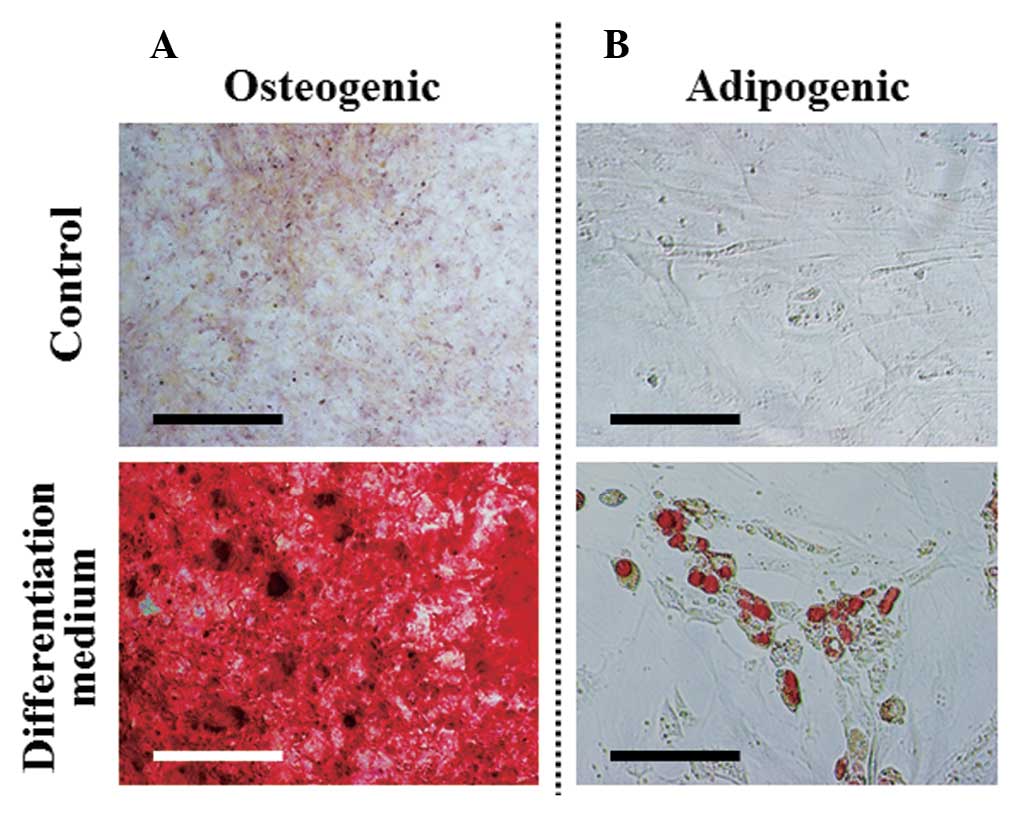
|
1
|
Thomson WM, Lawrence HP, Broadbent JM and
Poulton R: The impact of xerostomia on oral-health-related quality
of life among younger adults. Health Qual Life Outcomes. 4:862006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berk L: Systemic pilocarpine for treatment
of xerostomia. Expert Opin Drug Metab Toxicol. 4:1333–1340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pillemer SR, Matteson EL, Jacobsson LT,
Martens PB, Melton LJ III, O'Fallon WM and Fox PC: Incidence of
physician-diagnosed primary Sjögren syndrome in residents of
Olmsted County, Minnesota. Mayo Clin Proc. 76:593–599. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinheiro M and Freire-Maia N: Ectodermal
dysplasias: A clinical classification and a causal review. Am J Med
Genet. 53:153–162. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordgarden H, Storhaug K, Lyngstadaas SP
and Jensen JL: Salivary gland function in persons with ectodermal
dysplasias. Eur J Oral Sci. 111:371–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clauss F, Manière MC, Obry F, Waltmann E,
Hadj-Rabia S, Bodemer C, Alembik Y, Lesot H and Schmittbuhl M:
Dento-craniofacial phenotypes and underlying molecular mechanisms
in hypohidrotic ectodermal dysplasia (HED): A review. J Dent Res.
87:1089–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jensen SB, Pedersen AM, Vissink A,
Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L,
Lopes NN, et al: Salivary Gland Hypofunction/Xerostomia Section,
Oral Care Study Group, Multinational Association of Supportive Care
in Cancer (MASCC)/International Society of Oral Oncology (ISOO): A
systematic review of salivary gland hypofunction and xerostomia
induced by cancer therapies: Prevalence, severity and impact on
quality of life. Support Care Cancer. 18:1039–1060. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelson J, Manzella K and Baker OJ: Current
cell models for bioengineering a salivary gland: A mini-review of
emerging technologies. Oral Dis. 19:236–244. 2013.PubMed/NCBI
|
|
9
|
Ogawa M, Oshima M, Imamura A, Sekin Y,
Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T and
Tsuki T: Functional salivary gland regeneration by transplantation
of a bioengineered organ germ. Nat Commun. 4:24982013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rotter N, Oder J, Schlenke P, Lindner U,
Böhrnsen F, Kramer J, Rohwedel J, Huss R, Brandau S, Wollenberg B
and Lang S: Isolation and characterization of adult stem cells from
human salivary glands. Stem Cells Dev. 17:509–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu
O, Ding G, Gao R, Zhang C, Ding Y, et al: Allogeneic mesenchymal
stem cell treatment alleviates experimental and clinical Sjögren
syndrome. Blood. 120:3142–3151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumita Y, Liu Y, Khalili S, Maria OM, Xia
D, Key S, Cotrim AP, Mezey E and Tran SD: Bone marrow-derived cells
rescue salivary gland function in mice with head and neck
irradiation. Int J Biochem Cell Biol. 43:80–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Chang FH, Chen CY, Huang CY, Hu
FC, Huang WK, Ju SS and Chen MH: Cell therapy for salivary gland
regeneration. J Dent Res. 90:341–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim
HJ, Song SU and Kim YM: Intraglandular transplantation of bone
marrow-derived clonal mesenchymal stem cells for amelioration of
post-irradiation salivary gland damage. Oral Oncol. 49:136–143.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khalili S, Liu Y, Kornete M, Roescher N,
Kodama S, Peterson A, Piccirillo CA and Tran SD: Mesenchymal
stromal cells improve salivary function and reduce lymphocytic
infiltrates in mice with Sjögren's-like disease. PLoS One.
7:e386152012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weigert R, Porat-Shliom N and
Amornphimoltham P: Imaging cell biology in live animals: Ready for
prime time. J Cell Biol. 201:969–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaner NC, Patterson GH and Davidson MW:
Advances in fluorescent protein technology. J Cell Sci.
120:4247–4260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Labas YA, Gurskaya NG, Yanushevich YG,
Fradkov AF, Lukyanov KA, Lukyanov SA and Matz MV: Diversity and
evolution of the green fluorescent protein family. Proc Natl Acad
Sci USA. 99:4256–4261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matz MV, Fradkov AF, Labas YA, Savitsky
AP, Zaraisky AG, Markelov ML and Lukyanov SA: Fluorescent proteins
from nonbioluminescent Anthozoa species. Nat Biotechnol.
17:969–973. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Day RN and Schaufele F: Fluorescent
protein tools for studying protein dynamics in living cells: A
review. J Biomed Opt. 13:0312022008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohtsuka M, Ogiwara S, Miura H, Mizutani A,
Warita T, Sato M, Imai K, Hozumi K, Sato T, Tanaka M, et al:
Pronuclear injection-based mouse targeted transgenesis for
reproducible and highly efficient transgene expression. Nucleic
Acids Res. 38:e1982010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohtsuka M, Miura H, Gurumurthy CB, Kimura
M, Inoko H, Yoshimura S and Sato M: Fluorescent transgenic mice
suitable for multi-color aggregation chimera studies. Cell Tissue
Res. 350:251–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamoto T: Light microscopic
autoradiography for study of early changes in the distribution of
water-soluble materials. J Histochem Cytochem. 38:1805–1814. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamoto T and Shimizu M: A method for
preparing 2 to 50-micron-thick fresh-frozen sections of large
samples and undecalcified hard tissues. Histochem Cell Biol.
113:331–339. 2000.PubMed/NCBI
|
|
25
|
Aomatsu E, Takahashi N, Sawada S, Okubo N,
Hasegawa T, Taira M, Miura H, Ishisaki A and Chosa N: Novel
SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic
differentiation potential in mesenchymal stem cells. Sci Rep.
4:36522014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belema Bedada, Uchida S, Martire A, Kostin
S and Braun T: Efficient homing of multipotent adult mesenchymal
stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem
Cell. 2:566–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
2013.1307632013.PubMed/NCBI
|
|
29
|
Borowiec JA, Dean FB, Bullock PA and
Hurwitz J: Binding and unwinding - how T antigen engages the SV40
origin of DNA replication. Cell. 60:181–184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prives C: The replication functions of
SV40 T antigen are regulated by phosphorylation. Cell. 61:735–738.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arlt VM, Stiborová M, vom Brocke J, Simões
ML, Lord GM, Nortier JL, Hollstein M, Phillips DH and Schmeiser HH:
Aristolochic acid mutagenesis: Molecular clues to the aetiology of
Balkan endemic nephropathy-associated urothelial cancer.
Carcinogenesis. 28:2253–2261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gee CJ and Harris H: Tumorigenicity of
cells transformed by Simian virus 40 and of hybrids between such
cells and normal diploid cells. J Cell Sci. 36:223–240.
1979.PubMed/NCBI
|
|
33
|
Howell N: Suppression of transformation
and tumorigenicity in interspecies hybrids of human
SV40-transformed and mouse 3T3 cell lines. Cytogenet Cell Genet.
34:215–229. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kahn P, Topp WC and Shin S: Tumorigenicity
of SV40-transformed human and monkey cells in immunodeficient mice.
Virology. 126:348–360. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nitta M, Katabuchi H, Ohtake H, Tashiro H,
Yamaizumi M and Okamura H: Characterization and tumorigenicity of
human ovarian surface epithelial cells immortalized by SV40 large T
antigen. Gynecol Oncol. 81:10–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holsinger FC and Bui DT: Anatomy,
function, and evaluation of the salivary glands. In: Salivary
glands disorders. Myers EN and Ferris RL: Springer-Verlag Berlin
Heidelberg; Germany: pp. 1–16. 2007
|
|
37
|
Pringle S, Van Os R and Coppes RP: Concise
review: Adult salivary gland stem cells and a potential therapy for
xerostomia. Stem Cells. 31:613–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carpenter GH and Cotroneo E: Salivary
gland regeneration. Front Oral Biol. 14:107–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coppes RP and Stokman MA: Stem cells and
the repair of radiation-induced salivary gland damage. Oral Dis.
17:143–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okumura K, Nakamura K, Hisatomi Y, Nagano
K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto
T and Endo F: Salivary gland progenitor cells induced by duct
ligation differentiate into hepatic and pancreatic lineages.
Hepatology. 38:104–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hisatomi Y, Okumura K, Nakamura K,
Matsumoto S, Satoh A, Nagano K, Yamamoto T and Endo F: Flow
cytometric isolation of endodermal progenitors from mouse salivary
gland differentiate into hepatic and pancreatic lineages.
Hepatology. 39:667–675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sato A, Okumura K, Matsumoto S, Hattori K,
Hattori S, Shinohara M and Endo F: Isolation, tissue localization,
and cellular characterization of progenitors derived from adult
human salivary glands. Cloning Stem Cells. 9:191–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scott J, Liu P and Smith PM: Morphological
and functional characteristics of acinar atrophy and recovery in
the duct-ligated parotid gland of the rat. J Dent Res.
78:1711–1719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsumoto S, Okumura K, Ogata A, Hisatomi
Y, Sato A, Hattori K, Matsumoto M, Kaji Y, Takahashi M, Yamamoto T,
et al: Isolation of tissue progenitor cells from duct-ligated
salivary glands of swine. Cloning Stem Cells. 9:176–190. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lombaert IM, Brunsting JF, Wierenga PK,
Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G and
Coppes RP: Rescue of salivary gland function after stem cell
transplantation in irradiated glands. PLoS One. 3:e20632008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong J, Baek H, Kim YJ, Choi Y, Lee H,
Lee E, Kim ES, Hah JH, Kwon TK, Choi IJ and Kwon H: Human salivary
gland stem cells ameliorate hyposalivation of radiation-damaged rat
salivary glands. Exp Mol Med. 45:e582013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kawakami M, Ishikawa H, Tachibana T,
Tanaka A and Mataga I: Functional transplantation of salivary gland
cells differentiated from mouse early ES cells in vitro. Hum Cell.
26:80–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lim JY, Yi T, Lee S, Kim J, Kim SN, Song
SU and Kim YM: Establishment and characterization of mesenchymal
stem cell-like clonal stem cells from mouse salivary glands. Tissue
Eng Part C Methods. 21:447–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park YJ, Koh J, Gauna AE, Chen S and Cha
S: Identification of regulatory factors for mesenchymal stem
cell-derived salivary epithelial cells in a co-culture system. PLoS
One. 9:e1121582014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maria OM, Maria AM, Cai Y and Tran SD:
Cell surface markers CD44 and CD166 localized specific populations
ofsalivary acinar cells. Oral Dis. 18:162–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
52
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y,
Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori
T, et al: Prospective identification, isolation, and systemic
transplantation of multipotent mesenchymal stem cells in murine
bone marrow. J Exp Med. 206:2483–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Taichman RS, Wang Z, Shiozawa Y, Jung Y,
Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, et al:
Prospective identification and skeletal localization of cells
capable of multilineage differentiation in vivo. Stem Cells Dev.
19:1557–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen XD, Qian HY, Neff L, Satomura K and
Horowitz MC: Thy-1 antigen expression by cells in the osteoblast
lineage. J Bone Miner Res. 14:362–375. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|