Introduction
Bladder cancer is the second most common
genitourinary cancer and the eleventh most common malignancy
worldwide. It has been estimated that ~429,800 new cases of bladder
cancer and 165,100 bladder cancer-related mortalities occurred in
2012 worldwide, accounting for 3% of the total new cancer cases
(1). Furthermore, bladder cancer is
now the most frequent cancer of the urinary tract and the seventh
most frequent malignancy in men worldwide. An increasing trend in
the incidence and mortality rates of bladder cancer has been noted
in the past 30 years (2). Among all
newly diagnosed cases, ~75% of patients exhibit a
non-muscle-invasive tumor without invasion into bladder detrusor;
however, ~25% of patients present with muscle-invasive bladder
tumors, which means that the bladder detrusor has been invaded by
the cancer (3). Furthermore, between
50 and 70% of the cases of non-muscle-invasive tumors will recur
following transurethral resection, despite intravesical
chemotherapy or Bacillus Calmette-Guérin immunotherapy, and 10–20%
will progress to muscle-invasive bladder tumors in 5 years
(4). Notably, lymph node metastases
are confirmed by pathological examination in ~25% of patients with
muscle-invasive cancer who have undergone radical cystectomy, while
it is estimated that one-third of patients with muscle-invasive
cancer have undetected metastases at first diagnosis (5). There is, therefore, an active interest
in the study of the molecular mechanism of bladder cancer.
Focal adhesion kinase (FAK) is a 125-kDa tyrosine
kinase found in focal adhesions. It is a member of a growing family
that includes several structurally distinct protein tyrosine
kinases, such as related adhesion focal tyrosine kinase and
calcium-dependent protein tyrosine kinase (6). FAK can be activated by specific
extracellular stimuli, such as integrins and certain growth factors
(7). FAK is encoded by the protein
tyrosine kinase 2 (PTK2) gene, which is located at human chromosome
region 8q24.3. It has been found that the region is commonly
amplified in several types of cancer, such as serous ovarian
(8) and gastric (9) cancer. Abnormally increased FAK
expression has additionally been found in several types of cancer
(10), such as cervical (11), ovarian (12), breast (13) and lung (14) cancer. FAK plays an important role in
signal transduction in the tumor microenvironment (15), as it is a multifunctional scaffolding
molecule that links transmembrane input signals from growth factor
receptors and integrins to intracellular effectors, such as c-Jun
N-terminal kinase and phosphatidylinositol 3-kinase/Akt (PI3K/Akt)
(16). FAK proteins are known to
regulate cell survival and apoptosis via several pathways, such as
PI3K/Akt (17).
An increased FAK mRNA level is found in bladder
cancer (10); however, no study to
date has been performed to determine whether FAK is associated with
the survival and apoptosis of bladder cancer cells. The aim of the
present study, therefore, was to explore the potential role of FAK
in the apoptosis of bladder cancer cells.
Materials and methods
Cell culture and reagents
The T24 human bladder cancer cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium
(Gibco-BRL, Grand Island, NY, USA), supplemented with 10% fetal
bovine serum at 37°C in a humidified 5% CO2/95% air
atmosphere. The FAK inhibitor PF-573228 (PF-228), the Src inhibitor
PP2 and the PI3K inhibitor LY294002 were obtained from
Sigma-Aldrich (St. Louis, MO, USA). TGFβ was obtained from
Peprotech, Inc. (Rocky Hill, NJ, USA).
Preparation and transfection of small
interfering RNAs (siRNAs)
siRNAs against FAK [sense, 5′-UAA UAC UCG CUC CAU
UGC ACC(dT)(dT)-3′ and antisense, 5′-GGU GCA AUG GAG CGA GUA
UUA(dT)(dT)-3′] were designed as described previously (18). The siRNA duplexes were chemically
synthesized by GeneChem Co., Ltd. (Shanghai, China). Transfections
were performed in six-well plates (Corning Costar, Cambridge, MA,
USA) with Lipofectamine™ 2000 transfection reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
The treated cells were collected and the total RNA
was prepared using TRIzol® reagent (Invitrogen Life Technologies).
Subsequently, reverse transcription was performed with 2 µg total
RNA using a one-step RT-PCR system (Invitrogen Life Technologies).
qPCR was conducted in an Applied Biosystems 7300 Real-time PCR
Instrument (Applied Biosystems Life Technologies, Foster City, CA,
USA). The PCR cycling conditions were as follows: 95°C for 3 min,
followed by cycles at 95°C for 10 sec and 60°C for 20 sec, then
72°C for 15 sec. The PCR products were detected using SYBR® Green
dye (Applied Biosystems Life Technologies) according to the
manufacturer's instructions. Specific oligonucleotide primers were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Relative
quantification of the mRNA levels of target genes was performed
using the 2−ΔΔCT method, as described previously
(19).
Western blot analysis
Western blot analysis was performed as described
previously (20). Total protein (50
µg) from each sample was loaded for SDS-PAGE. The membrane was
exposed on an X-ray film (Eastman Kodak Co., Rochester, NY, USA)
using enhanced chemiluminescence western blot detection reagents
(Pierce Biotechnology, Inc., Rockford, IL, USA). Cumulative gray
levels of all bands were calculated using ImageJ software (National
Institutes of Health, Bethesda, MD, USA) for further relative
quantitative analysis. Primary antibodies that were specific
against following proteins were used: Rabbit polyclonal FAK (#3285;
1:1,000), rabbit polyclonal phosphorylated (p)FAK (#3283; 1:1,000),
rabbit mAb Src (#2109; 1:1,000), rabbit polyclonal pSrc (#2101;
1:1,000), mouse mAb Akt (#2966; 1:500), mouse mAb pAkt (#4051;
1:1,000), mouse mAb caspase-3 (#9668; 1:1,000) and cleaved
caspase-3 (#9579; 1:1,000) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA) and rabbit polyclonal β-actin (sc-1616-R;
1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
membrane was incubated with primary antibodies at 4°C overnight.
Horseradish peroxidase-labeled secondary antibodies were obtained
from Santa Cruz Biotechnology, Inc.
In situ deoxynucleotidyl
transferase-mediated dUTP-biotin nick end labeling assay
In situ TUNEL assay was employed to detect
the apoptotic cells. T24 cells were grown on slides and fixed with
4% buffered formaldehyde. TUNEL assay was performed using the in
situ Cell Death Detection kit (Boehringer Mannheim GmbH,
Mannheim, Germany) as described previously (18). The slides were counterstained with
hematoxylin. The apoptotic cells were stained brown under the
microscope.
Annexin V labeling
To measure the numbers and the ratio of apoptotic
cells, the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) was
employed as described previously (18). The treated cells were stained with
FITC, Annexin V and propidium iodide (PI), and the stained cells
were analyzed using a FACSort™ flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) and evaluated with the CellQuest™ software
system (BD Biosciences).
Cell viability assay
Cell viability was determined using the MTT assay as
described previously (18). Cells
were cultured in 96-well plates at a density of 2×104
cells/well. The cell viability was measured using the MTT assay.
Cells were incubated with 10 µl 0.5 mg/ml MTT at 37°C for 4 h. The
formazan crystals were dissolved using 200 µl dimethylsulfoxide and
quantified by measuring absorbance at 570 nm.
Statistical analysis
All experiments were repeated at least three times,
and the data were analyzed using the SPSS 12.0 statistical software
package (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance and the Student's t-test were employed to compare
the data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockdown of FAK induces apoptosis in
T24 bladder cancer cells
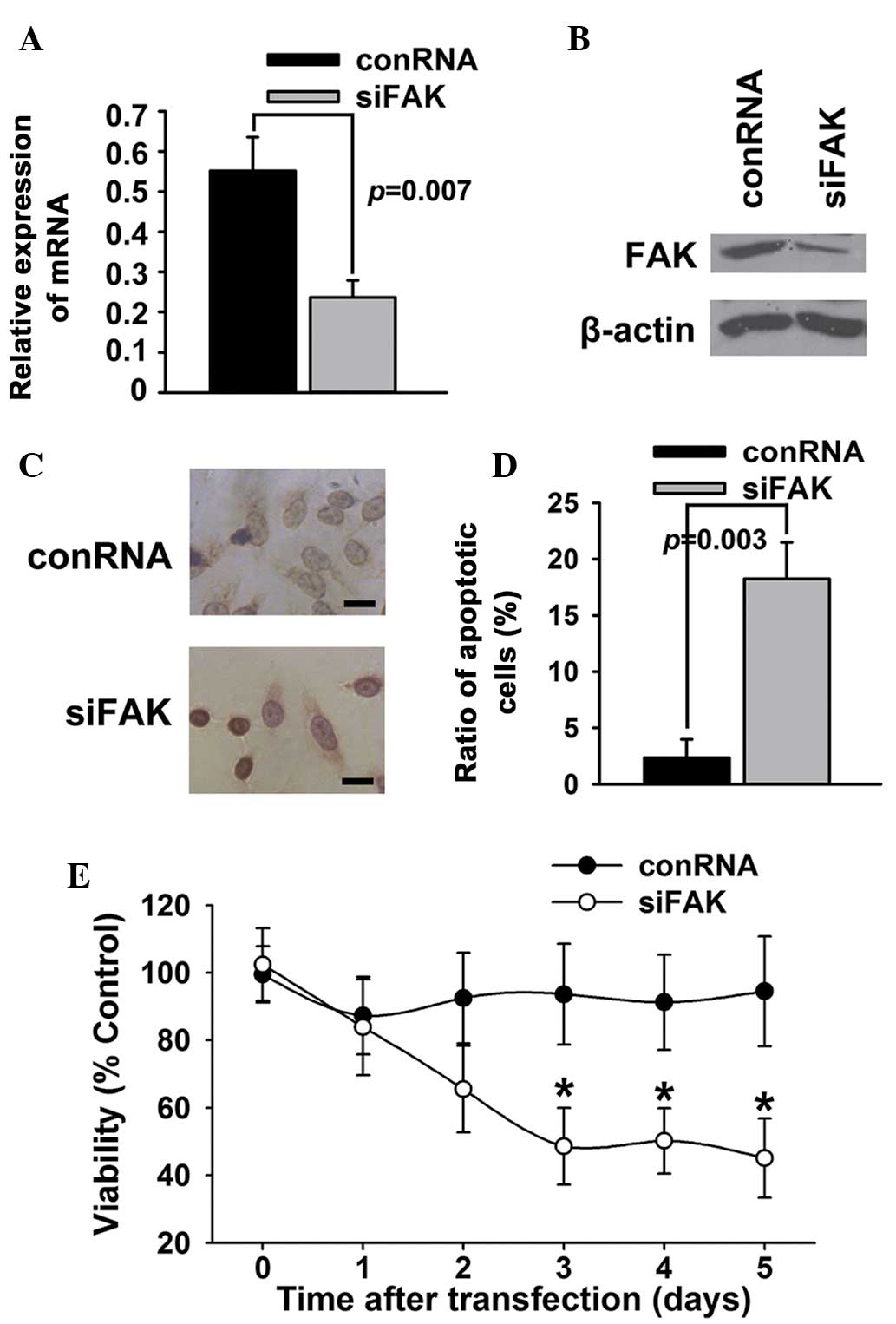
To identify whether FAK affected the survival or
apoptosis of T24 bladder cancer cells, FAK expression in T24
bladder cancer cells was knocked down using a specific siRNA duplex
targeting FAK. As shown in Fig. 1A and
B, the siRNA duplex caused a marked decrease of FAK mRNA and
protein expression in the T24 bladder cancer cells. The results of
TUNEL (Fig. 1C) and Annexin V/PI
(Fig. 1D) assays showed that siRNA
against FAK significantly increased the apoptosis of T24 bladder
cancer cells. The results of the MTT assays (Fig. 1E) showed that inhibition of FAK
expression by siRNA decreased the viability of the T24 cells
compared with control RNA.
Suppression of FAK phosphorylation
induces apoptosis in T24 bladder cancer cells
To further investigate whether the inhibition of FAK
tyrosine phosphorylation could promote the apoptosis of T24 bladder
cancer cells, PF-228, a selective inhibitor of FAK, was employed to
inhibit the transforming growth factor-β (TGFβ)-induced tyrosine
phosphorylation of FAK. The results of the TUNEL (Fig. 2A) and Annexin V/PI (Fig. 2B) assays showed that PF-228 was able
to induce the apoptosis of T24 bladder cancer cells. The western
blotting results indicated that PF-228 significantly reduced the
TGFβ-induced phosphorylation of FAK and Src, suppressed the
phosphorylation of Akt, an accepted signal of cell
survival/apoptosis, and activated caspase-3, an important
apoptosis-related protein (Fig. 2C and
D). The results of the MTT assays (Fig. 2E) showed that inhibition of FAK by
PF-228 decreased the viability of the T24 cells compared with the
control.
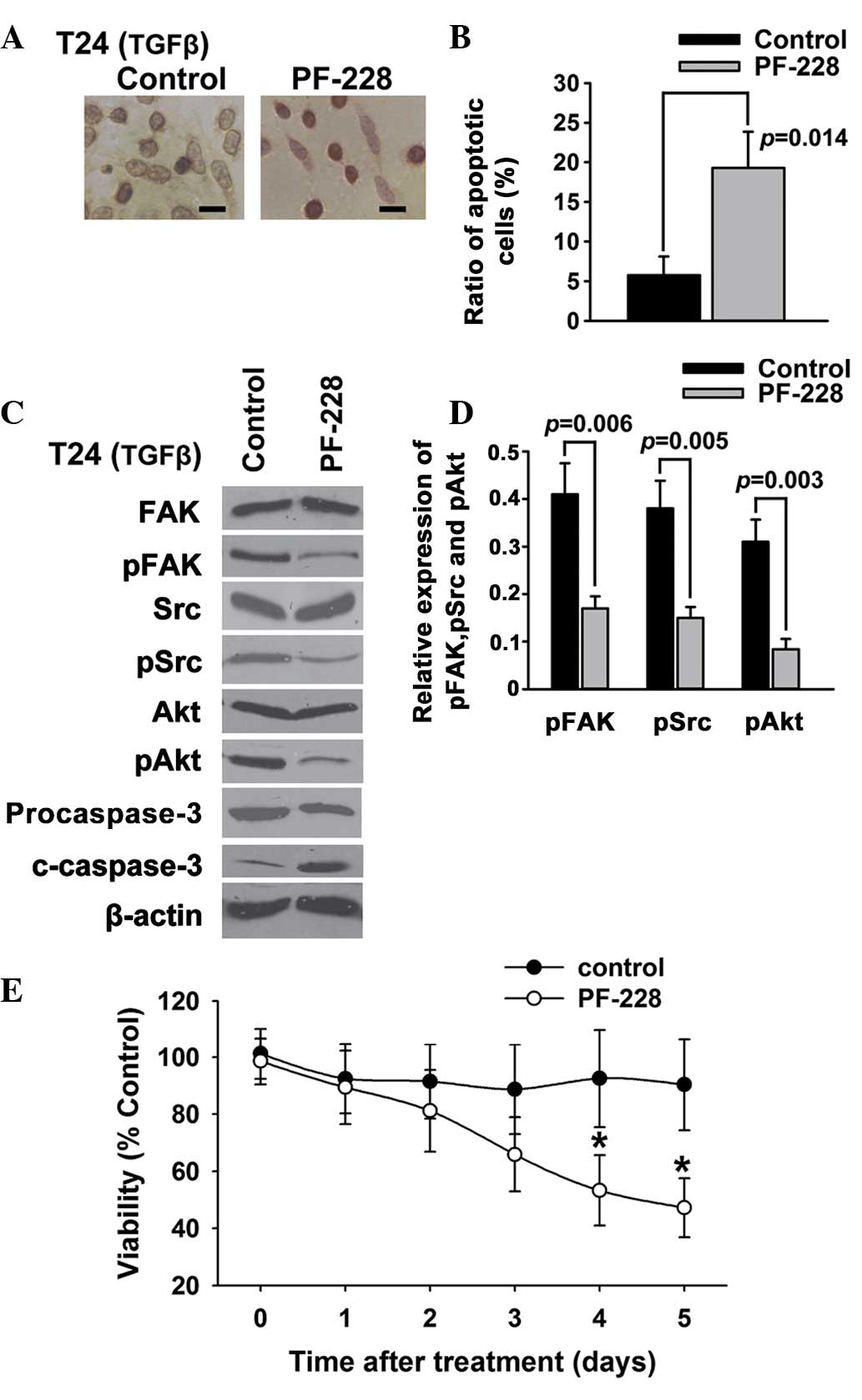 | Figure 2.Suppression of FAK phosphorylation
induces apoptosis in T24 bladder cancer cells. T24 bladder cancer
cells were treated with PF-228 and 5 ng/ml TGFβ. (A and B) Cell
apoptosis was examined using (A) deoxynucleotidyl
transferase-mediated dUTP-biotin nick end labeling assay and (B)
Annexin V/propidium iodide. (C and D) The expression of FAK, pFAK,
Src, pSrc, Akt, pAkt, caspase-3 and c-caspase-3 was examined using
western blotting. (E) Cell survival was examined using an MTT
assay. Scale bar, 200 µm. *P<0.05. FAK, focal adhesion kinase;
pFAK, phosphorylated FAK; c-caspase-3, cleaved caspase-3; TGFβ,
transforming growth factor-β. |
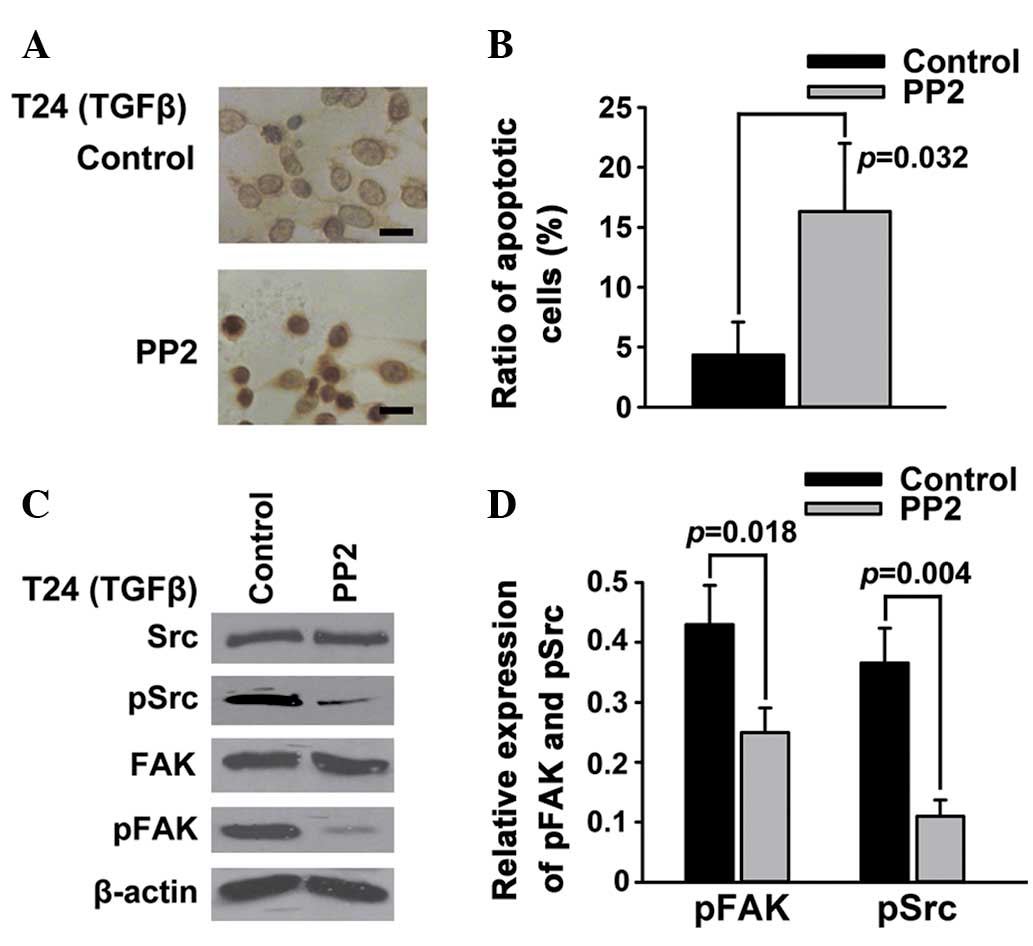
Src is an important mediator of
FAK-regulated apoptosis in T24 bladder cancer cells
FAK is a substrate for the oncogene protein tyrosine
kinase Src (21). To further whether
the inhibition of Src phosphorylation could also promote the
apoptosis of T24 bladder cancer cells, PP2, a selective inhibitor
of Src, was employed to inhibit the TGFβ-induced phosphorylation of
Src. The results of the TUNEL (Fig.
3A) and Annexin V/PI (Fig. 3B)
assays showed that PP2, similarly to the FAK inhibitor PF-228, was
able to induce the apoptosis of T24 bladder cancer cells. The
western blotting results demonstrated that PP2 significantly
reduced the TGFβ-induced phosphorylation of not only Src but also
FAK (Fig. 3C and D).
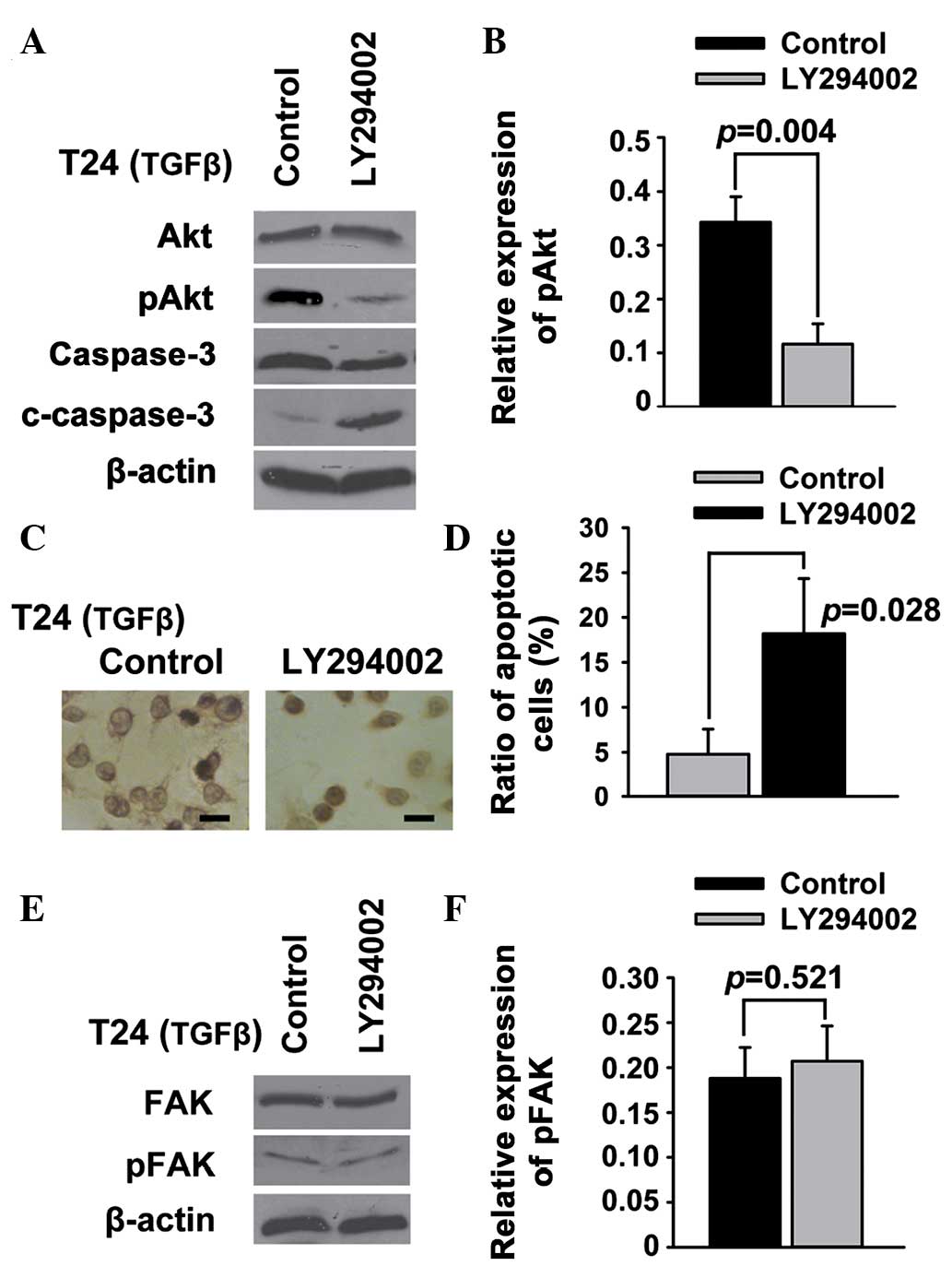
PI3K/Akt signaling acts downstream of
FAK to regulate apoptosis in T24 bladder cancer cells
PI3K/Akt is a potent pathway of survival and
apoptosis (22), and FAK is believed
to be an upstream signal protein of the PI3K/Akt pathway (23); therefore, it was investigated whether
PI3K/Akt acted downstream of FAK to regulate the apoptosis of T24
bladder cancer cells. Western blot analysis showed that Akt and FAK
phosphorylation was suppressed by PF-228 (Fig. 2C and D). In addition, LY294002
significantly downregulated pAkt in the T24 cells (Fig. 4A and B). The results of the TUNEL
(Fig. 4C) and Annexin V/PI (Fig. 4D) assays showed that LY294002 was
able to induce the apoptosis of T24 cells. Conversely, the
expression of general and tyrosine-phosphorylated FAK was not
regulated by inhibiting PI3K/Akt in T24 cells (Fig. 4E and F).
Discussion
FAK was first described by Linder and Burr in 1988
as a 120-kDa protein that was one of a large number of tyrosine
phosphoproteins in Rous sarcoma virus-transformed chicken embryo
fibroblasts (24). Reynolds et
al (25) reported the finding of
a 120-kDa protein whose phosphorylation was greatly enhanced in
cells expressing activated, oncogenic Src in 1989, and the same
research group generated monoclonal antibodies against the 120-kDa
protein in 1990 (26). Furthermore,
a 120-kDa protein was described to tyrosine-phosphorylate in
fibronectin-stimulated cells by Guan et al in 1991 (27). The protein was located in focal
contacts, where it codistributed with β1 integrins. Phosphorylation
of the protein was correlated with subsequent cell spreading. It
was suggested that the interaction of β1 integrins with
extracellular ligands, such as fibronectin, turned on the
phosphorylation of the 120-kDa protein, which may have been
involved in the responses of cells to attachment. Schaller et
al (28) identified a 125-kDa
phosphotyrosine-containing protein as a tyrosine phosphatase
substrate of v-Src in chicken embryo cells in 1992. In the study by
Schaller et al, cDNA of the protein was isolated, and the
predicted structure of the new protein, which was the prototype for
an additional family of protein-tyrosine kinases, was found. Since
the protein was localized to focal adhesions, they named the new
protein focal adhesion kinase. In the same year, Hanks et al
(29) found that the activation of
FAK via tyrosine phosphorylation was an important early step in
intracellular signal transduction pathways in response to
extracellular stimuli with the extracellular matrix. In 1994,
Schaller et al (30)
suggested that Tyr-397 was a major site of FAK autophosphorylation
and that FAK was physically associated with Src via their SH2
domains. More recent research has shown that FAK links
extracellular stimuli, such as integrins and growth factors, to
intracellular signaling and regulates cell movement, migration,
invasion, survival and cancer stem cell self-renewal (10,31). The
aim of the present study was to determine if FAK regulated the
survival and apoptosis of bladder cancer cells.
Apoptosis is the process of programmed cell death
that may occur in multicellular organisms and is characterized by
morphological changes of the cells, include blebbing, cell
shrinkage, nuclear fragmentation, chromatin condensation and
chromosomal DNA fragmentation (32).
The activation of FAK by the interaction of extracellular matrix
signals and integrins has been shown to be accompanied by
suppressed apoptosis in diverse cell types (33). Notably, FAK was found to be
tyrosine-phosphorylated by oxidative stress prior to the occurrence
of apoptosis (34). Sonoda et
al (34) found that FAK retained
tyrosine phosphorylation at least up to 5 h and gradually lost
tyrosine phosphorylation after 8 h, concomitant with apoptosis.
While FAK was inhibited by inhibitor of protein tyrosine kinases or
antisense oligonucleotide against FAK, apoptosis was accelerated.
Sonoda et al suggested that tyrosine phosphorylation of FAK
played a suppressive role in cell apoptosis. Since it has been
shown that specific siRNAs targeting mRNA of the PTK2 gene are
effective in inhibiting the expression of FAK (35–39), RNA
interference was employed in the present study as a potent tool to
explore the role of FAK in the survival and apoptosis of bladder
cancer cells. The results showed that apoptosis was induced in T24
bladder cancer cells when FAK was suppressed by siRNA targeting
FAK. Similar results were observed following the administration of
PF-228, an exclusive inhibitor of FAK tyrosine phosphorylation.
These results suggest that not only the general expression but also
the tyrosine phosphorylation level of FAK is associated with the
apoptosis of bladder cancer cells.
FAK is an important mediator of TGFβ signaling
(40). TGFβ predominantly regulates
FAK via tyrosine phosphorylation of FAK (41). FAK was first described as a 120-kDa
protein that was tyrosine-phosphorylated following induction by Src
(24). FAK is autophosphorylated at
Tyr-397 due to input signals from integrins and growth factor
receptors, to which Src proteins subsequently bind. Afterwards,
FAK-Src complexes are formed, and FAK is phosphorylated at Tyr-576
and Tyr-577. The complexes play an important role in mediating the
extracellular signal to downstream molecules such as extracellular
signal-regulated kinases and paxillin (42,43);
therefore, Src is one of the most important regulatory proteins in
FAK-related signals. PP2 is a selective inhibitor of Src family
members and is able to block the Tyr-416 phosphorylation of Src
(44). In the present study,
therefore, the effect of Src tyrosine dephosphorylation on FAK and
the apoptosis of bladder cancer cells was explored using PP2. The
results showed that PP2 was able to induce the apoptosis of T24
cells, while tyrosine phosphorylation of not only Src but also FAK
was inhibited in TGFβ-stimulated bladder cancer cells.
Notably, Wen et al (45) found that FAK is cleaved into two
different fragments in early apoptosis, which is mediated by
caspase-7 and caspase-3 (45). They
suggested that the disruption of FAK may contribute to the
morphological changes of cells in apoptosis. Levkau et al
(46) found that cleavage of FAK
affected its association with signaling and other cytoskeletal
components of the focal adhesion complex, such as paxillin. They
suggested that the caspase-mediated cleavage of FAK disturbed
survival signals from the extracellular matrix and propagated the
cell death program. Van de Water et al (47) found that the inhibition of caspase
activity blocked FAK cleavage and apoptosis, but not FAK
dephosphorylation. They suggested that caspases are required for
FAK cleavage, but not for FAK dephosphorylation, during apoptosis.
Sonoda et al (23)
demonstrated that the tyrosine phosphorylation of FAK, the
association of FAK with PI3K and the serine phosphorylation of Akt
occurred during oxidative stress-induced apoptosis. They suggested
that FAK was an upstream signal protein of the PI3K/Akt pathway
during oxidative stress-induced apoptosis. In another study, Sonoda
et al (48) found that the
PI3K/Akt survival pathway was activated, and the activation of
procaspase-3 to caspase-3 was inhibited, in FAK-transfected cells,
which had resistance to apoptotic stimuli. They suggested that FAK
activated the PI3K/Akt survival pathway, as well as its downstream
signals, and finally inhibited apoptosis by blocking the caspase-3
cascade. The role of the PI3K/Akt pathway and caspase-3 during the
apoptosis of bladder cancer cells induced by inhibiting FAK was
therefore explored in the present study. The results showed that
the phosphorylation of Akt was suppressed and the activation of
procaspase-3 to caspase-3 was induced during apoptosis, while FAK
was dephosphorylated by a tyrosine phosphorylation inhibitor.
LY294002, a common inhibitor of PI3K, was additionally utilized to
investigate the effect of inhibiting the PI3K/Akt pathway on the
general expression and tyrosine phosphorylation level of FAK. The
results showed that inhibiting the PI3K/Akt pathway was able to
induce the apoptosis of T24 cells, but did not regulate either the
general expression or the tyrosine phosphorylation of FAK. These
results suggested that PI3K/Akt acted downstream of FAK signaling
to regulate apoptosis in bladder cancer cells.
In this study, the regulatory role of FAK signaling
on the apoptosis and survival of bladder cancer cells was
demonstrated. Both the knockdown of FAK and the suppression of FAK
phosphorylation were able to induce apoptosis in bladder cancer
cells. Caspase-3 was activated during the apoptosis induced by the
suppression of FAK phosphorylation. Src was involved in
FAK-regulated apoptosis in bladder cancer cells, while the
suppression of Src phosphorylation was able to inhibit FAK tyrosine
phosphorylation and induce apoptosis. Furthermore, PI3K/Akt
signaling was inhibited via the suppression of FAK tyrosine
phosphorylation. Conversely, neither the expression of general nor
tyrosine-phosphorylated FAK was regulated by inhibiting PI3K/Akt.
These results suggested that PI3K/Akt acted downstream of FAK
signaling to regulate apoptosis in bladder cancer cells.
Collectively, the data indicate that FAK is an important regulator
of apoptosis and survival signaling in bladder cancer cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 30901499 and 81272862),
the Zhejiang Provincial Natural Science Foundation of China (nos.
LY12H16020 and LY13H160020) and the Zhejiang Provincial Medical
Science and Technology Program (nos. 2013RCA014 and
2014KYA117).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgan TM, Keegan KA and Clark PE: Bladder
cancer. Curr Opin Oncol. 23:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Rhijn BW, Burger M, Lotan Y, Solsona
E, Stief CG, Sylvester RJ, Witjes JA and Zlotta AR: Recurrence and
progression of disease in non-muscle-invasive bladder cancer: From
epidemiology to treatment strategy. Eur Urol. 56:430–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee CT, Dunn RL, Ingold C, Montie JE and
Wood DP Jr: Early-stage bladder cancer surveillance does not
improve survival if high-risk patients are permitted to progress to
muscle invasion. Urology. 69:1068–1072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prout GR Jr, Griffin PP and Shipley WU:
Bladder carcinoma as a systemic disease. Cancer. 43:2532–2539.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lechertier T and Hodivala-Dilke K: Focal
adhesion kinase and tumour angiogenesis. J Pathol. 226:404–412.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okamoto H, Yasui K, Zhao C, Arii S and
Inazawa J: PTK2 and EIF3S3 genes may be amplification targets at
8q23-q24 and are associated with large hepatocellular carcinomas.
Hepatology. 38:1242–1249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH, Lee BL, Yoon J, Kim J, Kim MA,
Yang HK and Kim WH: Focal adhesion kinase (FAK) gene amplification
and its clinical implications in gastric cancer. Hum Pathol.
41:1664–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oktay MH, Oktay K, Hamele-Bena D, Buyuk A
and Koss LG: Focal adhesion kinase as a marker of malignant
phenotype in breast and cervical carcinomas. Hum Pathol.
34:240–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas N Network:
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu H, Wang L, Gao W, Meng J, Dai B, Wu S,
Minna J, Roth JA, Hofstetter WL, Swisher SG and Fang B: IGFBP2/FAK
pathway is causally associated with dasatinib resistance in
non-small cell lung cancer cells. Mol Cancer Ther. 12:2864–2873.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schober M and Fuchs E: Tumor-initiating
stem cells of squamous cell carcinomas and their control by TGF-β
and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad
Sci USA. 108:10544–10549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wendt MK, Smith JA and Schiemann WP:
Transforming growth factor-β-induced epithelial-mesenchymal
transition facilitates epidermal growth factor-dependent breast
cancer progression. Oncogene. 29:6485–6498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo M and Guan JL: Focal adhesion kinase:
A prominent determinant in breast cancer initiation, progression
and metastasis. Cancer Lett. 289:127–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sima N, Wang W, Kong D, Deng D, Xu Q, Zhou
J, Xu G, Meng L, Lu Y, Wang S and Ma D: RNA interference against
HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical
cancer cells and apoptosis via upregulation of Rb and p53.
Apoptosis. 13:273–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li BH, Zhou JS, Ye F, Cheng XD, Zhou CY,
Lu WG and Xie X: Reduced miR-100 expression in cervical cancer and
precursors and its carcinogenic effect through targeting PLK1
protein. Eur J Cancer. 47:2166–2174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sima N, Wang S, Wang W, Kong D, Xu Q, Tian
X, Luo A, Zhou J, Xu G, Meng L, et al: Antisense targeting human
papillomavirus type 16 E6 and E7 genes contributes to apoptosis and
senescence in SiHa cervical carcinoma cells. Gynecol Oncol.
106:299–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan JL and Shalloway D: Regulation of
focal adhesion-associated protein tyrosine kinase by both cellular
adhesion and oncogenic transformation. Nature. 358:63881992.
View Article : Google Scholar
|
|
22
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sonoda Y, Watanabe S, Matsumoto Y,
Aizu-Yokota E and Kasahara T: FAK is the upstream signal protein of
the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen
peroxide-induced apoptosis of a human glioblastoma cell line. J
Biol Chem. 274:10566–10570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linder ME and Burr JG: Nonmyristoylated
p60v-src fails to phosphorylate proteins of 115–120 kDa in chicken
embryo fibroblasts. Proc Natl Acad Sci USA. 85:2608–2612. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reynolds AB, Roesel DJ, Kanner SB and
Parsons JT: Transformation-specific tyrosine phosphorylation of a
novel cellular protein in chicken cells expressing oncogenic
variants of the avian cellular src gene. Mol Cell Biol. 9:629–638.
1989.PubMed/NCBI
|
|
26
|
Kanner SB, Reynolds AB, Vines RR and
Parsons JT: Monoclonal antibodies to individual
tyrosine-phosphorylated protein substrates of oncogene-encoded
tyrosine kinases. Proc Natl Acad Sci USA. 87:3328–3332. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan JL, Trevithick JE and Hynes RO:
Fibronectin/integrin interaction induces tyrosine phosphorylation
of a 120-kDa protein. Cell Regul. 2:951–964. 1991.PubMed/NCBI
|
|
28
|
Schaller MD, Borgman CA, Cobb BS, Vines
RR, Reynolds AB and Parsons JT: pp125FAK a structurally distinctive
protein-tyrosine kinase associated with focal adhesions. Proc Natl
Acad Sci USA. 89:5192–5196. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanks SK, Calalb MB, Harper MC and Patel
SK: Focal adhesion protein-tyrosine kinase phosphorylated in
response to cell attachment to fibronectin. Proc Natl Acad Sci USA.
89:8487–8491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schaller MD, Hildebrand JD, Shannon JD,
Fox JW, Vines RR and Parsons JT: Autophosphorylation of the focal
adhesion kinase, pp125FAK, directs SH2-dependent binding of
pp60src. Mol Cell Biol. 14:1680–1688. 1994.PubMed/NCBI
|
|
31
|
Park MS, Kim YH and Lee JW: FAK mediates
signal crosstalk between type II collagen and TGF-beta 1 cascades
in chondrocytic cells. Matrix Biol. 29:135–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duvall E, Wyllie AH and Morris RG:
Macrophage recognition of cells undergoing programmed cell death
(apoptosis). Immunology. 56:351–358. 1985.PubMed/NCBI
|
|
33
|
Frisch SM, Vuori K, Ruoslahti E and
Chan-Hui PY: Control of adhesion-dependent cell survival by focal
adhesion kinase. J Cell Biol. 134:793–799. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sonoda Y, Kasahara T, Yokota-Aizu E, Ueno
M and Watanabe S: A suppressive role of p125FAK protein tyrosine
kinase in hydrogen peroxide-induced apoptosis of T98G cells.
Biochem Biophys Res Commun. 241:769–774. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clemente CF, Tornatore TF, Theizen TH,
Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JR and Franchini KG:
Targeting focal adhesion kinase with small interfering RNA prevents
and reverses load-induced cardiac hypertrophy in mice. Circ Res.
101:1339–1348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duxbury MS, Ito H, Benoit E, Zinner MJ,
Ashley SW and Whang EE: RNA interference targeting focal adhesion
kinase enhances pancreatic adenocarcinoma gemcitabine
chemosensitivity. Biochem Biophys Res Commun. 311:786–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan KT, Cortesio CL and Huttenlocher A:
FAK alters invadopodia and focal adhesion composition and dynamics
to regulate breast cancer invasion. J Cell Biol. 185:357–370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thamilselvan V, Craig DH and Basson MD:
FAK association with multiple signal proteins mediates
pressure-induced colon cancer cell adhesion via a Src-dependent
PI3K/Akt pathway. FASEB J. 21:1730–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Q, Bao S, Song L, Wu Q, Bigner DD,
Hjelmeland AB and Rich JN: Targeting SPARC expression decreases
glioma cellular survival and invasion associated with reduced
activities of FAK and ILK kinases. Oncogene. 26:4084–4094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leask A: Focal adhesion kinase: A key
mediator of transforming growth factor beta signaling in
fibroblasts. Adv Wound Care (New Rochelle). 2:247–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang SE, Xiang B, Zent R, Quaranta V,
Pozzi A and Arteaga CL: Transforming growth factor beta induces
clustering of HER2 and integrins by activating Src-focal adhesion
kinase and receptor association to the cytoskeleton. Cancer Res.
69:475–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schaller MD: Cellular functions of FAK
kinases: Insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chiang GJ, Billmeyer BR, Canes D, Stoffel
J, Moinzadeh A, Austin CA, Kosakowski M, Rieger-Christ KM,
Libertino JA and Summerhayes IC: The src-family kinase inhibitor
PP2 suppresses the in vitro invasive phenotype of bladder carcinoma
cells via modulation of Akt. BJU Int. 96:416–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wen LP, Fahrni JA, Troie S, Guan JL, Orth
K and Rosen GD: Cleavage of focal adhesion kinase by caspases
during apoptosis. J Biol Chem. 272:26056–26061. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Levkau B, Herren B, Koyama H, Ross R and
Raines EW: Caspase-mediated cleavage of focal adhesion kinase
pp125FAK and disassembly of focal adhesions in human endothelial
cell apoptosis. J Exp Med. 187:579–586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van de Water B, Nagelkerke JF and Stevens
JL: Dephosphorylation of focal adhesion kinase (FAK) and loss of
focal contacts precede caspase-mediated cleavage of FAK during
apoptosis in renal epithelial cells. J Biol Chem. 274:13328–13337.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sonoda Y, Matsumoto Y, Funakoshi M,
Yamamoto D, Hanks SK and Kasahara T: Anti-apoptotic role of focal
adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins
and apoptosis suppression by the overexpression of FAK in a human
leukemic cell line, HL-60. J Biol Chem. 275:16309–16315. 2000.
View Article : Google Scholar : PubMed/NCBI
|