Introduction
Cardiac natriuretic peptides (NPs), such as the
atrial NP (ANP) and B-type natriuretic peptide (BNP), as well as
their associated peptides, comprise a complex family of peptide
hormones, which are produced and secreted by the human heart
(1,2). The physiological role of BNP is to
facilitate the adaptation of the myocardium to strain or stress
imposed by a volume and/or pressure load. BNP has emerged as a
promising marker for the diagnosis of heart failure (HF), as well
as the determination of prognosis and monitoring of treatment
effects in adult patients with the disease (3,4).
Prognosis permits clinicians to separate patients
with HF into subgroups based on likely health outcomes. This
separation encourages effective targeting of therapies to subgroups
that are most likely to require and benefit from treatment, while
minimizing risks to the other subgroups, such as adverse effects.
The Canadian National Institute for Health and Clinical Excellence
(NICE) guidelines for adult chronic HF note that higher BNP and
N-terminal proBNP (NT-proBNP) levels are associated with poorer
prognosis in HF. For adults the NICE guidelines recommend
transthoracic Doppler 2D echocardiography and specialist assessment
for persons with suspected HF, and BNP ≥100 pg/ml or NT-proBNP ≥400
pg/ml. The 2013 Canadian guidelines recommend using BNP and
NT-proBNP to obtain prognostic information on patients with HF
(5). In addition, the European HF
guidelines contain a table of prognostic factors for adults, which
includes both peptides (6).
HF is a condition in which the heart cannot, at a
normal filling pressure, pump blood at a rate proportionate to the
demands of the metabolizing tissues (5,7–10). The diagnosis of HF remains a clinical
challenge in all settings, particularly with regard to pediatric
patients. Pneumonia is a frequently encountered disease in
children, and, in severe cases, it may influence their
cardiovascular function or even cause HF. It is usually an
extremely difficult task to determine whether severe pneumonia is
accompanied by HF, mostly due to limited communication between the
pediatrician and the child, but also due to the fact that the
symptoms often manifest more severely than would be expected from
the underlying condition (2). For
that reason, the present study focused on investigating the
differential diagnostic and therapeutic analysis of the plasma BNP
assay levels in pediatric pneumonia accompanied by HF.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of The First People's Hospital of Zhangjiagang
(Zhangjiagang, China), and informed consent was provided by the
parents of the patients. Between August 2012 and February 2014, 80
pediatric patients (<3 years old) hospitalized with pneumonia
met the inclusion criteria and were enrolled in the present study.
Among them were 42 males and 38 females, with a mean age of 1.2±0.9
years. The patients were divided into two groups according to their
diagnosis. In group 1, which comprised patients with simple
pneumonia (24 males and 22 females; mean age, 1.2±0.9 years), all
patients presented with dyspnea, but the dyspnea was not
accompanied by HF. Group 2 comprised patients with pneumonia
accompanied by HF (18 males and 16 females; mean age, 1.1±0.8
years). The diagnostic criteria for pneumonia and HF used in the
present study were based on the Nelson Textbook of Pediatrics
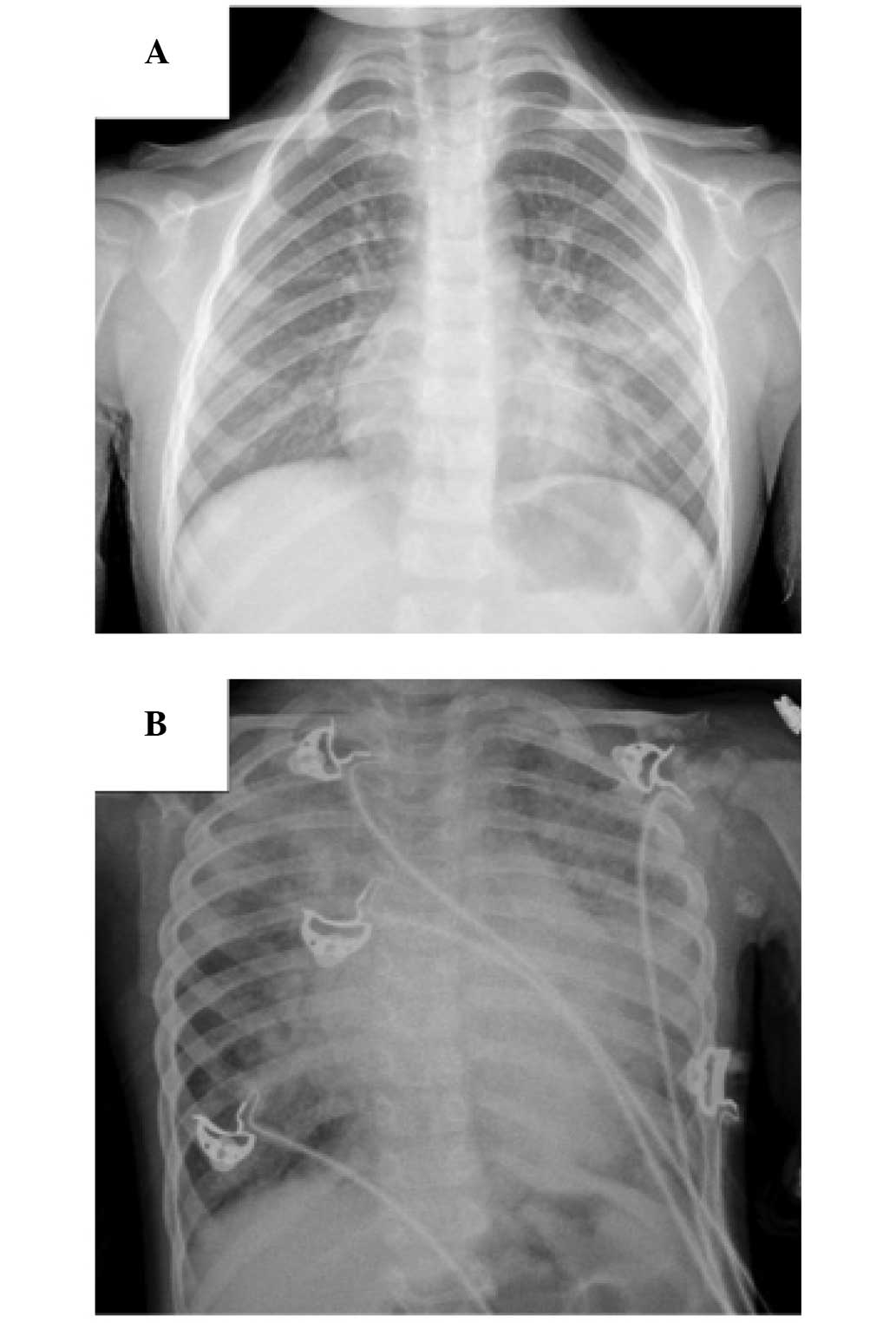
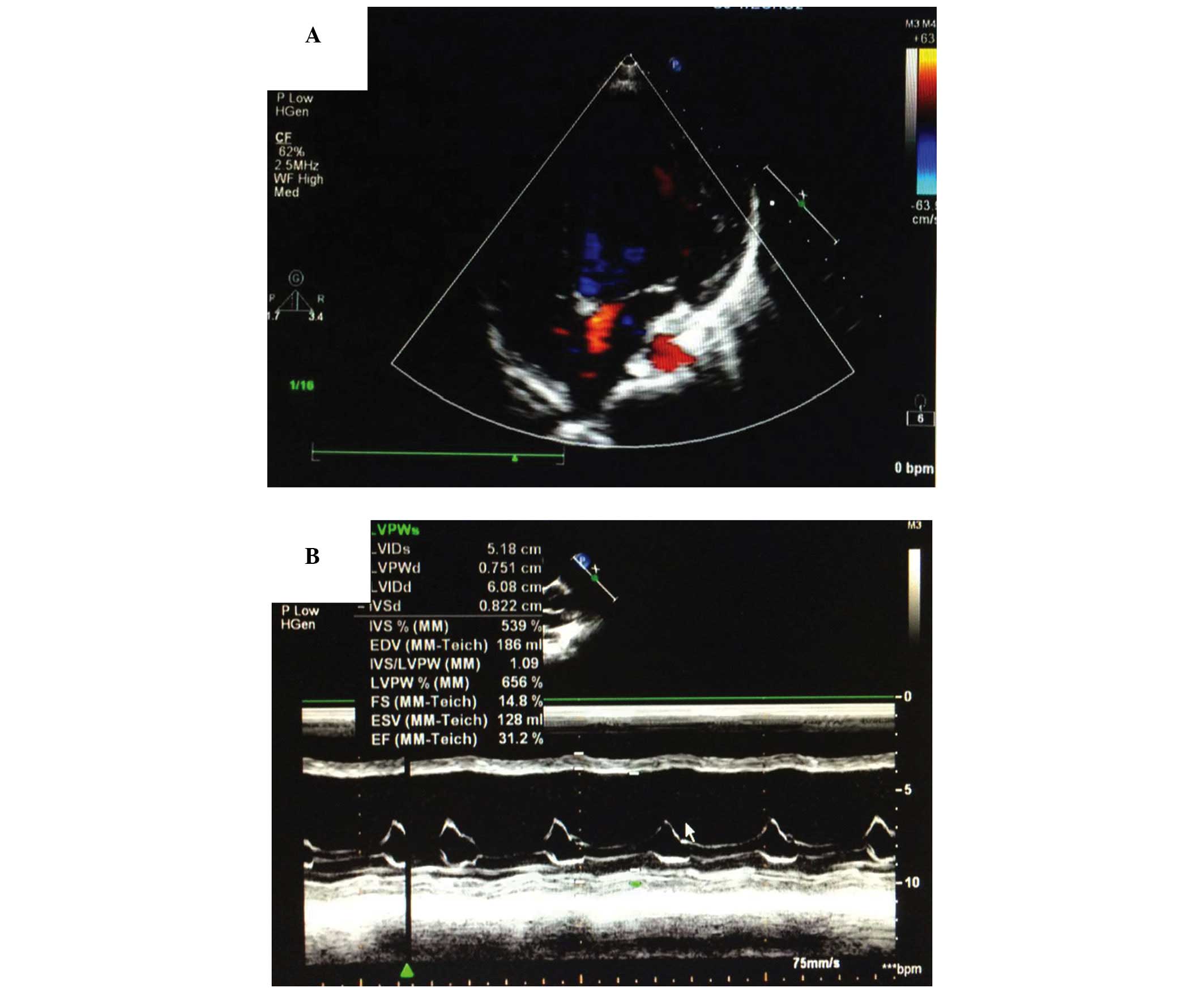
(11) (Figs. 1 and 2). In addition, 20 healthy children were
enrolled in the study as negative controls. There were no
statistical differences in the age and gender of the subjects among
the three groups. All patients who participated in this study were
confirmed to not suffer from any congenital heart disease or
hepatic or renal disorders. The patients received treatments that
were in accordance with the guidelines for pneumonia and/or HF.
Briefly, for pneumonia, antibiotics were administered, and oxygen
support, cough relief and sputum reduction were performed. For
pneumonia accompanied by HF, alongside the previously mentioned
treatments, conscious sedation, cardiotonic treatment, diuresis,
and blood vessel expansion were performed.
Clinical specimens
Venous blood (2 ml) was collected from all patients
twice for plasma BNP detection. The first samples were collected on
the day the patients were enrolled in the study, prior to
treatment, and the second samples on the day the patients were
discharged from the hospital, following the disappearance of the
symptoms. The Triage® BNP automated immunoassay systems and
reagents (Biosite Diagnostics, Inc., San Diego, CA, USA) were used
to detect the plasma BNP levels, according to the manufacturer's
instructions (12). During the
evaluation, blood samples were collected in tubes, which contained
potassium EDTA. A fluorescence immunoassay kit (Triage; Biosite
Diagnostics, Inc.) was used for the quantitative determination of
BNP in the plasma specimens. The analytic sensitivity, stability
characteristics and precision of the system have been previously
described (13,14).
Statistical analysis
The data are presented as the mean ± standard
deviation. Analysis of variance and χ2 tests were
performed in order to determine the statistical differences among
the different groups. All P-values were determined by two-sided
tests. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis of the data was
performed using SPSS 10.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Plasma BNP levels in patients prior to
treatment
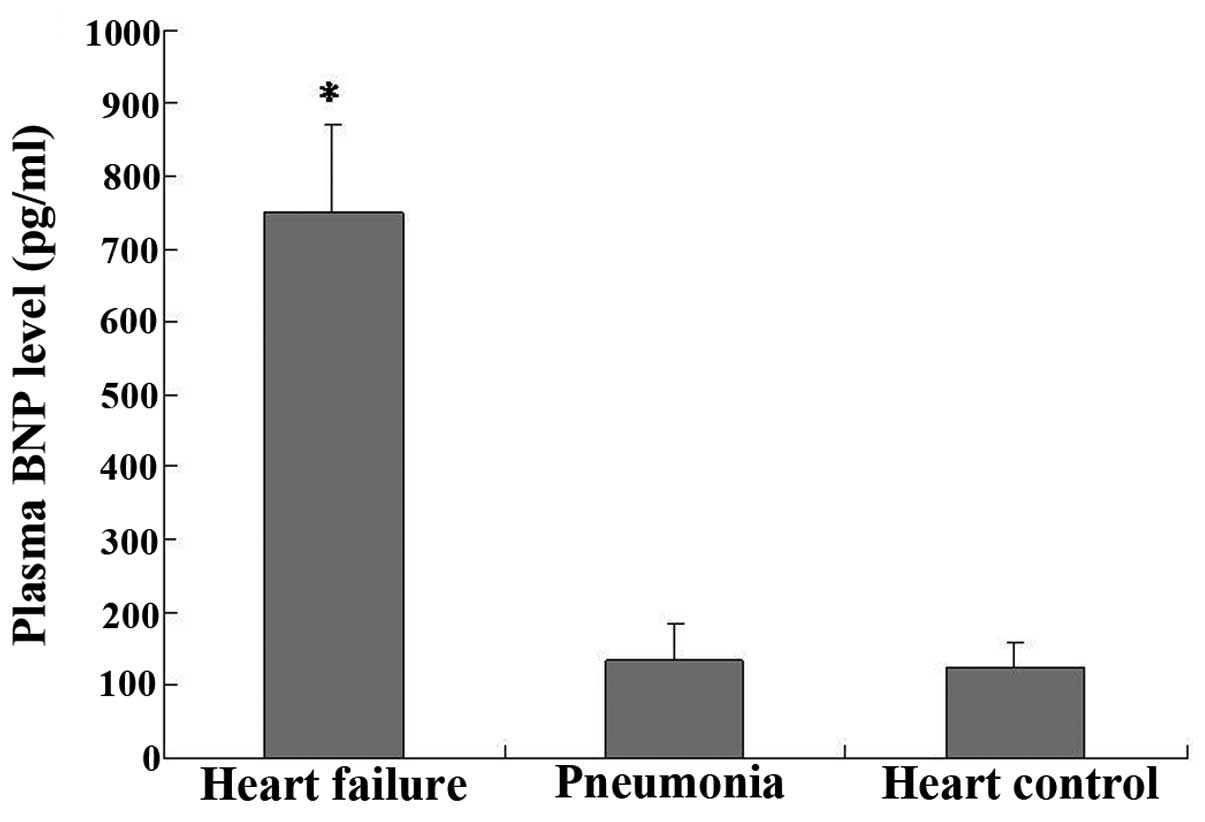
The plasma BNP assays performed upon the enrollment
of the patients in the study showed that the BNP levels of group 2
(750±120 pg/ml) were significantly higher than those of group 1
(135±50 pg/ml) (P<0.05, group 2 vs. group 1; Fig. 3); however, no significant difference
was observed in the BNP levels between group 1 and the negative
control (125±34 pg/ml) (P>0.05).
Plasma BNP level in patients following
treatment
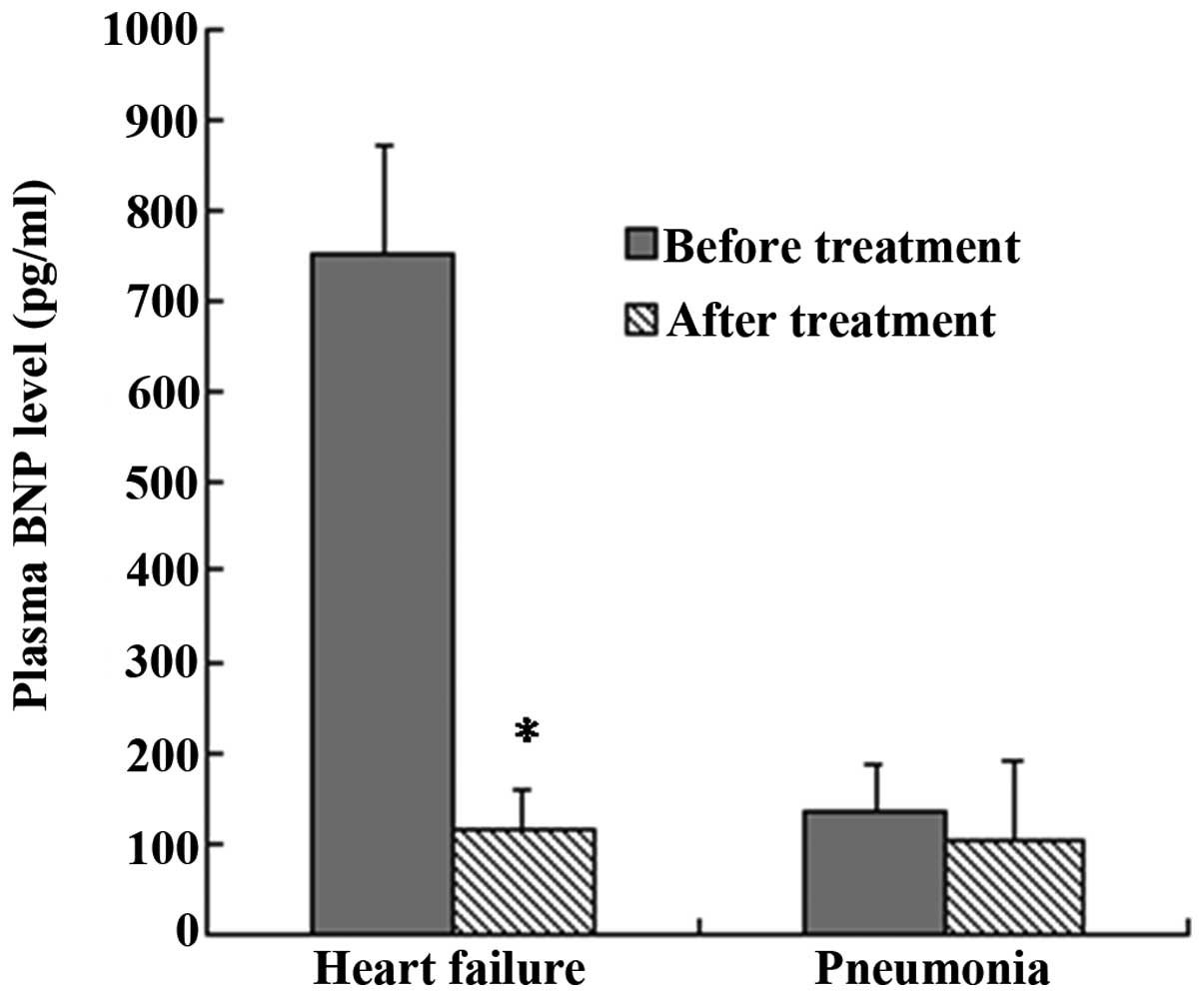
The results of the plasma BNP assays performed upon
the enrollment and prior to the discharge of the patients showed
that, in group 2, the BNP levels of the patients (750±120 pg/ml)
were significantly higher upon enrollment than those prior to
discharge (115±45 pg/ml) (P<0.05, group 2 at enrollment vs.
group 2 at discharge; Fig. 4). The
BNP levels in group 1, however, showed no significant difference
between the specimens collected upon enrollment (135±50 pg/ml) and
those collected prior to discharge (105±86 pg/ml) (P>0.05, group
1 at enrollment vs. group 1 at discharge; Fig. 4).
Discussion
The discovery of ANP >30 years ago (15) was a milestone for the implementation
of a laboratory marker of HF in the clinical setting; soon
thereafter, it was discovered that the heart also secretes BNP, a
second NP (16). It is now apparent
that the heart is also an endocrine gland releasing NPs from the
hemodynamically stressed myocardium as a result of increased atrial
or ventricular myocardial stretch or strain. BNP is expressed in
the myocardium, brain and adrenal glands; however, the main source
of BNP synthesis and secretion is the heart (1). The BNP system is considered to be one
of the most important hormonal regulators of cardiovascular
homeostasis and function (1,2,4).
Furthermore, it has been reported that this family of
cardiovascular NPs plays a paracrine and autocrine role in the
maintenance of the myocardial and vascular structure and function
(3,4). Soon after its discovery, the first
finding of increased ANP plasma concentrations in patients with HF
was reported (17). The potential
for a plasma marker in HF was thereby raised, and investigations in
this area have since received significant attention, with a
particular focus on clinical applications. In subsequent
comparative clinical studies, BNP emerged as a superior diagnostic
marker compared with ANP-derived forms (1,12). The
significance of BNP in the diagnosis and risk stratification of
patients with HF, as well as various other cardiac conditions, has
also been largely investigated during the last two decades
(12–14). Studies have focused on the impact of
the NPs on disease and treatment monitoring, and their effect on
patient management has been demonstrated (1,2,4,12,13,18).
In clinical terms, HF is a syndrome with typical
symptoms and signs, such as fatigue and breathlessness, and
pulmonary crackles and elevated jugular venous pressure,
respectively. Patients with HF may have either a reduced or
preserved left ventricular ejection fraction. The diagnosis of HF
can be complicated, since the clinical features of the condition
are not always sensitive or specific, particularly in pediatric
patients. The challenges of diagnosing HF in children emphasize the
importance of evaluating whether other biomarkers can assist the
diagnosis of the condition. Furthermore, the characteristics of
these other biomarkers should be examined for their prognostic
utility and usefulness in guiding therapy. The NPs, including BNP,
have been shown to be useful in facilitating the diagnosis,
prognosis, and management of HF in adults. BNP is secreted into the
bloodstream by cardiac myocytes in response to increased
ventricular wall stress, hypertrophy and volume overload, and its
levels are increased in HF; therefore, BNP appears to be a
promising marker for HF (5,6).
The present study focused on the differential
diagnostic value of BNP in pneumonia accompanied by HF in
pediatrics patients. The Biosite Triage BNP assay, which was the
first device to receive the Food and Drug Administration approval
in the United States, was used for the definitive diagnosis of
congestive HF in patients within an acute setting with dyspnea
(12). In the present study, the
plasma BNP levels in patients with pneumonia, pneumonia accompanied
by HF and healthy pediatric patients were detected, and it was
found that the BNP levels in patients with pneumonia accompanied by
HF were significantly higher than those in the patients with simple
pneumonia. No differences were observed in the BNP levels between
the patients with simple pneumonia and those in the healthy control
group. The BNP levels of the patients were also compared prior to
and following treatment. It was observed that, prior to treatment,
the BNP levels in the patients with pneumonia accompanied by HF
were significantly higher than those following treatment, which was
not the case with the patients in the simple pneumonia group. No
differences were observed in the BNP levels of the patients of the
simple pneumonia group prior to treatment compared with those
following treatment.
Previous studies have investigated the prognostic
value of BNP in HF and consistent findings have shown that BNP is
an independent predictor of all-cause and cardiovascular mortality,
as well as other cardiac outcomes, such as worsening HF and
hospitalization (19–22). In addition, these studies suggested
that BNP levels that were measured prior to discharge or
post-treatment were better predictors of prognosis than those
measured at other time-points; however, the aforementioned studies
focused mainly on adult patients. By contrast, the present study
investigated the differential diagnostic value of BNP in pediatric
patients with pneumonia accompanied by HF without congenital
cardiac disease.
In conclusion, BNP may represent a useful biomarker
that could be used for therapeutic monitoring and to help
distinguish pediatric patients with pneumonia accompanied by HF
from pediatric patients with simple pneumonia. BNP may therefore
become a useful biomarker with diagnostic and prognostic value in
pediatric patients with pneumonia accompanied by HF.
Acknowledgements
This study was supported by grants from the Science
and Technology Research Development Project of Suzhou City, China
(grant no. SYSD2012001).
References
|
1
|
Clerico A, Giannoni A, Vittorini S, et al:
Thirty years of the heart as an endocrine organ: Physiological role
and clinical utility of cardiac natriuretic hormones. Am J Physiol
Heart Circ Physiol. 301:H12–H20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cantinotti M, Giovannini S, Murzi B and
Clerico A: Diagnostic, prognostic and therapeutic relevance of
B-type natriuretic hormone and related peptides in children with
congenital heart diseases. Clin Chem Lab Med. 49:567–580. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunt PJ, Richards AM, Nicholls MG, et al:
Immunoreactive amino-terminal pro-brain natriuretic peptide
(NT-PROBNP): A new marker of cardiac impairment. Clin Endocrinol
(Oxf). 47:287–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mair J, Friedl W, Thomas S and Puschendorf
B: Natriuretic peptides in assessment of left-ventricular
dysfunction. Scand J Clin Lab Invest Suppl. 230:132–142. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKelvie RS, Moe GW, Ezekowitz JA, et al:
The 2012 Canadian Cardiovascular Society heart failure management
guidelines update: Focus on acute and chronic heart failure. Can J
Cardiol. 29:168–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McMurray JJ, Adamopoulos S, Anker SD, et
al: ESC Committee for Practice Guidelines: ESC Guidelines for the
diagnosis and treatment of acute and chronic heart failure: 2012
The Task Force for the Diagnosis and Treatment of Acute and Chronic
Heart Failure 2012 of the European Society of Cardiology. Developed
in collaboration with the Heart Failure Association (HFA) of the
ESC. Eur Heart J. 33:1787–1847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metra M, Ponikowski P, Dickstein K, et al:
Advanced chronic heart failure: A position statement from the Study
Group On Advanced Heart Failure of the Heart Failure Association of
the European Society of Cardiology. Eur J Heart Fail. 9:684–694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heart Failure Society of America.
Lindenfeld J, Albert NM, Boehmer JP, et al: HFSA 2010 comprehensive
heart failure practice guideline. J Card Fail. 16:e1–e194. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Felker GM, Adams KF Jr, Konstam MA, et al:
The problem of decompensated heart failure: Nomenclature,
classification and risk stratification. Am Heart J. 145(Suppl 2):
18–25. 2003. View Article : Google Scholar
|
|
10
|
Arnold LM, Crouch MA, Carroll NV and
Oinonen MJ: Outcomes associated with vasoactive therapy in patients
with acute decompensated heart failure. Pharmacotherapy.
26:1078–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kliegman RM, Stanton BMD, St. Geme J,
Schor N and Behrman RE: Part XIX, Respiratory System. Nelson
Textbook of Pediatrics (19th). (Philadephia, PA). Saunders
(Elsevier). 2015.
|
|
12
|
Maisel A, Krishnaswamy P, Nowak RM, et al:
Rapid measurement of B-type natriuretic peptide in the emergency
diagnosis of heart failure. N Engl J Med. 347:161–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrison LK, Harrison A, Krishnaswamy P,
et al: Utility of a rapid B-natriuretic peptide assay in
differentiating congestive heart failure from lung disease in
patients presenting with dyspnea. J Am Coll Cardiol. 39:202–209.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mair J, Hammerer-Lercher A and Puschendorf
B: The impact of cardiac natriuretic peptide determination on the
diagnosis and management of heart failure. Clin Chem Lab Med.
39:571–588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Bold AJ, Borenstein HB, Veress AT, et
al: A rapid and potent natriuretic response to intravenous
injection of atrial myocardial extracts in rats. Life Sci.
28:89–94. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saito Y, Nakao K, Itoh H, et al: Brain
natriuretic peptide is a novel cardiac hormone. Biochem Biophys Res
Commun. 158:360–368. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burnett JC, Kao PC, Hu DC, et al: Atrial
natriuretic peptide elevation in congestive heart failure in human.
Science. 231:1145–1147. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frankenstein L, Nelles M, Slavutsky M, et
al: Beta-blockers influence the short-term and long-term prognostic
information of natriuretic peptides and catecholamines in chronic
heart failure independent from specific agents. J Heart Lung
Transplant. 26:1033–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Angelantonio E, Chowdhury R, Sarwar N,
et al: B-type natriuretic peptides and cardiovascular risk:
Systematic review and meta-analysis of 40 prospective studies.
Circulation. 120:2177–2187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doust JA, Pietrzak E, Dobson A and
Glasziou P: How well does B-type natriuretic peptide predict death
and cardiac events in patients with heart failure: systematic
review. BMJ. 330:6252005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vesbianu D, Vesbianu C, Bernstein P, et
al: Plasma brain natriuretic peptide-an independent predictor of
mortality and rehospitalization in congestive heart failure-a
meta-analysis. World Heart J. 1:349–354. 2008.
|
|
22
|
Oremus M, Raina PS, Santaguida P, et al: A
systematic review of BNP as a predictor of prognosis in persons
with coronary artery disease. Clin Biochem. 41:260–265. 2008.
View Article : Google Scholar : PubMed/NCBI
|