Introduction
Men in Western countries and developing nations are
frequently diagnosed with cancers of the prostate gland (1). Statistically, prostate cancer has
overtaken heart diseases and lung disorders in old people as a
cause of mortality. According to estimates, >200,000 new cases
of prostate cancer were reported with >30,000 mortalities during
the year 2013 (2,3). A similar or higher number of cases has
been projected for the year 2014 (4). Mortality due to prostate cancer has
declined in recent years; however, the expense of therapy remains
very high (5). The treatment for
prostate cancer mainly relies on chemotherapy, surgical
intervention and ionizing radiation therapy. Conventional
chemotherapy often results in low therapeutic effects with
undesirable side-effects. The exposure of normal cells to
anticancer drugs results in severe organ-related side-effects
(6). Surgical interventions have a
risk of reoccurrence and pain to the patient. Radiation therapy
also results in adverse effects on rapidly dividing healthy cells
(7). Therefore, a therapeutic drug
delivery system that can enhance the chemotherapeutic efficacy of
an anticancer drug while reducing its organ-related toxicity is
highly desired.
Nanomedicine-based therapeutic approaches have been
gaining increasing popularity. Nanotechnology-based delivery
systems have attracted the attention of researchers from across the
globe due to their tumor-targeting ability (8). This type of drug delivery system
provides the sustained release of anticancer drug to target cancer
cells and thereby avoids the exposure of normal cells. This unique
approach can greatly enhance the therapeutic outcome while reducing
drug-related severe toxicity (9). In
this regard, several researchers have successfully demonstrated the
potential of a nanomedical approach in the treatment of various
cancers. In optimal cases, it decreases the mortality and morbidity
rates associated with the cancers. Among various nanoparticulate
delivery systems (lipid nanoparticles, nanomicelles, inclusion
complexes, nanosuspensions and liposomes), biodegradable
polymer-based nanoparticles have shown enormous potential for use
in cancer drug delivery (10–12).
Specifically, the polylactide (PLA)- and polyglycolide (PGA)-based
polymer known as poly(D,L-lactide-co-glycolide) (PLGA) has been
extensively studied owing to its biocompatible, biodegradable,
non-toxic, non-immunogenic and noncarcinogenic properties (13,14).
PLGA-based nanomedicine products are being investigated in
different phases of clinical trials (15–17). In
particular, a docetaxel-loaded PLGA formulation has completed phase
I clinical trial for the treatment of prostate cancer (4). Such proof-of-success inspired the
present study in which an anticancer drug was encapsulated in PLGA
nanoparticles to obtain a potential therapeutic for prostate
cancer.
Bicalutamide (BLT), a nonsteroidal antiandrogen, was
selected in the present study as a model drug for incorporation
into PLGA nanoparticles in order to investigate its therapeutic
effect in prostate cancers. BLT is an FDA-approved antineoplastic
hormonal agent primarily used in the treatment of prostate cancer
(18). It is widely used in the
treatment of locally advanced and metastatic prostate cancer,
either as a monotherapy or combined with other anticancer agents.
It acts primarily by inhibiting the binding of androgen with
androgen receptors. Moreover, BLT is a biopharmaceutics
classification system (BCS) class II drug with low aqueous
solubility and high permeability (19). The incorporation of BLT into PLGA
nanoparticles should improve its dispersity and aqueous solubility,
making it ideal for cancer drug delivery.
The present study aimed to develop a BLT-loaded PLGA
nanoparticulate system in order to improve the therapeutic efficacy
of BLT in prostate cancer and to mitigate its toxicity. The drug
was loaded into PLGA nanoparticles and its size distribution and
release characteristics were evaluated. The morphology of the
nanoparticles was observed using a transmission electron microscope
(TEM). The cytotoxic potential of free BLT and PLGA-BLT was studied
by MTT assay, and a caspase-3 assay was carried out to investigate
the apoptosis of cancer cells. Finally, the inhibitory effects of
the drug-loaded formulation on cell growth and colony formation
were studied in two prostate cancer cell lines. The LNCaP and C4-2
cell lines were selected as androgen-dependent and
androgen-independent cells.
Materials and methods
Materials
PLGA (molecular weight, ~25,000) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). BLT was procured from Beijing
Guolian Chenhui Pharmaceutical Technology Co., Ltd. (Beijing,
China). C4-2 human prostate cancer cells and lymph node prostate
adenocarcinoma (LNCaP) cells were purchased from American Type
Culture Collection (ATCC; Manassas, VA, USA). All other chemicals
were of reagent grade and used without further modifications.
Preparation of BLT-loaded PLGA
nanoparticles
BLT-loaded PLGA nanoparticles were prepared by a
nanoprecipitation method. In brief, 5 mg BLT and 50 mg PLGA were
dissolved in 10 ml acetone and dissolved by vortexing and
sonication. To this solution, 0.5% poloxamer 407 solution
(Sigma-Aldrich, St. Louis, MO, USA) (10 ml) was added drop-by-drop
in a controlled manner. The solution was stirred at a high speed in
a magnetic stirrer and kept overnight at room temperature.
Following evaporation of the organic solvents, drug-loaded
nanoparticles were collected by centrifuging at 5,000 × g for 20
min at 37°C. The supernatant was removed and the pellet was
re-dispersed with water and stored until use.
Particle size analysis
The particle size and size distribution of the
PLGA-BLT nanoparticles were determined by a laser diffraction
technique. A Malvern Zetasizer (Nano-ZS; Malvern Instruments,
Malvern, UK) was employed to measure the size characteristics. The
samples were diluted with double distilled water and the particle
size was evaluated in triplicate. All measurements were performed
at a fixed angle of 90° at 25°C room temperature. The results were
expressed as the mean size ± standard deviation (SD).
Morphology
The morphological examination of PLGA-BLT was
carried out using a TEM (Hitachi® 800-MT; Hitachi, Tokyo, Japan).
The liquid samples were counterstained with phosphotungstic acid,
then placed over a carbon-coated copper grid and air-dried.
Loading efficiency
The amount of drug loaded into the nanoparticles was
evaluated by calculating the amount of drug added and the amount
entrapped in the nanoparticles. For the latter, BLT-loaded PLGA
nanoparticles were combined with HCl and stirred for 24 h. The
mixture was centrifuged 8,000 × g for 20 min at 37°C, and the
supernatant was collected to analyze the BLT via high-performance
liquid chromatography (HPLC) using an LC 1200 system (Agilent
Technologies, Santa Clara, CA, USA) with a reverse-phase C18 column
(250×4.6×5 mm) at 25C. The entrapment efficiency was calculated
using the following formula: Entrapment efficiency (%) = amount of
BLT in PLGA nanoparticles/total amount of BLT × 100.
Release study
The release of BLT in phosphate-buffered saline
(PBS) and acetate-buffered saline (ABS) was evaluated by a dialysis
method. For this purpose, 1 ml PLGA-BLT suspension, suspended in
release buffer and containing 1 mg BLT, was transferred into a
dialysis tube (molecular weight cutoff, 3,000; Membra-Cel®;
Viskase, Darien, IL, USA), sealed, and placed in a tube containing
20 ml release medium. The whole assembly was kept in a horizontal
laboratory shaker (80 × g) and maintained at 37°C. At specific time
intervals, 1 ml sample was removed and replaced with equal amount
of fresh release medium. The amount of drug present in the release
media was analyzed using a HPLC method. The mobile phase consisted
of PBS (pH 3.0 adjusted with phosphoric acid) and acetonitrile in a
55:45 v/v ratio. The mobile phase was filtered using a 0.45-µm
filter, degassed and run at a rate of 1.1 ml/min. BLT was detected
using a 112 UV detector (Gilson, Middleton, WI, USA) at 270 nm.
Cell culture
C4-2 and LNCaP prostate cancer cells were cultured
in RPMI-1640 medium (Sigma-Aldrich), which was supplemented with
10% fetal bovine serum (FBS), 1% penicillin-streptomycin mixture
and 1% sodium pyruvate. The cells were incubated in ambient
conditions of 5% CO2 at 37°C.
Cytotoxicity assay
The cytotoxic potentials of free BLT and PLGA-BLT
were studied by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. C4-2 and LNCaP cells were seeded in a 96-well plate at a
seeding density of 4×103 cells/well and were allowed to
attach for 48 h until they reached 90% confluency. The cells were
treated with free BLT or PLGA-BLT and further incubated for 24 h at
concentrations of 0.1, 0.5, 1, 5, 10 and 50 µg/ml. The
drug-containing media were removed carefully and replaced with
fresh growth media containing MTT solution (0.5 mg), and the cells
were then incubated for additional 3 h. The supernatant was
discarded carefully and 100 µl DMSO was added to extract the
formazan crystals. The intensity of the purple coloration was
measured using a SpectraMax® Plus 384 UV-Visible microplate reader
(Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 570
nm.
Caspase-3 activity
Caspase-3 activity of the C4-2 and LNCaP cells was
analyzed using a Caspase-Glo 3 assay kit according to the
instructions provided by the manufacturer (Promega, Madison, WI,
USA). Cells were seeded in a 12-well plate at a density of
2×105 cells/well, treated with 5 µg free BLT or an
equivalent amount of PLGA-BLT and incubated for 24 h at 37°C. Cells
were washed with PBS and harvested using 0.25% trypsin. The cells
were centrifuged at 500 × g for 30 sec at 37°C, re-dispersed and
treated with 100 µl Caspase-Glo and incubated for 1 h. The
solutions were transferred to culture tubes and examined using a
luminometer (Lumat3 LB 9508; Berthold Technologies GmbH & Co.
KG, Bad Wildbad, and Germany).
Clonogenic assay
C4-2 and LNCaP cells were seeded in a 6-well plate
at a density of 500 cells/well. The cells were allowed to form
colonies for 3 days and then treated with 1, 5 and 10 µg/ml free
BLT or PLGA-BLT for a week. The drug-containing media were removed
and replaced with fresh growth media and the cells were further
incubated for 2 weeks. The cells were washed twice with PBS, fixed,
and stained with hematoxylin. The colonies were observed using a
MultiImage™light cabinet (Alpha Innotech Corporation, San Leandro,
CA, USA) with the aid of AlphaEaseFC™(Alpha Imager HP automatic
image capture) software. The number of colonies was counted and
quantified using a stereomicroscope (SMZ745; Nikon Corporation,
Tokyo, Japan).
Statistical analysis
Statistical significance was analyzed using
Student's t-test for two groups and one way analysis of variance
for multiple groups. The level of significance was set at
P<0.05. All the studies were performed in triplicate and data
are presented as the mean ± SD.
Results and Discussion
The treatment options for prostate cancer that are
commonly used at present are chemotherapy, surgical intervention
and ionizing radiation therapy. However none of these therapeutic
approaches effectively controls tumor proliferation, with 20–30%
reoccurrence rates in patients (1–3). In this
regard, BLT, an FDA-approved antineoplastic hormonal agent is
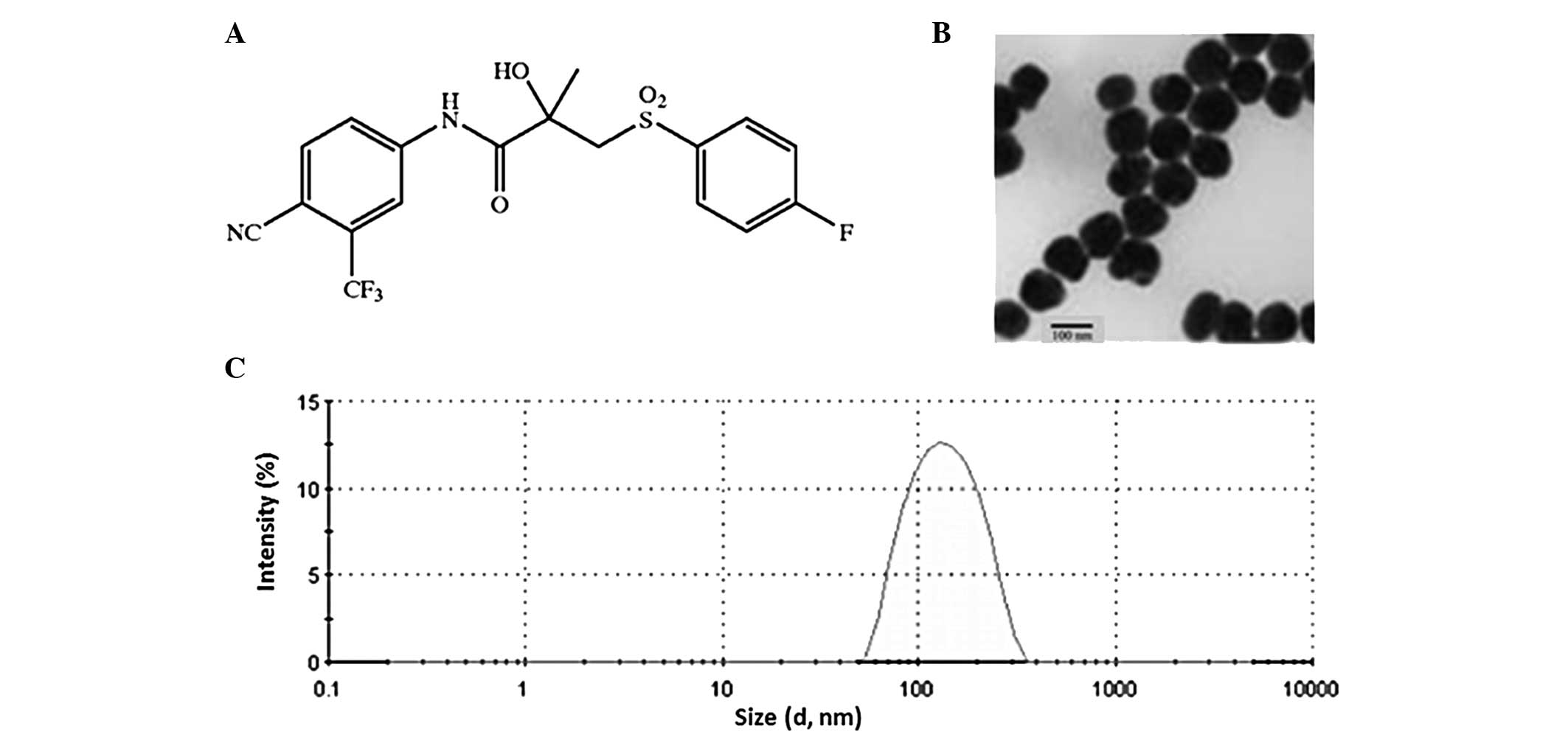
primarily indicated for the treatment of prostate cancer (Fig. 1A). It is widely used in the treatment
of locally advanced and metastatic prostate cancer, either as a
monotherapy or in combination with other anticancer agents.
However, the clinical translation of BLT faces numerous challenges,
since the drug has poor pharmacokinetics and insufficient aqueous
solubility (20). Therefore, the
incorporation of BLT into a nanoparticulate system is expected to
improve its therapeutic efficacy towards prostate cancers. The aim
of the present study was to evaluate the anticancer effects of free
BLT and PLGA-BLT in androgen-dependent and androgen-independent
prostate cancer cell lines. PLGA has been extensively studied owing
to its biocompatible, biodegradable, non-toxic, non-immunogenic and
noncarcinogenic properties (13–15).
PLGA-based nanomedicine products are being evaluated in clinical
trials (16). Inspired by the
clinical success of PLGA nanocarriers, a PLGA polymer was employed
to encapsulate BLT in the present study. In addition, BLT is
hydrophobic with poor aqueous solubility, making it difficult to
administer intravenously. The loading of BLT into PLGA
nanoparticles is likely to improve its systemic characteristics and
physicochemical properties.
Physicochemical analysis of
PLGA-BLT
A nanoprecipitation method was employed to prepare
the BLT-loaded PLGA nanoparticles. As shown in Fig. 1B, the particles were spherical in
shape with a dense core complex. When observed under a TEM, the
mean size of the particles was found to be ~100 nm with a uniform
distribution of the particles on the surface of the TEM grid
(Fig. 1B). No aggregation or
distortion of the nanoparticles was observed during the dry state
analysis. Furthermore, size was further confirmed with a dynamic
light scattering (DLS) technique using laser diffraction. This
revealed a uniform size distribution with an average size between
100 and 120 nm (Fig. 1C). It should
be noted that the TEM measured the particle in a dried state while
DLS measured the particle in a hydrodynamic state. A particle size
of ~100 nm should be ideal for cancer drug delivery (21). A nanosized particle is able to take
advantage of passive targeting and the enhanced permeability
retention (EPR) effect.
Release study
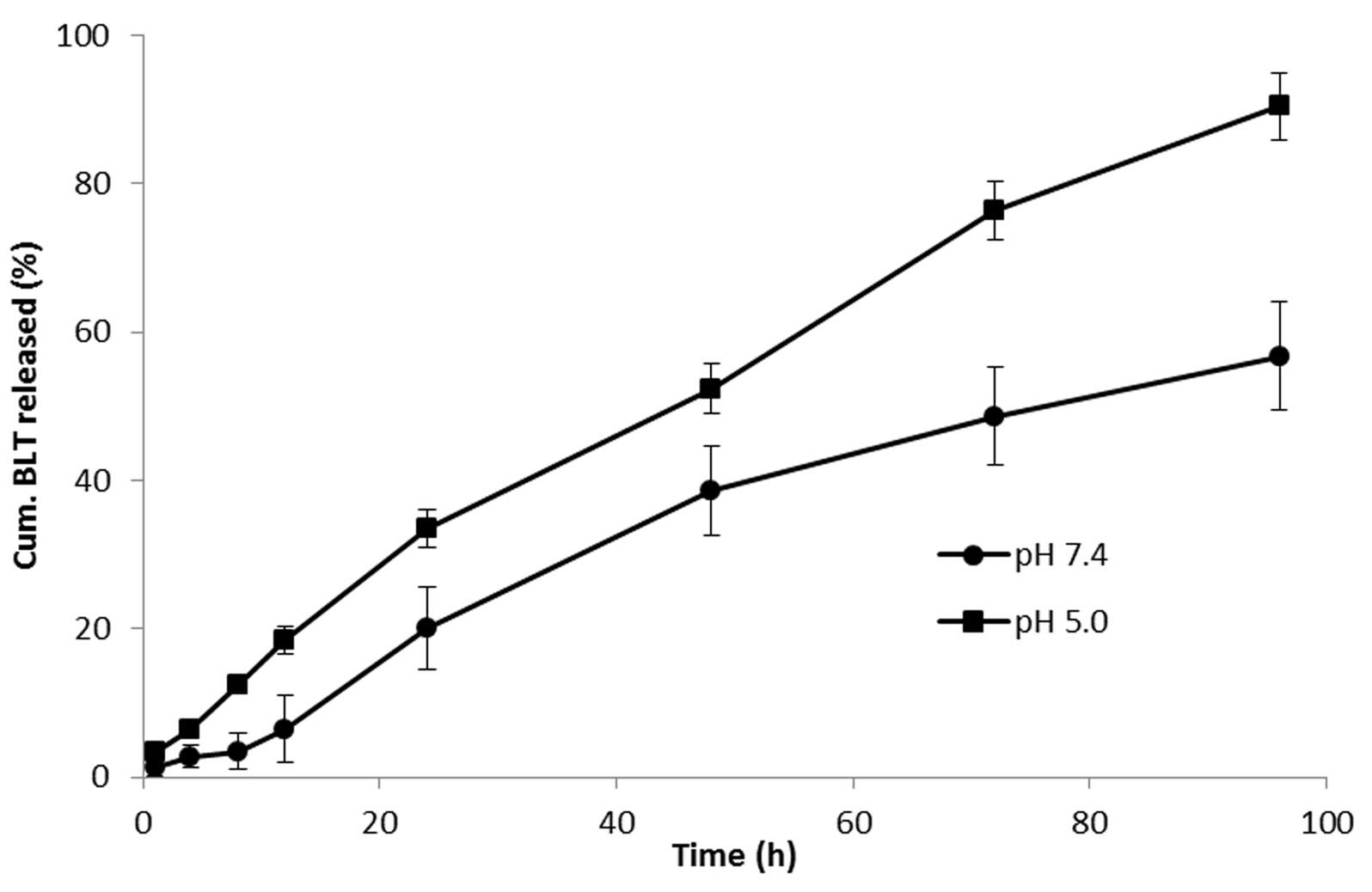
The release of BLT from the PLGA nanoparticles was
studied in PBS and ABS at 37°C. As shown in Fig. 2, BLT presented a sustained-release
profile at the two pH conditions. No initial burst release
phenomenon was observed at any point of the study period. At pH
7.4, the drug was released in a sustained manner and nearly 60% of
the drug was released by the end of 96 h. Accelerated drug release
was observed at pH 5.0, indicating the pH-responsive behavior of
the PLGA nanocarrier. At lower pH, >90% of the drug was
released. The sustained release of the drug in the region of a
tumor should increase the chemotherapeutic efficiency of the
anticancer drug. Moreover, it can be expected that the delivery
system will protect healthy cells from exposure to the drug and
provide a constant dose level to cancer cells. It should be noted
that the release of the drug from the nanoparticle matrix may occur
either by drug diffusion or by erosion of the nanoparticle
architecture. Slow release of the drug in this manner upon systemic
administration should provide constant exposure of the cancer cells
to the drug, thereby enhancing the cytotoxic effect. Moreover,
sustained release is likely to avoid unnecessary drug-related
side-effects in normal organs of the body.
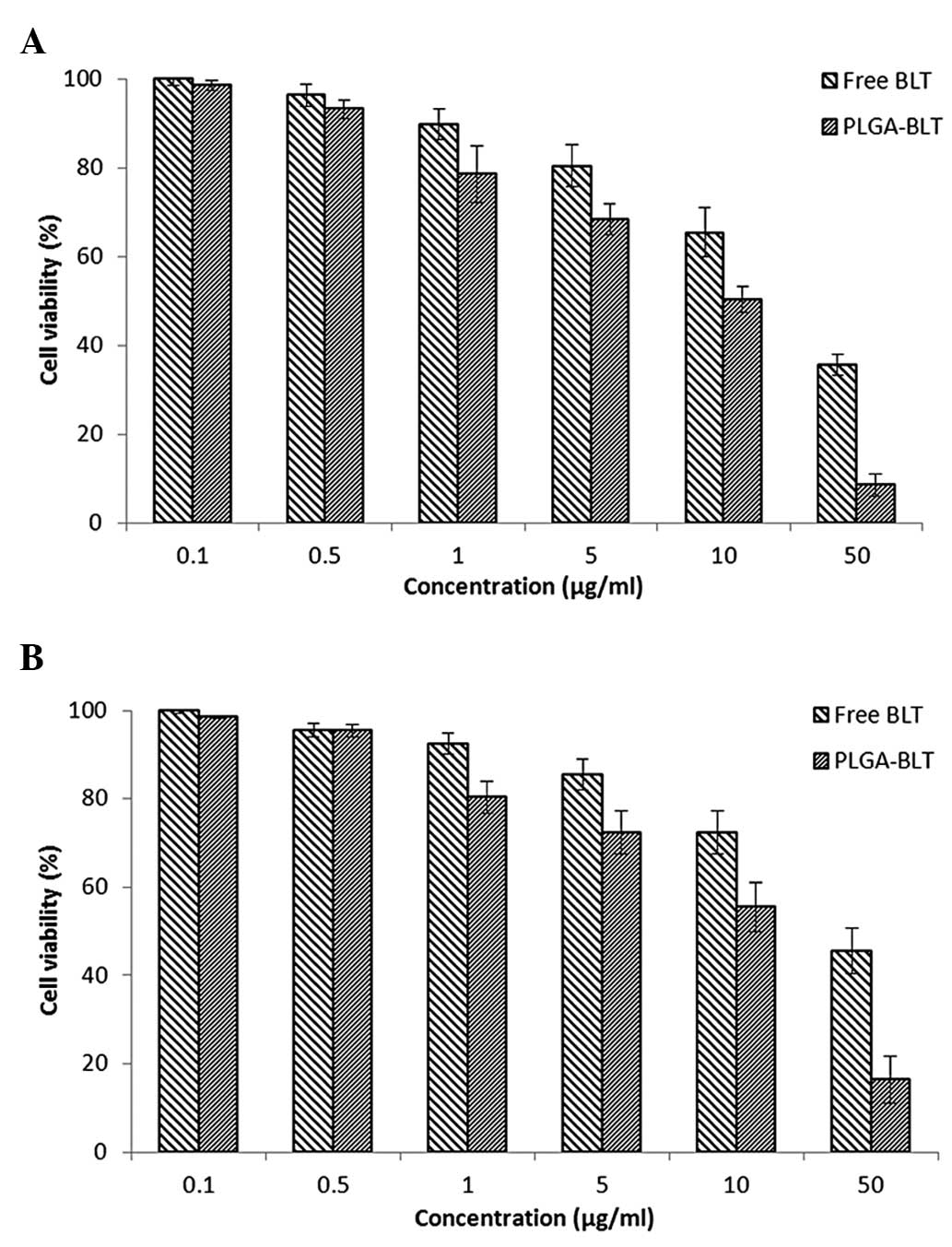
In vitro cell viability
The cytotoxic potential of PLGA-BLT and free BLT in
LNCaP (androgen dependent) and C4-2 (androgen independent) cancer
cells was then investigated. The cell viability of each cell line
was evaluated using MTT assay. Cells were treated with increasing
concentrations of drug ranging from 0.1 to 50 µg/ml. As shown in
Fig. 3, the drug-loaded formulation
had a concentration-dependent cytotoxic effect on cell
proliferation. PLGA-BLT exhibited a pronounced cell killing effect
comparing with that of free BLT. Another important observation is
that neither BLT nor PLGA-BLT were effective at lower
concentrations while at higher concentrations (>10 µg/ml) they
demonstrated significant cytotoxicity. In order to quantify the
effect of the two formulations, the IC50 value of each
was calculated. The IC50 values of PLGA-BLT in LNCaP and
C4-2 cells were 45.4 and 58.5 µg/ml, respectively. However, free
BLT exhibited a higher IC50 value in the two cancer cell
lines (48.9 and 65.8 µg/ml) respectively. The results, therefore,
clearly showed that nanocarrier-based BLT remarkably enhanced the
therapeutic or anticancer effect of the drug. The superior
cytotoxicity of PLGA-BLT may be attributed to its sustained-release
characteristics and higher cellular internalization, whereas the
free drug enters cells by passive diffusion (22).
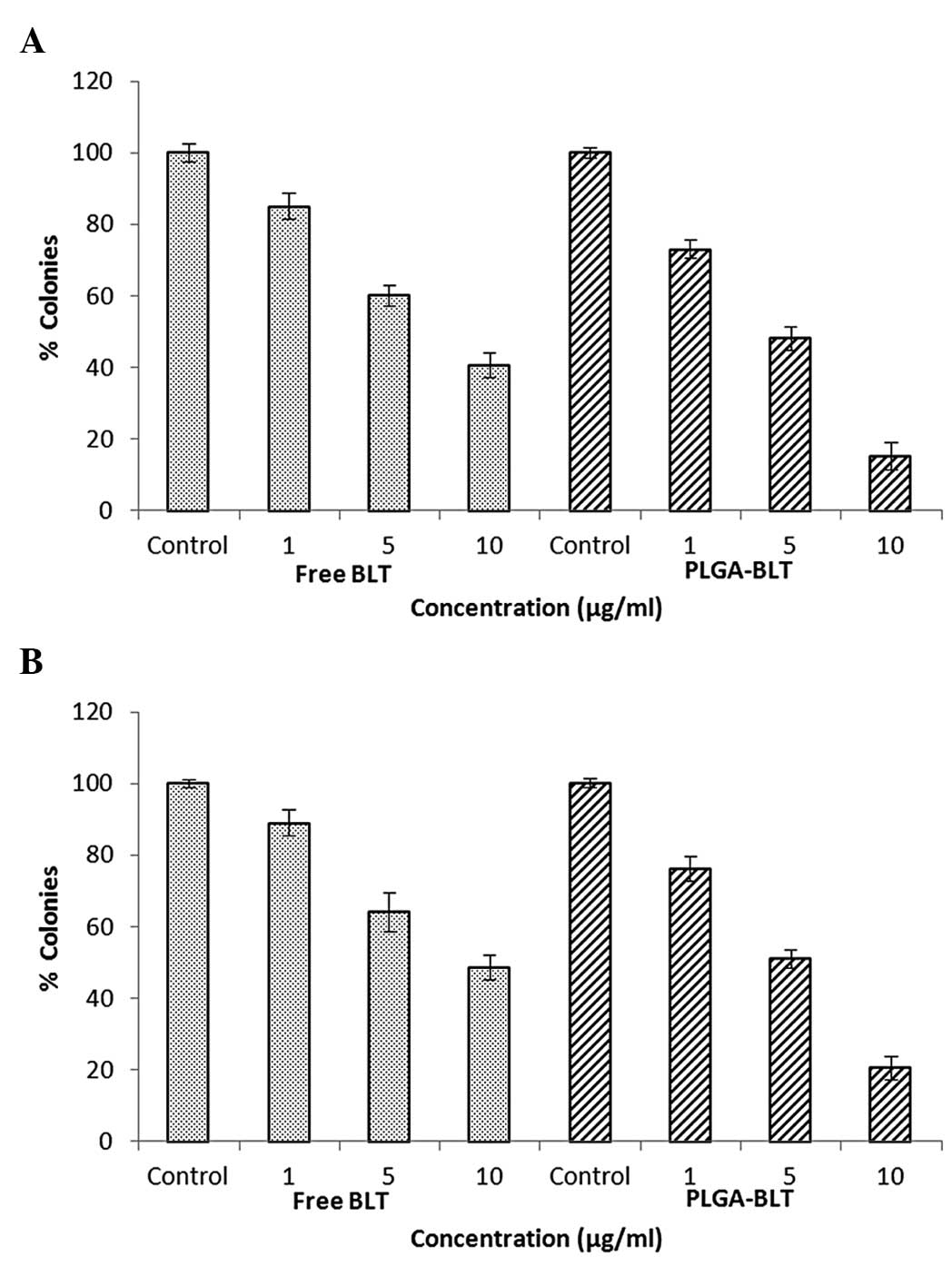
Clonogenic assay
The anticancer effects of the free drug and
drug-loaded formulation were also determined by evaluation in a
clonogenic assay. Untreated cells produced large colonies while
drug-treated cells showed markedly fewer colonies. The
colony-forming effect was studied in the presence of increasing
concentrations of the drug. As shown in (Fig. 4), the colony-forming ability of the
cells was inhibited by the two formulations in a strictly
dose-dependent manner. Specifically, PLGA-BLT demonstrated a
greater inhibitory effect on colony formation than was observed for
free BLT. At all concentrations, the two drug formulations
inhibited the colony forming ability of the cells. Although, BLT at
a concentration of 1 µg/ml showed mild inhibitory effects on colony
formation, marked inhibition was observed at a concentration of 10
µg/ml. Notably, at each concentration, the drug-loaded nanocarriers
induced a prominent reduction in colony formation. Although a
slight difference in inhibitory level was observed between the two
cell lines, the trend observed in the cell lines was similar.
Blockage of androgen activity does not have high efficiency in C4-2
cells, which are androgen independent. Overall, the results suggest
that the slow and sustained release of BLT from PLGA nanoparticles
resulted in PLGA-BLT exhibiting a superior performance compared
with free BLT. This indicates that the overall performance of BLT
may be improved by incorporating it into nanoparticles.
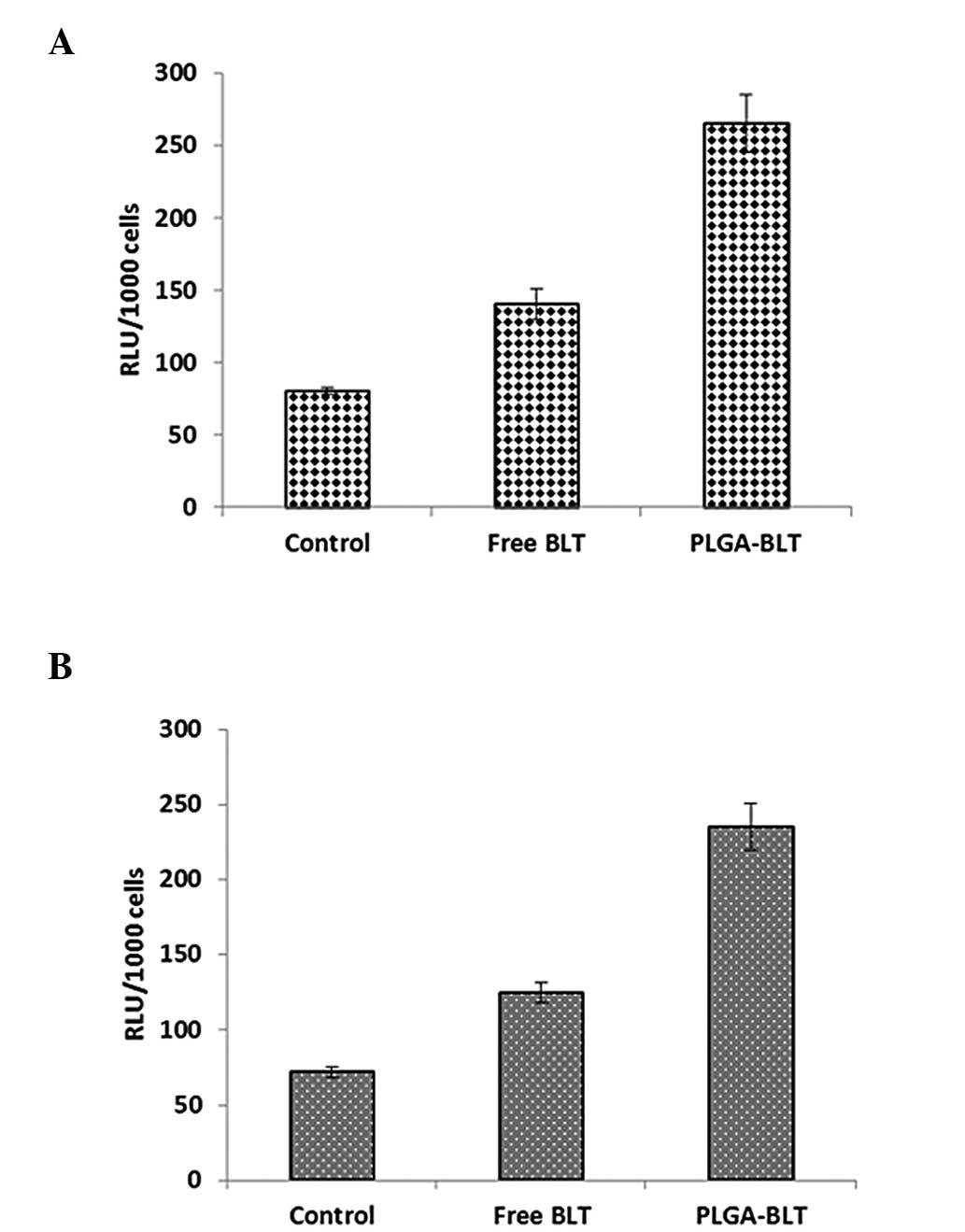
Apoptotic activity by caspase-3
assay
The superior cell inhibitory effect of PLGA-BLT was
further confirmed by the evaluation of caspase-3 activity (Fig. 5). Significantly higher caspase-3
activity was observed in the PLGA-BLT-treated cells compared with
that in the cells treated with free BLT. High caspase-3 activity is
an indication of a greater degree of cell apoptosis (23). This result is consistent with the
marked cytotoxic potential and growth inhibitory effect of the
PLGA-BLT nanoparticles on colony formation.
Conclusion
BLT-loaded PLGA nanoparticles were successfully
prepared and their anticancer effect evaluated in LNCaP and C4-2
cancer cells. Nanosized PLGA-BLT particles with a uniform size
distribution and spherical shape were developed. The PLGA
nanoparticles released BLT in a slow and sustained manner, such
that cancer cells should be exposed to a constant level of the
drug. The PLGA-BLT nanoparticles showed a pronounced cytotoxic
effect on LNCaP and C4-2 cancer cells. The superior cell killing
effect of PLGA-BLT nanoparticles compared with that of free BLT may
be due to the sustained drug release characteristics and high
cellular internalization of the particles. In addition, PLGA-BLT
has been demonstrated to significantly inhibit the colony formation
activity of the two cell lines. Finally, the caspase-3 activity of
PLGA-BLT-treated cancer cells has been indicated to be enhanced
compared with that of cancer cells treated with free BLT,
indicating the cell apoptosis-inducing potential of PLGA-BLT.
Overall, these results suggest that nanotechnology-based BLT
treatment should be able to effectively target and kill cancer
cells. This study may pave the way for the successful
chemotherapeutic treatment of prostate cancers.
Acknowledgements
The study was supported by a University Grant
allocated for research.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gianino MM, Galzerano M, Minniti D, Di
Novi C, Martin B, Davini O and Barbaro S: A comparative costs
analysis of brachytherapy and radical retropubic prostatectomy
therapies for clinically localized prostate cancer. Int J Technol
Assess Health Care. 25:411–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snyder CF, Frick KD, Blackford AL, Herbert
RJ, Neville BA, Carducci MA and Earle CC: How does initial
treatment choice affect short-term and long-term costs for
clinically localized prostate cancer? Cancer. 116:5391–5399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Djenaba JA, Soman A, Rim SH and
Master VA: Recent trends in prostate cancer incidence by age,
cancer stage, and grade, the United States, 2001–2007. Prostate
Cancer. 2012:691382012. View Article : Google Scholar
|
|
5
|
Niraula S and Tannock IF: Broadening
horizons in medical management of prostate cancer. Acta Oncol.
50(Suppl 1): 141–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sowery RD, So AI and Gleave ME:
Therapeutic options in advanced prostate cancer: Present and
future. Curr Urol Rep. 8:53–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mishra B, Patel BB and Tiwari A: Colloidal
nanocarriers: A review on formulation technology, types and
applications toward targeted drug delivery. Nanomedicine. 6:9–24.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishiyama N and Kataoka K: Current state,
achievements and future prospects of polymeric micelles as
nanocarriers for drug and gene delivery. Pharmacol Ther.
112:630–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torchilin VP: Micellar nanocarriers:
Pharmaceutical perspectives. Pharm Res. 24:1–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murthy RS and Umrethia ML: Optimization of
formulation parameters for the preparation of flutamide liposomes
by 3(3) factorial 26-term logit model. Pharm Dev Technol.
9:369–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madhusudhan B, Rambhau D, Apte SS and
Gopinath D: Oral bioavailability of flutamide from
1-o-alkylglycerol stabilized o/w nanoemulsions. J Disp Sci Technol.
28:1254–61. 2007. View Article : Google Scholar
|
|
12
|
Zeng X, Tao W, Mei L, Huang L, Tan C and
Feng SS: Cholic acid-functionalized nanoparticles of star-shaped
PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical
cancer. Biomaterials. 34:6058–6067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thamake SI, Raut SL, Gryczynski Z, Ranjan
AP and Vishwanatha JK: Alendronate coated poly-lactic-co-glycolic
acid (PLGA) nanoparticles for active targeting of metastatic breast
cancer. Biomaterials. 33:7164–7173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hrkach J, Von Hoff D, Ali MM, Andrianova
E, Auer J, Campbell T, de Witt D, Figa M, Figueiredo M, Horhota A,
et al: Preclinical development and clinical translation of a
PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128–39. 2012. View Article : Google Scholar
|
|
15
|
Ganju A, Yallapu MM, Khan S, Behrman SW,
Chauhan SC and Jaggi M: Nanoways to overcome docetaxel resistance
in prostate cancer. Drug Resist Updat. 17:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hrkach JI, Von Hoff D, Ali Mukkaram M,
Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M,
Horhota A, et al: Preclinical development and clinical translation
of a PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128ra392012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yallapu MM, Gupta BK, Jaggi M and Chauhan
SC: Fabrication of curcumin encapsulated PLGA nanoparticles for
improved therapeutic effects in metastatic cancer cells. J Colloid
Interface Sci. 351:19–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanfer I: Report on the international
workshop on the biopharmaceutics classification system (BCS):
Scientific and regulatory aspects in practice. J Pharm Pharm Sci.
5:1–4. 2002.PubMed/NCBI
|
|
19
|
Le Y, Ji H, Chen JF, Shen Z, Yun J and Pu
M: Nanosized bicalutamide and its molecular structure in solvents.
Int J Pharm. 370:175–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meer T, Fule R, Khanna D and Amin P:
Solubility modulation of bicalutamide using porous silica. Int J
Pharm Invest. 43:279–285. 2013. View Article : Google Scholar
|
|
21
|
Sundaramoorthy P, Baskaran R, Mishra SK,
Jeong KY, et al: Novel self-micellizing anticancer lipid
nanoparticles induce cell death of colorectal cancer cells.
Colloids Surf B Biointerfaces: Biointerfaces. 135:793–801. 2015.
View Article : Google Scholar
|
|
22
|
Yallapu MM, Othman SF, Curtis ET, Gupta
BK, Jaggi M and Chauhan SC: Multi-functional magnetic nanoparticles
for magnetic resonance imaging and cancer therapy. Biomaterials.
32:1890–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solito E, de Coupade C, Canaider S,
Goulding NJ and Perretti M: Transfection of annexin 1 in monocytic
cells produces a high degree of spontaneous and stimulated
apoptosis associated with caspase-3 activation. Br J Pharmacol.
133:217–228. 2001. View Article : Google Scholar : PubMed/NCBI
|