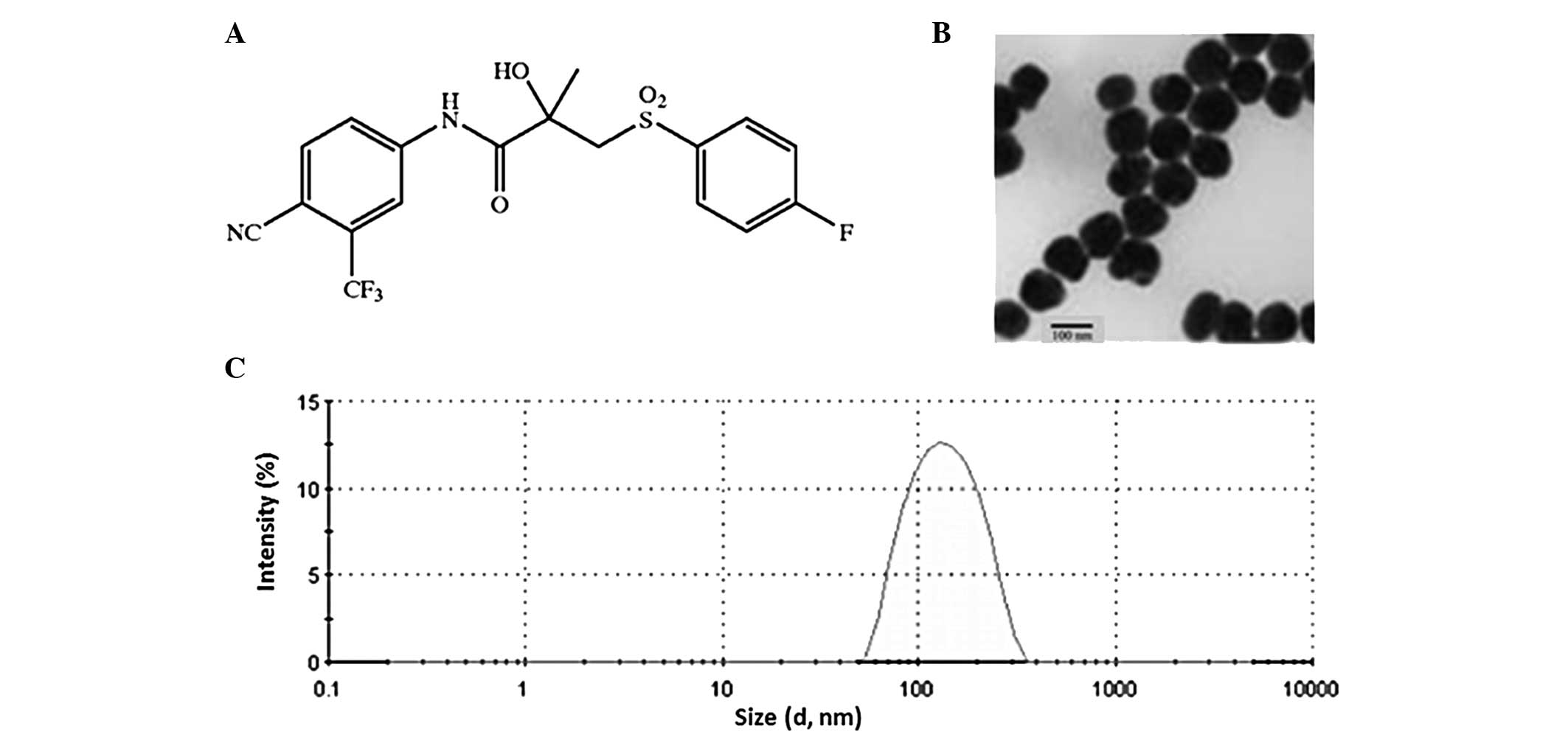
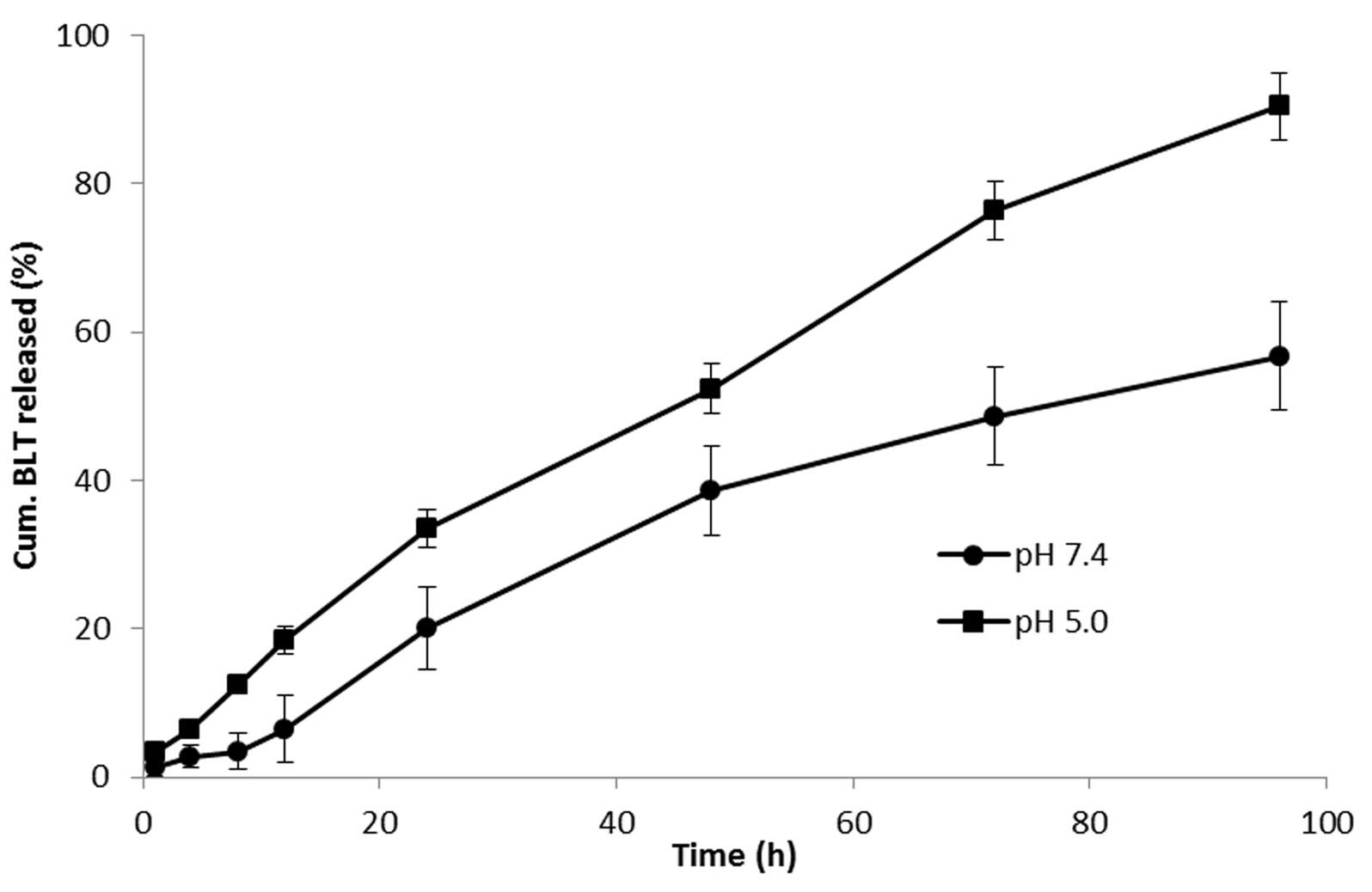
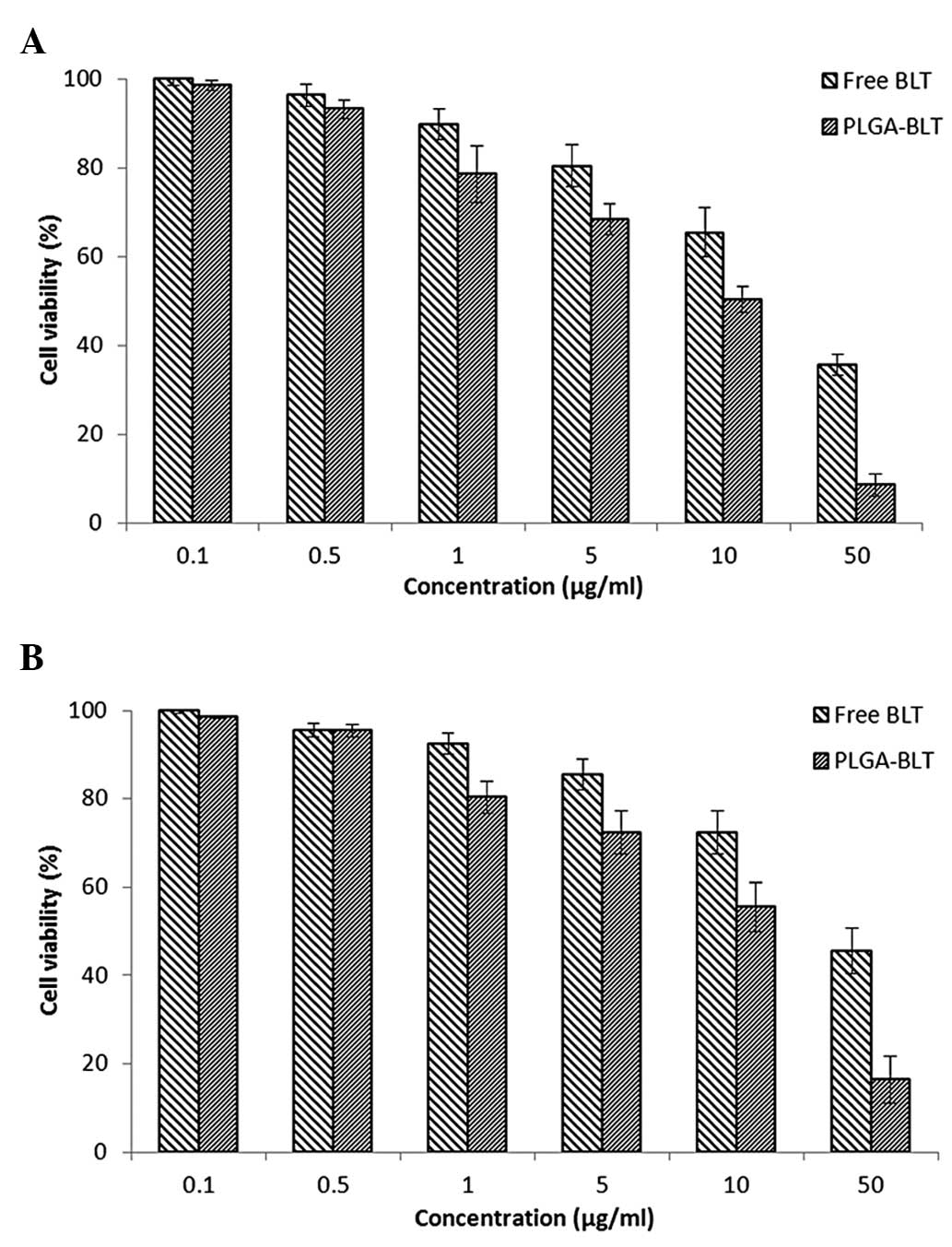
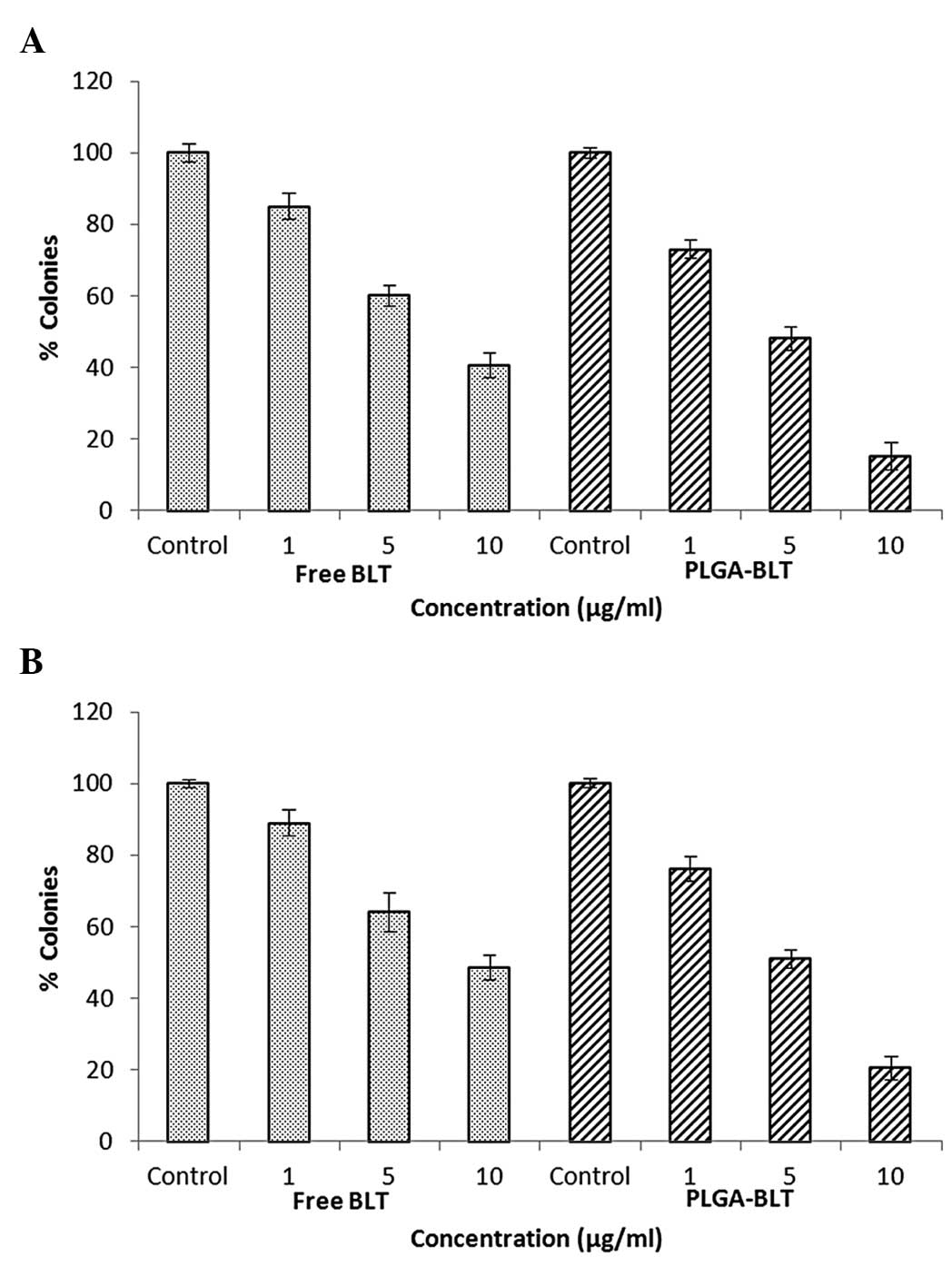
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gianino MM, Galzerano M, Minniti D, Di
Novi C, Martin B, Davini O and Barbaro S: A comparative costs
analysis of brachytherapy and radical retropubic prostatectomy
therapies for clinically localized prostate cancer. Int J Technol
Assess Health Care. 25:411–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snyder CF, Frick KD, Blackford AL, Herbert
RJ, Neville BA, Carducci MA and Earle CC: How does initial
treatment choice affect short-term and long-term costs for
clinically localized prostate cancer? Cancer. 116:5391–5399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Djenaba JA, Soman A, Rim SH and
Master VA: Recent trends in prostate cancer incidence by age,
cancer stage, and grade, the United States, 2001–2007. Prostate
Cancer. 2012:691382012. View Article : Google Scholar
|
|
5
|
Niraula S and Tannock IF: Broadening
horizons in medical management of prostate cancer. Acta Oncol.
50(Suppl 1): 141–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sowery RD, So AI and Gleave ME:
Therapeutic options in advanced prostate cancer: Present and
future. Curr Urol Rep. 8:53–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mishra B, Patel BB and Tiwari A: Colloidal
nanocarriers: A review on formulation technology, types and
applications toward targeted drug delivery. Nanomedicine. 6:9–24.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishiyama N and Kataoka K: Current state,
achievements and future prospects of polymeric micelles as
nanocarriers for drug and gene delivery. Pharmacol Ther.
112:630–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torchilin VP: Micellar nanocarriers:
Pharmaceutical perspectives. Pharm Res. 24:1–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murthy RS and Umrethia ML: Optimization of
formulation parameters for the preparation of flutamide liposomes
by 3(3) factorial 26-term logit model. Pharm Dev Technol.
9:369–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madhusudhan B, Rambhau D, Apte SS and
Gopinath D: Oral bioavailability of flutamide from
1-o-alkylglycerol stabilized o/w nanoemulsions. J Disp Sci Technol.
28:1254–61. 2007. View Article : Google Scholar
|
|
12
|
Zeng X, Tao W, Mei L, Huang L, Tan C and
Feng SS: Cholic acid-functionalized nanoparticles of star-shaped
PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical
cancer. Biomaterials. 34:6058–6067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thamake SI, Raut SL, Gryczynski Z, Ranjan
AP and Vishwanatha JK: Alendronate coated poly-lactic-co-glycolic
acid (PLGA) nanoparticles for active targeting of metastatic breast
cancer. Biomaterials. 33:7164–7173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hrkach J, Von Hoff D, Ali MM, Andrianova
E, Auer J, Campbell T, de Witt D, Figa M, Figueiredo M, Horhota A,
et al: Preclinical development and clinical translation of a
PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128–39. 2012. View Article : Google Scholar
|
|
15
|
Ganju A, Yallapu MM, Khan S, Behrman SW,
Chauhan SC and Jaggi M: Nanoways to overcome docetaxel resistance
in prostate cancer. Drug Resist Updat. 17:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hrkach JI, Von Hoff D, Ali Mukkaram M,
Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M,
Horhota A, et al: Preclinical development and clinical translation
of a PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128ra392012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yallapu MM, Gupta BK, Jaggi M and Chauhan
SC: Fabrication of curcumin encapsulated PLGA nanoparticles for
improved therapeutic effects in metastatic cancer cells. J Colloid
Interface Sci. 351:19–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanfer I: Report on the international
workshop on the biopharmaceutics classification system (BCS):
Scientific and regulatory aspects in practice. J Pharm Pharm Sci.
5:1–4. 2002.PubMed/NCBI
|
|
19
|
Le Y, Ji H, Chen JF, Shen Z, Yun J and Pu
M: Nanosized bicalutamide and its molecular structure in solvents.
Int J Pharm. 370:175–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meer T, Fule R, Khanna D and Amin P:
Solubility modulation of bicalutamide using porous silica. Int J
Pharm Invest. 43:279–285. 2013. View Article : Google Scholar
|
|
21
|
Sundaramoorthy P, Baskaran R, Mishra SK,
Jeong KY, et al: Novel self-micellizing anticancer lipid
nanoparticles induce cell death of colorectal cancer cells.
Colloids Surf B Biointerfaces: Biointerfaces. 135:793–801. 2015.
View Article : Google Scholar
|
|
22
|
Yallapu MM, Othman SF, Curtis ET, Gupta
BK, Jaggi M and Chauhan SC: Multi-functional magnetic nanoparticles
for magnetic resonance imaging and cancer therapy. Biomaterials.
32:1890–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solito E, de Coupade C, Canaider S,
Goulding NJ and Perretti M: Transfection of annexin 1 in monocytic
cells produces a high degree of spontaneous and stimulated
apoptosis associated with caspase-3 activation. Br J Pharmacol.
133:217–228. 2001. View Article : Google Scholar : PubMed/NCBI
|