Introduction
Ideal sedatives for use in intensive care units
(ICUs) should have following properties: Rapid action, easy control
of the depth of sedation, minor impact on respiratory function, no
accumulation of metabolites, no obvious interference with other
medicine and multiple in vivo metabolic pathways,
eliminating without relying on liver, kidney or pulmonary function,
low cost and minimal side effects (1). At present, no medicine exhibits all of
the aforementioned conditions, and benzodiazepines, opioid agonist,
propofol and α2-epinephrine agonists are commonly used in ICUs.
However, it has been demonstrated that high speed injection and
high dose of midazolam may result in respiratory depression and
reduced blood pressure, particularly in those elderly, hypovolemic
patients or patients with respiratory failure (2). Furthermore, high-dose infusion of
propofol for an extended period may cause propofol infusion
sydrome, which may involve serious lactic acidosis, hyperlipemia,
liver fatty infiltration, rhabdomyolysis and mortality (3). Therefore, these is a requirement for
alternative sedative agents for use in ICUs
Dexmedetomidine (DEX) is a highly selective
α2-adrenoceptor agonist with sedative, analgesic,
anti-anxiety and sympathetic nerve inhibitory activities. Patients
receiving DEX may be awakened easily without producing respiratory
arrest. Therefore, DEX has been considered to be an ideal sedative
and analgesic agent for use in intensive care unit (ICU) patients
(4,5). DEX is the dextro-isomer of
medetomidine, which exerts sedative and analgesic effects. In
addition, DEX has been shown to exert neuroprotective effects
(6) by agitating α2 receptor
mediated the receptor tyrosine kinase phosphorylation. Furthermore,
DEX promotes the release of various growth factors by agitating
astrocytes to participate in neural protection (7). DEX is able to activate survival
promoting enzymes by activating α2 adrenoceptor may
exertcardioprotective effects by adjusting the protein kinase,
protein kinase B and endothelial nitric oxide synthase pathways
extracellularly (8).
Currently, the infusion speed of DEX in mechanical
ventilation patients ranges between 0.2 and 0.7 µg/kg/h. Previous
studies have shown that DEX is safe for use in healthy patients at
10–15 times the normal dosage, producing no obvious side effects or
loss of blood pressure and heart rate (9–11).
However, a previous study indicated that DEX may reduce blood
pressure and heart rate in critical patients, potentially
necessitating drug discontinuation (12).
It was observed in our clinical practice that
certain patients receiving the recommended loading dose (1.0
µg/kg/10 min) plus the maintenance dose (0.2–0.7 µg/kg/h) of DEX
for sedation developed hypotension and bradycardia (13). In specific patients, DEX
administration had to be suspended, even in certain serious cases.
These observations are consistent with the most commonly known
adverse reactions of DEX, which include hypotension, nausea,
bradycardia and dry mouth (14).
However, critically ill patients in ICUs are physiopathologically
different from patients undergoing selective surgery, since they
frequently exhibit multi-organ dysfunctions involving the heart,
liver and kidney (15); therefore,
the in vivo metabolic process of DEX in ICU patients may
also be different.
The aim of the present study was to determine the
optimal dose of DEX for use in ICU patients by observing and
comparing the sedative effect of different doses of DEX on the
circulatory system of critically ill patients, in an attempt to
provide experimental references for the safe and effective clinical
use of DEX.
Materials and methods
General patient data
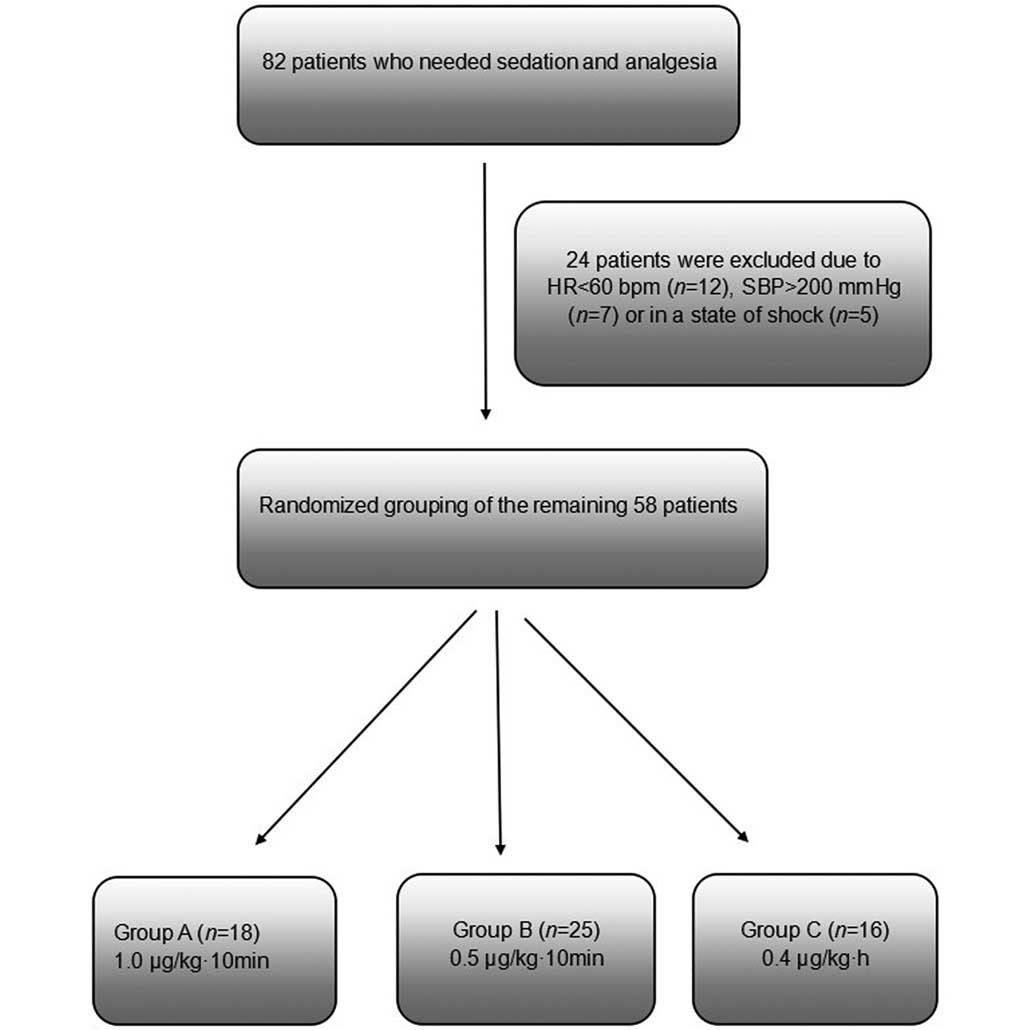
This study initially considered a total of 82
patients who were retained in the ICU at the First People's
Hospital Affiliated to Shanghai Jiao Tong University (Shanghai,
China) and required sedation between January and March 2014.
Patients were excluded if they presented blood pressure (BP) of
>200 mmHg and heart rate (HR) of <60 bpm, or if they
exhibited circulatory shock. A total of 24 patients were excluded
for these reasons. The remaining 58 patients were randomized into
three groups: Group A, high-dose group (1.0 µg/kg/10 min DEX;
n=18); group B, mid-dose group (0.5 µg/kg/10 min DEX; n=24); and
group C, routine-dose control group (0.4 µg/kg/h DEX; n=16). The
patients in the three groups initially received the designated
doses of DEX via an intravenous (IV) infusion pump for 10 min, and
were subsequently maintained continuously at the same dose of 0.4
µg/kg/h DEX (Fig. 1).
The study complied with the Declaration of Helsinki.
The data collection protocol was approved by the Shanghai First
People's Hospital Institutional Review Board. All participants
signed informed consent statements that allowed access to their
medical records.
Drug administration
DEX (2 ml; Sichuan Guorui Pharmaceutical Co., Ltd.,
Sichuan, China) was first diluted with 0.9% sodium chloride
solution to a total volume of 4 ml. Patients in the three groups
received the designated doses of DEX via an IV infusion pump for 10
min, and were then administered continuously with the same dose of
0.4 µg/kg/h DEX in order to maintain an ideal state of sedation, if
no significant adverse reaction occurred. DEX IV infusion was
controlled at a rate of 0.2–1.4 µg/kg/h. Propofol (Fresenius Kabi
Deutschland GmbH, Langenhagen, Germany) or midazolam (Jiangsu Enhua
Herun Medicine Co., Ltd., Xuzhou, China) was added to achieve
appropriate depth of sedation if necessary. If adequate sedation
was not achieved using DEX, fentanyl (Yichang Renfu Pharmaceutical
Co., Ltd., Yichang, China) was administered in a single dose of 1–4
µg/kg. IV pump infusion was suspended in patients with an HR of
<50 bpm or HR that had reduced by >30%, and a systolic blood
pressure (SBP) of <90 mmHg.
Observation parameters
Ramsay score, HR, SBP, diastolic blood pressure
(DBP), breathing rate (BR) and peripheral capillary oxygen
saturation (SpO2) were recorded prior to the IV pump
infusion (0 min) and at 2, 4, 6, 8, 10, 60, 120, 180 and 240 min
following infusion. Blood routine, electrolyte, liver/kidney
function and blood gas measurements were obtained prior to IV pump
infusion. Ramsay Score was evaluated by a physician according to
the Ramsay rating scale. HR and SBP were determined using an MP50
electrocardiogram monitor (Philips Healthcare, DA Best, The
Netherlands). Blood routine, electrolyte, liver/kidney function,
blood gas measurements were obtained using a Beckman Power
Processor Sample-Handling System (Beckman Coulter, Inc., Brea, CA,
USA). Acute Physiology and Chronic Health Evaluation II (APACHE II)
score was calculated (16).
Statistical treatment
Data analysis was performed using SPSS software,
version 16.0 (SPSS, Inc., Chicago, IL, USA). The results are
expressed as the mean ± standard deviation. The Shapiro-Wilk test
was used in combination with a histogram to determine whether data
were normally distributed. Data of normal distribution were
analyzed by analysis of variance, and data of non-normal
distribution were compared for inter-group difference using the
Mann-Whitney U nonparametric test. Categorical data were tested
using the χ2 test. P<0.05 was considered to indicate
a statistically significant difference.
Results
General data comparison
General patient characteristics in the three groups
are listed in Table I. No
statistically significant difference was observed in the APACHE II
score, gender, age and primary diagnosis between the three groups
(P>0.05).
 | Table I.General data comparison between the
three groups. |
Table I.
General data comparison between the
three groups.
| Parameter | Group A (n=18) | Group B (n=24) | Group C (n=16) |
|---|
| Gender
(male/female) | 6/12 | 19/5 | 11/5 |
| Age
(years)a | 57.72±16.50 | 50.83±20.53 | 50.25±21.43 |
| APACHE II
scorea | 12.78±5.31 | 8.54±4.84 | 10.44±7.07 |
| Primary diagnosis
(n) |
|
|
|
|
AECOPD | 2 | 2 | 1 |
| Severe
pneumonia | 1 | 0 | 1 |
|
Pneumothorax | 0 | 1 | 0 |
|
Cholecystitis | 2 | 1 | 0 |
|
Pancreatitis | 0 | 1 | 0 |
|
Digestive tract
perforation | 1 | 0 | 1 |
|
Cirrhosis | 0 | 1 | 0 |
| Upper
digestive tract hemorrhage | 0 | 2 | 0 |
|
Intestinal obstruction | 1 | 0 | 1 |
|
Sepsis | 1 | 1 | 0 |
|
Multiple trauma | 3 | 7 | 8 |
|
Post-operation | 3 | 4 | 2 |
|
Encephalitis | 0 | 2 | 0 |
|
Cerebral infarction | 1 | 1 | 2 |
|
Poisoning | 1 | 0 | 0 |
| HELLP
syndrome | 0 | 1 | 0 |
| Post
CPR | 1 | 0 | 0 |
|
Multiple myeloma | 1 | 0 | 0 |
| Organ dysfunction
(n) |
|
|
|
|
Respiratory failure | 1 | 2 | 3 |
| Cardiac
dysfunction | 2 | 0 | 0 |
| Renal
failure | 0 | 0 | 1 |
| Concomitant use of
sedatives (n) |
|
|
|
|
Midazolam | 1 | 1 | 0 |
|
Propofol | 0 | 5 | 0 |
|
Midazolam + propofol | 0 | 0 | 3 |
| Concomitant use of
vasoactive agents (n) |
|
|
|
|
Norepinephrine | 1 | 1 | 0 |
|
Dopamine | 1 | 1 | 1 |
|
Norepinephrine + dopamine | 2 | 0 | 0 |
| Use of mechanical
ventilation (n) | 11 | 8 | 8 |
| Drug
discontinuation (n) | 4 | 4 | 0 |
Achievement of the sedative state
Patients in all three groups achieved an ideal state
of sedation at 1 h after IV pump infusion of DEX, with a Ramsay
score of 3–4 (P>0.05). Groups A and B achieved an ideal state of
sedation at 6 min, which was more rapid compared with group C
(P<0.05; Fig. 2).
Differences in HR, SBP and DBP
following IV pump infusion of DEX
A decreasing tendency was observed in the HR, SBP
and DBP values following the initial IV pump infusion of DEX in all
three groups (Figs. 3–5). HR decreased more notably in groups A
and B compared with group C at 8 min and 60 min after IV pump
infusion of DEX (P<0.05), while there was no significant
difference in HR between groups A and B (P>0.05; Fig. 3). At 8 min after IV pump infusion of
DEX, SBP decreased more evidently in group A compared with the
value in group C (P<0.05; Fig.
4). In addition, at 10 min after IV pump infusion, SBP
decreased more evidently in group A compared with the value in both
groups B and C (P<0.05). However, no statistically significant
differences were detected in SBP reduction among the three groups
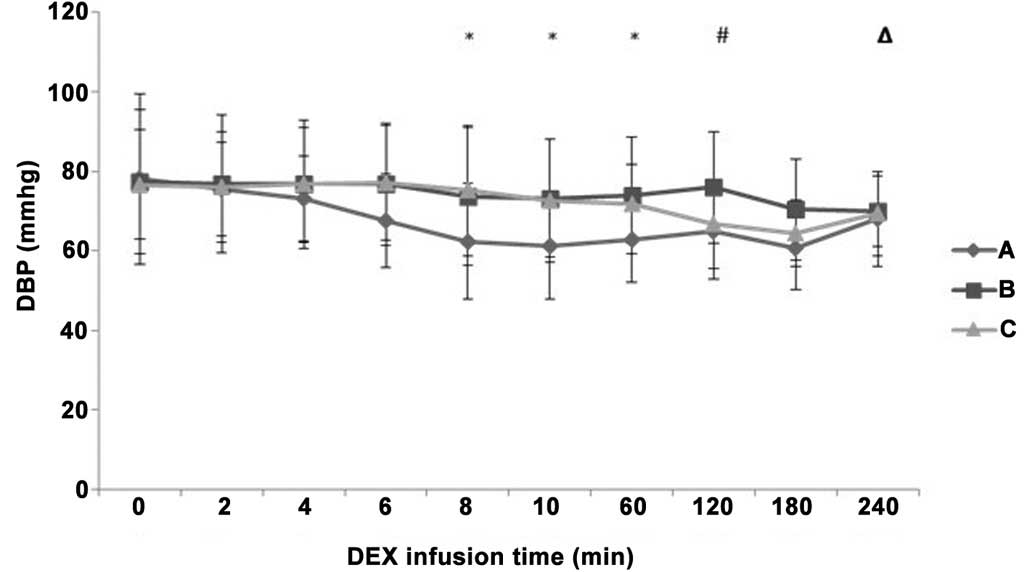
during the maintenance period (P>0.05). DBP was significantly
decreased in group A compared with groups B and C at 8 min and 60
min after IV pump infusion (P<0.05; Fig. 5). DBP in group B was increased
compared with groups A and C at 120 min after IV pump infusion
(P<0.05), while it decreased more significantly in group A
compared with group B at 240 min after IV pump infusion
(P<0.05).
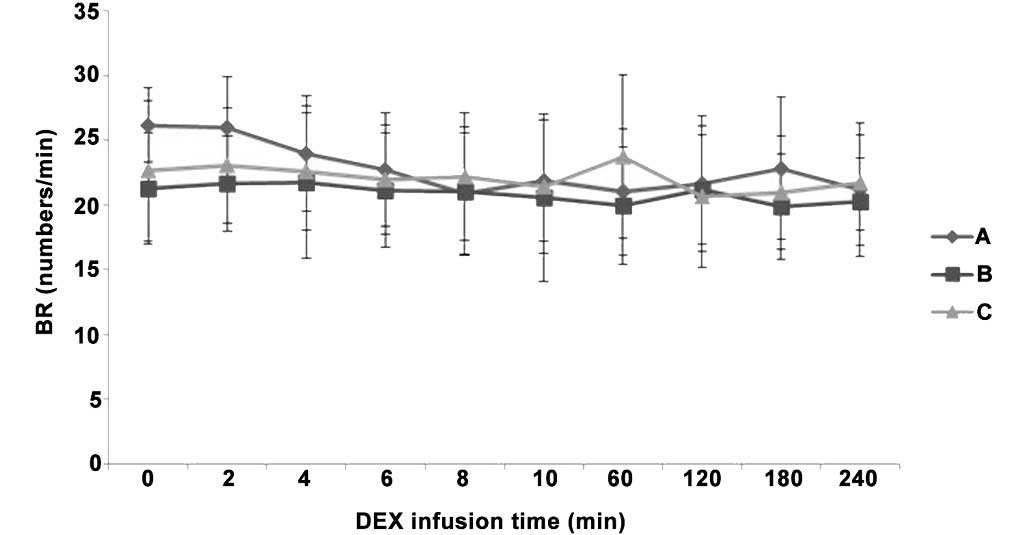
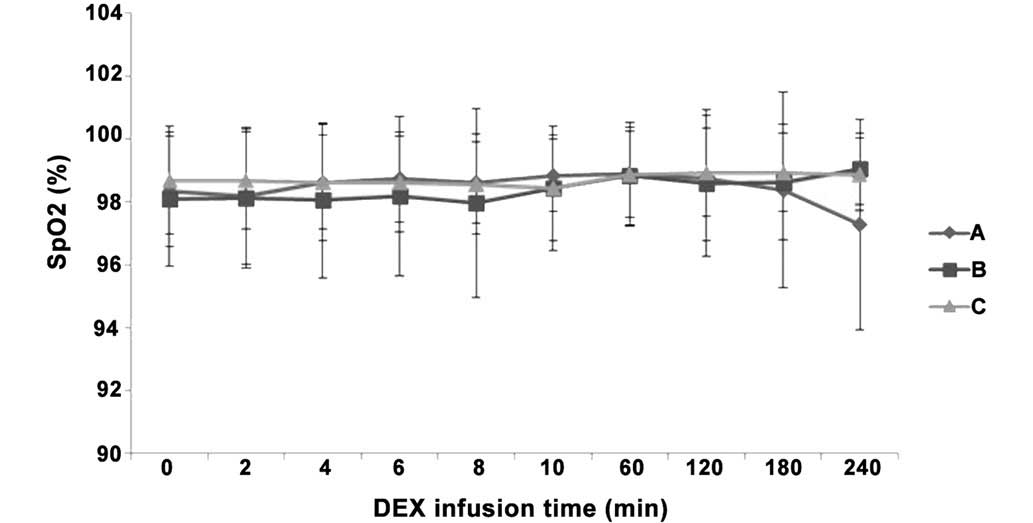
Differences in BR and SpO2 following IV
pump infusion of DEX. There were no indications of respiratory
arrest in any of the three groups, as well as no significant
differences in BR and SpO2 values between the three
groups (P>0.05). In addition, SpO2 was >97% at all
time points in all three groups (Figs.
6 and 7), indicating no indirect
inhibition of respiration.
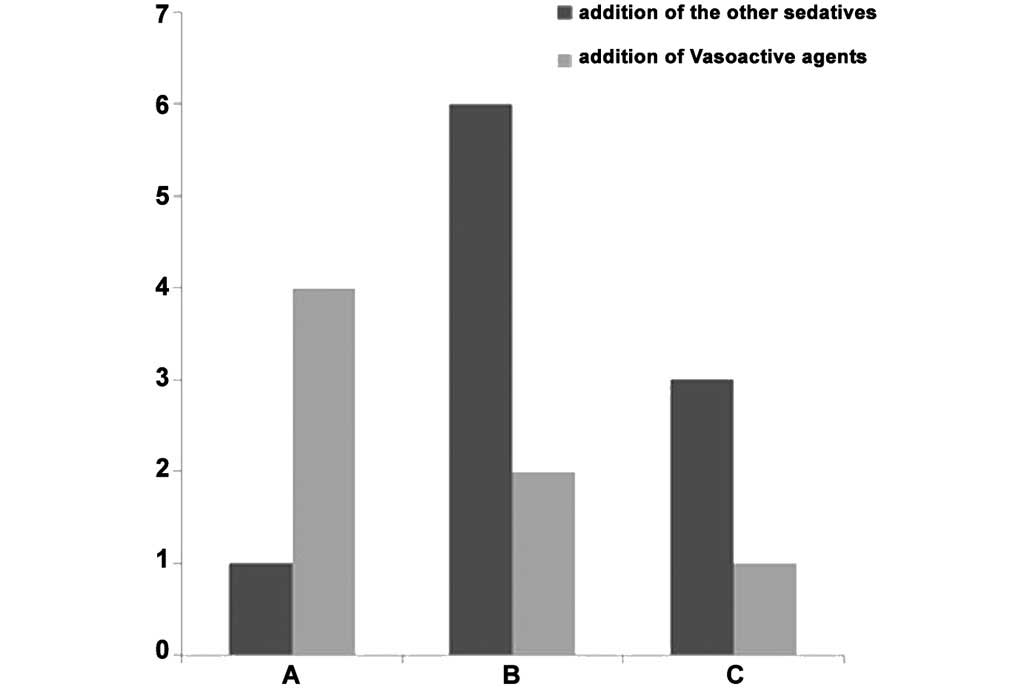
Concomitant use of medications
There was no significant difference between the
three groups following addition of other sedatives (P>0.05).
Vasoactive agents were administered to 4 patients in group A, 2
patients in group B and 1 patient in group C, and the difference
was not statistically significant (P>0.05; Table I; Fig.
8).
Drug withdrawal
DEX administration was suspended in 4 patients in
group A, 4 patients in group B and no patients in group C. Drug
withdrawal in group A was due to HR reduction of >30% in 2
patients and an SBP value of <90 mmHg in the other 2 patients.
Drug suspension in the 4 patients of group B was due to HR
reduction of >30% in 1 patient and an SBP value of <90 mmHg
in the other 3 patients. HR and BP values gradually returned to the
normal ranges following drug discontinuation, with no severe
consequences in any of the 8 patients (Table II).
 | Table II.Details of patients that underwent
drug withdrawal in the three groups. |
Table II.
Details of patients that underwent
drug withdrawal in the three groups.
|
|
|
|
|
|
|
| Abnormal laboratory
results |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Patient | Time of drug
suspension after medication | Age (years) | Gender | APACHE II
score | Primary
diagnosis | Mechanical
ventilation | Complete blood
count (Hb; g/l) | Blood gas
(PaCO2; mmHg) |
|---|
| A1 | 3 h | 88 | Female | 9 | AECOPD, pleural
effusion, pericardial effusion | BIPAP | 63.3 | 57.4 |
| A2 | 2 h | 55 | Male | 8 | Total hip
replacement | IPPV | Normal | Normal |
| A3 | 1 h | 59 | Female | 16 | Multiple
myeloma | IPPV | Normal | 68 |
| A4 | 3 h | 74 | Female | 3 | Postoperatively -
left femoral intertrochanteric | No | Normal | Normal |
|
|
|
|
|
| fracture |
| B1 | 4 h | 70 | Male | 16 | Upper digestive
tract hemorrhage, postoperation - gastric cancer | No | 37.6 | Normal |
| B2 | 3 h | 49 | Male | 8 | Cerebral
infarction | No | 73.3 | Normal |
| B3 | 10 min | 77 | Male | 20 | AECOPD, pleural
effusion | BIPAP | Normal | 66.7 |
| B4 | 1 h | 83 | Male | 6 | AECOPD, pulmonary
encephalopathy | BIPAP | Normal | 77.3 |
Discussion
Critically ill patients in ICUs frequently require
various supportive therapies, including mechanical ventilation,
vital sign monitoring, critical nursing care and constant
illumination to maintain the patients in a long-term sleep-deprived
state (17,18). Therefore, appropriate sedative
treatment is often required for ICU patients, as well as for
postoperative patients and patients with severe multiple injuries.
Midazolam, propofol and DEX are among the primary sedatives used in
ICUs. DEX is a highly selective α2-adrenoceptor agonist
that exerts its sedative and anti-anxiety effects by agonizing the
brainstem locus ceruleus, which is the most concentrated area of
α2 receptor in the central nervous system (19–21). DEX
exerts an analgesic effect and ameliorates stress response;
however, DEX simultaneously inhibits respiration by acting on
α2 receptors in the presynaptic membrane of the spinal
dorsal horn and postsynaptic membrane of interneurons (22,23). DEX
may be the preferred option for inducing sedation in ICU patients
as it is able to reduce the occurrence of delirium and minimize the
period of mechanical ventilation required (24,25).
The results of the present study showed that
continuous IV pump infusion of DEX was able to induce an ideal
state of sedation (Ramsay score, 3–4) in all three groups of
patients. The target level of sedation was achieved at ~6 min after
DEX infusion in groups A (1.0 µg/kg/10 min) and B (0.5 µg/kg/10
min). This is particularly crucial for the sedation of ICU
patients, as antagonism between spontaneous breathing and
mechanical ventilation may occur in patients if adequate sedation
cannot be achieved rapidly, which may affect the target tidal
volume, or even aggravate the existing pulmonary injury. Therefore,
a key aim of using DEX is to enable the patient to enter an ideal
state of sedation (24,26). In addition, continuous IV pump
infusion of DEX is able to maintain a Ramsay score of 3–4, at which
the patient is in an arousable ‘dormant’ state, which may attenuate
the injury from severe pathological factors while allowing the
patient to be awakened if necessary in order to perform actions for
the convenience of observing the condition and assessing
neurological functions.
A previous study demonstrated that DEX has the
function of bidirectional regulation of the cardiovascular system
(27). DEX initially agonizes the
α2B receptor of the postsynaptic membrane of the
vascular smooth muscle to induce tachycardia and hypertension via
vascular constriction. Subsequently, hypotension is induced via
vascular dilation under the central sympatholytic effect produced
by the continuous infusion of DEX. Therefore, DEX exerts a
predictable effect on hemodynamics (28). In the present patients, HR, SBP and
DBP tended to decrease following IV pump infusion with DEX;
however, all the mean values of these parameters were within the
normal ranges at all time points (HR, >60 bpm; SBP, >115
mmHg; and SBP, >60 mmHg). DEX administration was suspended in 8
patients in the present study due to unstable hemodynamics. The HR
and BP values of these patients were restored gradually to within
normal ranges following the withdrawal of DEX, with no severe
consequences.
Analysis of 8 patients (4 with hypercapnia, 3 with
anemia and 1 with hypercapnia and anemia) who were withdrawn from
DEX indicates that hypercapnia and anemia are two high-risk factors
contributing to unstable hemodynamics. The primary causes
underlying these results may be explained as follows: i) Patients
with acute exacerbation of chronic obstructive pulmonary disease
(AECOPD) are the primary group of patients in ICUs that typically
require mechanical ventilation due to unconsciousness and severe
hypercapnia. A previous study demonstrated that COPD may excite the
sympathetic nerve system and induce the reflectory release of
norepinephrine from the heart, causing hypertension and increasing
the HR (29). In COPD patients with
hypercapnia, the sympathetic system may be in a state of persistent
excitement even under normal blood gas conditions (30,31). ii)
Peripheral vascular dilation, decline in BP, excitement of the
sympathetic system, increase in HR and cardiac output and
subsequent renal vascular restriction, water-sodium retention and
increased BP are the primary physiopathological changes observed in
anemic patients. iii) The α2A receptor subtype serves a
crucial role in the pharmacology of DEX. This receptor exists in
pre- and postsynapses, primarily serving the function of inhibiting
norepinephrine release and neural excitement. DEX inhibits
norepinephrine release by agonizing the presynaptic membrane
α2 receptor, thus terminating transmission of the pain
signal. In addition, DEX inhibits the sympathetic activity by
agonizing the postsynaptic membrane α2 receptor. When
the in vivo blood concentration of DEX is sufficient to
inhibit sympathetic activity, the effect of sympathetic excitement
induced by AECOPD and anemia is also inhibited. This mechanism may
underlie the observed reduction in cardiovascular function and the
more marked differences in HR and BP in patients with AECOPD and
anemia (32–34). This mechanism is consistent with the
results of the present study, which identified that the majority of
patients that were withdrawn from DEX suffered from hypercapnia and
anemia.
An ideal state of sedation may be achieved using the
maintenance dose of DEX (0.4 µg/kg/h); however, this effect is
induced slowly. In order to achieve a more rapid sedative effect,
we suggest the use of a loading dose of 0.5 µg/kg/10 min. In
patients with AECOPD and anemia, high loading doses at a rapid rate
of IV pump infusion should be avoided. Combination medication may
be considered if necessary. As the sample size of the present study
is relatively small, the results obtained may not fully reflect the
effect of DEX on the circulatory system. Multi-center randomized
controlled trials in larger samples are required to verify the
present conclusions.
References
|
1
|
Oldham M and Pisani MA: Sedation in
critically ill patients. Crit Care Clin. 31:563–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Genta PR, Eckert DJ, Gregorio MG, Danzi
NJ, Moriya HT, Malhotra A and Lorenzi-Filho G: Critical closing
pressure during midazolam-induced sleep. J Appl Physiol (1985).
111:1315–1322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perrier ND, Baerga-Varela Y and Murray MJ:
Death related to propofol use in an adult patient. Crit Care Med.
28:3071–3074. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pichot C, Ghignone M and Quintin L:
Dexmedetomidine and clonidine: From second- to first-line sedative
agents in the critical care setting? J Intensive Care Med.
27:219–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rayner SG, Weinert CR, Peng H, Jepsen S
and Broccard AF: Study Institution: Dexmedetomidine as adjunct
treatment for severe alcohol withdrawal in the ICU. Ann Intensive
Care. 23:122012. View Article : Google Scholar
|
|
6
|
Yang L, Xu JM, Jiang X, Ruan W, Cui Y and
He L: Effect of dexmedetomidine on plasma brain-derived
neurotrophic factor: A double-blind, randomized and
placebo-controlled study. Ups J Med Sci. 118:235–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chrysostomou CI, Beerman L, Shiderly D,
Berry D, Morell VO and Munoz R: Dexmedetomidine: A novel drug for
the treatment of atrial and junctional tachyarrhythmias during the
perioperative period for congenital cardiac surgery: A preliminary
study. Anesth Analg. 107:1514–1522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jalowiecki P, Rudner R, Gonciarz M,
Kawecki P, Petelenz M and Dziurdzik P: Sole use of dexmedetomidine
has limited utility for conscious sedation during outpatient
colonoscopy. Anesthesiology. 103:269–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giovannitti JA Jr, Thoms SM and Crawford
JJ: Alpha-2 adrenergic receptor agonists: A review of current
clinical applications. Anesth Prog. 62:31–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Longrois D, Conti G, Mantz J, Faltlhauser
A, Aantaa R and Tonner P: Sedation in non-invasive ventilation: Do
we know what to do (and why)? Multidiscip Respir Med. 9:562014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishizawa T, Suzuki H, Sagara S, Kanai T
and Yahagi N: Dexmedetomidine versus midazolam for gastrointestinal
endoscopy: A meta-analysis. Dig Endosc. 27:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodwin HE, Gill RS, Murakami PN, Thompson
CB, Lewin JJ 3rd and Mirski MA: Dexmedetomidine preserves
attention/calculation when used for cooperative and short-term
intensive care unit sedation. J Crit Care. 28:1113.e7–1113.e10.
2013. View Article : Google Scholar
|
|
13
|
Ludtke KA, Stanley KS, Yount NL and Gerkin
RD: Retrospective review of critically ill patients experiencing
alcohol withdrawal: Dexmedetomidine versus propofol and/or
lorazepam continuous infusions. Hosp Pharm. 50:208–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhana N, Goa KL and McClellan KJ:
Dexmedetomidine. Drugs. 59:263–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassett R, Adams KM, Danesh V, Groat PM,
Haugen A, Kiewel A, Small C, Van-Leuven M, Venus S and Ely EW:
Rethinking critical care: Decreasing sedation, increasing delirium
monitoring, and increasing patient mobility. Jt Comm J Qual Patient
Saf. 41:62–74. 2015.PubMed/NCBI
|
|
16
|
Del Bufalo C, Morelli A, Bassein L, Fasano
L, Quarta CC, Pacilli AM and Gunella G: Severity scores in
respiratory intensive care: APACHE II predicted mortality better
than SAPS II. Respir Care. 40:1042–1047. 1995.PubMed/NCBI
|
|
17
|
Maraboto E: ABCDEs of ICU: Choice of
sedative. Crit Care Nurs Q. 36:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Devabhakthuni S, Armahizer MJ, Dasta JF
and Kane-Gill SL: Analgosedation: A paradigm shift in intensive
care unit sedation practice. Ann Pharmacother. 46:530–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gudmundsson G, Ulrik CS, Gislason T,
Lindberg E, Brøndum E, Bakke P and Janson C: Long-term survival in
patients hospitalized for chronic obstructive pulmonary disease: A
prospective observational study in the Nordic countries. Int J
Chron Obstruct Pulmon Dis. 7:571–576. 2012.PubMed/NCBI
|
|
20
|
Ai-Ping C, Lee KH and Lim TK: In-hospital
and 5-year mortality of patients treated in the ICU for acute
exacerbation of COPD: A retrospective study. Chest. 128:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andreas S, Haarmann H, Klarner S,
Hasenfuss G and Raupach T: Increased sympathetic nerve activity in
COPD is associated with morbidity and mortality. Lung. 192:235–241.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerlach AT and Dasta JF: Dexmedetomidine:
An updated review. Ann Pharmacother. 41:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riker RR, Shehabi Y, Bokesch PM, Ceraso D,
Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW and
Rocha MG: SEDCOM (Safety and Efficacy of Dexmedetomidine Compared
With Midazolam) Study Group: Dexmedetomidine vs. midazolam for
sedation of critically ill patients: A randomized trial. JAMA.
301:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pandharipande PP, Pun BT, Herr DL, Maze M,
Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen
SA, et al: Effect of sedation with dexmedetomidine vs lorazepam on
acute brain dysfunction in mechanically ventilated patients: The
MENDS randomized controlled trial. JAMA. 298:2644–2653. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maldonado JR, Wysong A, van der Starre PJ,
Block T, Miller C and Reitz BA: Dexmedetomidine and the reduction
of postoperative delirium after cardiac surgery. Psychosomatics.
50:206–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozaki M, Takeda J, Tanaka K, Shiokawa Y,
Nishi S, Matsuda K, Doi M, Kakihana Y, Fujino Y, Takinami M and
Kawai M: Safety and efficacy of dexmedetomidine for long-term
sedation in critically ill patients. J Anesth. 28:38–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takata K, Adachi YU, Suzuki K, Obata Y,
Sato S and Nishiwaki K: Dexmedetomidine-induced atrioventricular
block followed by cardiac arrest during atrial pacing: A case
report and review of the literature. J Anesth. 28:116–120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bharati S, Pal A, Biswas C and Biswas R:
Incidence of cardiac arrest increases with the indiscriminate use
of dexmedetomidine: A case series and review of published case
reports. Acta Anaesthesiol Taiwan. 49:165–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakamaki F, Satoh T, Nagaya N, Kyotani S,
Nakanishi N and Ishida Y: Abnormality of left ventricular
sympathetic nervous function assessed by (123)
I-metaiodobenzylguanidine imaging in patients with COPD. Chest.
116:1575–1581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Victor RG and Shafiq MM: Sympathetic
neural mechanisms in human hypertension. Curr Hypertens Rep.
10:241–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown SJ, Raman A, Barnes MJ and Mundel T:
Autonomic cardiovascular response to acute hypoxia and passive
head-up tilting in humans. Eur J Appl Physiol. 113:1731–1736. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vincent JL, Baron JF, Reinhart K,
Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G and
Peres-Bota D: ABC (Anemia and Blood Transfusion in Critical Care)
Investigators: Anemia and blood transfusion in critically ill
patients. JAMA. 288:1499–1507. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dünser MW and Hasibeder WR: Sympathetic
overstimulation during critical illness: Adverse effects of
adrenergic stress. J Intensive Care Med. 24:293–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Franchitto N, Despas F, Labrunée M,
Roncalli J, Boveda S, Galinier M, Senard JM and Pathak A: Tonic
chemoreflex activation contributes to increased sympathetic nerve
activity in heart failure-related anemia. Hypertension.
55:1012–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|