Introduction
In liver surgery, hepatic blood flow is typically
blocked in order to reduce bleeding; however, this inevitably
results in hepatic ischemia/reperfusion injury (HI/R) (1). HI/R injury does not only cause
significant direct damage to liver cells; but also alters the
regenerative ability of these cells, which is a critical factor
influencing the success rate of liver surgery and the postoperative
survival rate of patients (2). Under
physiological conditions, reactive oxygen species (ROS) are
continuously generated and cleared in the body, in order to
maintain a state of dynamic equilibrium (3). However, the hypoxic environment of
ischemic liver cells following HI/R injury promotes the elevated
catabolism of adenosine triphosphate, which in turn results in
accumulation of the decomposition product, hypoxanthine (4). Furthermore, hypoxia results in
inactivation or depletion of endogenous antioxidants, including
superoxide dismutase (SOD) (5).
Tumor necrosis factor-α (TNF-α) is a mononuclear
factor that is primarily produced by monocytes and macrophages.
TNF-α is able to increase the phagocytosis of neutrophils, promote
the secretion of interleukin (IL)-1 and IL-6 from endothelial
cells, and strengthen the adhesion of neutrophils and endothelial
cells, thereby stimulating the body to expand local inflammation
and the tissue response to injury (6,7).
Furthermore, TNF-α may stimulate monocytes and macrophages to
secrete IL-1, which in turn further induces the production of TNF-α
(8). Following excessive TNF-α
production in the acute phase of HI/R injury, due to toxic
stimulation within the body, IL-1 serves as a pro-inflammatory
cytokine that activates various immune and inflammatory cells
(9). The levels of IL-1 not only
reflect the extent of the inflammatory response, but also direct
clinical treatment (9). IL-6 is
secreted by activated macrophages, lymphocytes and epithelial
cells, and may be induced by IL-1β and TNF-α (10). Notably, the levels of IL-6 have been
demonstrated to decrease following attenuation of HI/R injury, thus
indicating that IL-6 exists as a result of HI/R injury and has an
important role in the development of HI/R injury (11).
Paeoniflorin (PF), which is the main active
ingredient in the traditional Chinese medicine peony, is a
monoterpene glycoside compound. Previous studies have studied the
pharmacological effects of PF, and detected activities of the
compound, including anti-radical damage, intracellular
calcium-overload inhibition and anti-neurotoxicity, as well as
numerous biological effects, including blood vessel dilatation,
microcirculation improvement, anti-oxidization and anti-convulsion
(12–14). Furthermore, Cao et al
(15) demonstrated a protective
effect of PF on the blood-brain barrier following cerebral
ischemia, as well as on local cerebral blood flow and brain edema.
Furthermore, it has been indicated that PF may have a significant
protective role in focal cerebral ischemic injury, by inhibiting
intracellular calcium overload, protecting against free radicals,
and improving cerebral vasomotor dysfunction caused by ischemia and
anoxia (16). In addition, PF has
been shown to protect the blood-brain barrier following cerebral
perfusion during ischemia, and promote the recovery of cerebral
blood flow in the early period of reperfusion. PF can also
significantly increase SOD levels in rat brain tissue, decrease
malondialdehyde (MDA) levels, and alleviate oxidative stress injury
in brain tissue caused by cerebral ischemia (16). Previous studies have demonstrated
that PF may markedly reduce the expression levels of nuclear factor
(NF)-κB (15,17). NF-κB and B-cell lymphoma-2 (Bcl-2)
expression levels decreased gradually with increasing PF
concentrations (18–20). These studies have also suggested that
PF possesses anti-oxidative and anti-apoptotic properties; however,
to the best of our knowledge, no previous research has assessed the
ability of PF to relieve HI/R in mice. Therefore, the present study
aimed to investigate the potential application of PF in the
treatment of HI/R, as well as to determine the underlying
mechanisms.
Materials and methods
Animals and establishment of HI/R
model
Thirty male BALB/c mice (age, 6–8 weeks old, weight,
23±4 g) were maintained in a standard animal laboratory under
controlled conditions (light between 7:00 a.m. and 6:00 p.m.;
50–70% humidity; 24°C) and with ad libitum access to food
and water. The mice were purchased from SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). All experimental procedures were approved
by the Institutional Animal Care and Ethics Committee of The First
Affiliated Hospital of Dalian Medical University (Dalian,
China).
HI/R method was performed according to previous
guidelines, with minor modifications (21–24).
Briefly, mice were anesthetized with 99% ether (Invitrogen, Grand
Island, NY, USA). Following anesthesia, the abdomen was shaved and
cleansed with 10% povidone-iodine. Subsequently, mice underwent a
median laparotomy. The hepatoportal vein, hepatic artery and
hepatic duct were sought out and separated, after which they were
clamped for 30 min, and the abdomen was temporarily closed with a
suture. Mice were sacrificed by decollation for experimental assays
following a 6 h reperfusion period with an atraumatic vascular
clamp, during which they were maintained at a constant temperature
via the use of a heated blanket.
Drug preparation
The chemical structure of PF is shown in Fig. 1. PF (Sigma-Aldrich China, Inc.,
Shanghai, China), with a purity >98%, was dissolved in
physiological saline, according to the manufacturer's instructions
and injected into the tail vein of the mice 5 min prior to HI/R
injury. A total of 30 mice were randomized into five groups, each
containing six animals: Sham, Vehicle, and PF treatment groups (5,
10 and 20 mg/kg) (25). The sham
(control) group did not undergo the clamping operation, whereas the
vehicle group underwent the procedure before injections of the same
volume of physiological saline. PF treatment groups underwent the
procedure of HI/R prior to injection with 5, 10, or 20 mg/kg of PF
via the tail vein. Following the reperfusion period, and 24 h
following clip removal, the blood and liver samples of all mice
were collected and stored at −80°C prior to analysis. Blood samples
were taken from the suprahepatic inferior vena cava and were
centrifuged at 3,500 × g for 5 min, in order to obtain serum at
room temperature for determination of ALT and AST levels. Liver
tissue samples from each animal were measured for hepatic tissue
SOD, MDA, glutathione (GSH), glutathione peroxidase (GSH-PX, NF-κB,
TNF-α, IL-1β and IL-6 levels, together with evaluating the
activities of caspase-3.
Detection of serum ALT and AST
levels
Liver injury was measured by serum levels of ALT and
AST. Blood samples were centrifuged at 3,500 × g for 5 min, in
order to obtain serum at room temperature. Serum levels of ALT and
AST were measured using an automated analyzer (Model 200; Toshiba,
Tokyo, Japan). ALT and AST levels were determined as described
previously (26) and expressed as
international units per liter (U/l).
NF-κB, TNF-α, IL-1β and IL-6 secretion
analysis
Liver tissue was homogenized using a homogenizer
(ZW-800D, Wenzhou Weike Biological Laboratory equipment Co., Ltd.,
Wenzhou, China) and centrifuged at 13,200 × g for 20 min at 4°C.
Following centrifugation, the supernatant was retained for storage
at −80°C. Subsequently, 15 µg nuclear protein, extracted from liver
tissue using a powder with liquid nitrogen and lysed in
radioimmunoprecipitation assay buffer, was analyzed, in order to
detect NF-κB, TNF-α, IL-1β and IL-6 levels using commercially
available ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA),
and absorbance was measured using a TECAN Sunrise ELISA Reader
(Tecan Group Ltd., Männedorf, Switzerland) at 450 nm.
Assay of SOD, MDA, GSH, and GSH-PX
activities
Blood samples were centrifuged at 1,000 × g for 10
min to obtain serum at room temperature. The supernatant was stored
at −80°C until assays for SOD, MDA GSH, and GSH-PX were
established. The enzymatic activities of SOD, MDA GSH, and GSH-PX
in the serum homogenate was evaluated according to the
manufacturer's instructions (Shimadzu Corporation, Kyoto,
Japan).
Liver tissue was homogenized using a homogenizer
(ZW-800D, Wenzhou Weike Biological Laboratory equipment Co., Ltd.),
and the enzymatic activity of SOD in hepatic tissue homogenate was
evaluated by determining the rate (U/mg protein) of inhibition of
nucleotide oxidation. The level of MDA (nmol/mg protein) in the
serum homogenate was measured spectrophotometrically using the
Sunrise Remote R-microplate reader at 532 nm. The level of GSH
(mmol/mg protein) in the serum homogenate was evaluated by
monitoring the reduction of dithiobis-2-nitrobenzoic acid (4 mg/ml
in methanol), after which, readings were taken
spectrophotometrically using the Sunrise Remote R-microplate reader
at 412 nm. GSH-PX activity (U/mg protein) in the serum homogenate
was determined at the end of the reaction, using the Sunrise Remote
R-microplate reader, by measuring absorption at 412 nm.
Analysis of caspase-3 activity
Caspase-3 activity was analyzed 6 h following HI/R
injury. Liver tissue was homogenized using a homogenizer (Haimen
Botai Experimental Equipment) and centrifuged at 13,200 × g for 20
min at 4°C. Following centrifugation, the supernatant was stored at
−80°C for subsequent analysis. In accordance with the
manufacturer's instructions, the activity of caspase-3 was analyzed
by the cleavage of chromogenic caspase substrates (Beyotime
Institute of Biotechnology, Nantong, China).
Statistical analysis
All results were expressed as the mean ± standard
deviation. Comparisons between groups were performed using
Student's t-test and one-way analysis of variance. All statistical
analyses were performed using SPSS 15.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with PF reduces serum levels
of ALT and AST following HI/R in murine hepatic tissue
Levels of serum ALT in the vehicle group were
significantly increased from 18.53±3.23 to 1956.53±34.89 U/l
(P<0.01, n=6) 6 h following HI/R, as compared with the sham
group (Fig. 2A). Following treatment
with PF, the levels of serum ALT were significantly reduced from
1956.53±34.89 to 200.89±36.44 (5 mg/kg treatment group, P<0.01,
n=6), 189.53±26.89 (10 mg/kg treatment group, P<0.01, n=6) and
168.46±20.14 U/l (20 mg/kg treatment group, P<0.01, n=6), as
compared with the vehicle group (Fig.
2A). Similarly, the serum levels of AST in the vehicle group
were significantly enhanced from 20.58±3.45 to 3546.89±45.89 U/l
(P<0.01, n=6) 6 h following HI/R, as compared with the sham
group (Fig. 2B). Conversely, the
serum AST levels of the PF-treated groups markedly decreased from
3546.89±45.89 to 486.56±26.78 (5 mg/kg treatment group, P<0.01,
n=6), 351.46±32.89 (10 mg/kg treatment group, P<0.01, n=6) and
298.46±46.11 U/l (20 mg/kg treatment group, P<0.01, n=6), as
compared with the vehicle group (Fig.
2B).
Treatment with PF relieves HI/R injury
via its anti-oxidative activity
In order to corroborate the effects of PF on
oxidative stress during HI/R injury of mice, the levels of MDA, and
the activities of SOD, GSH and GSH-PX in hepatic tissue were
evaluated (Fig. 3A–D). The levels of
MDA in the vehicle group were significantly increased from
2.15±0.12 to 6.23±0.52 nmol/mg protein (P<0.01, n=6) 6 h
following HI/R, as compared with the sham group (Fig. 3A). Following treatment with PF, the
levels of MDA were significantly reduced from 6.23±0.52 to
4.12±0.32 (5 mg/kg treatment group, P<0.01, n=6), 3.62±0.31 (10
mg/kg treatment group, P<0.01, n=6) and 2.89±0.45 nmol/mg
protein (20 mg/kg treatment group, P<0.01, n=6), as compared
with the vehicle group (Fig.
3A).
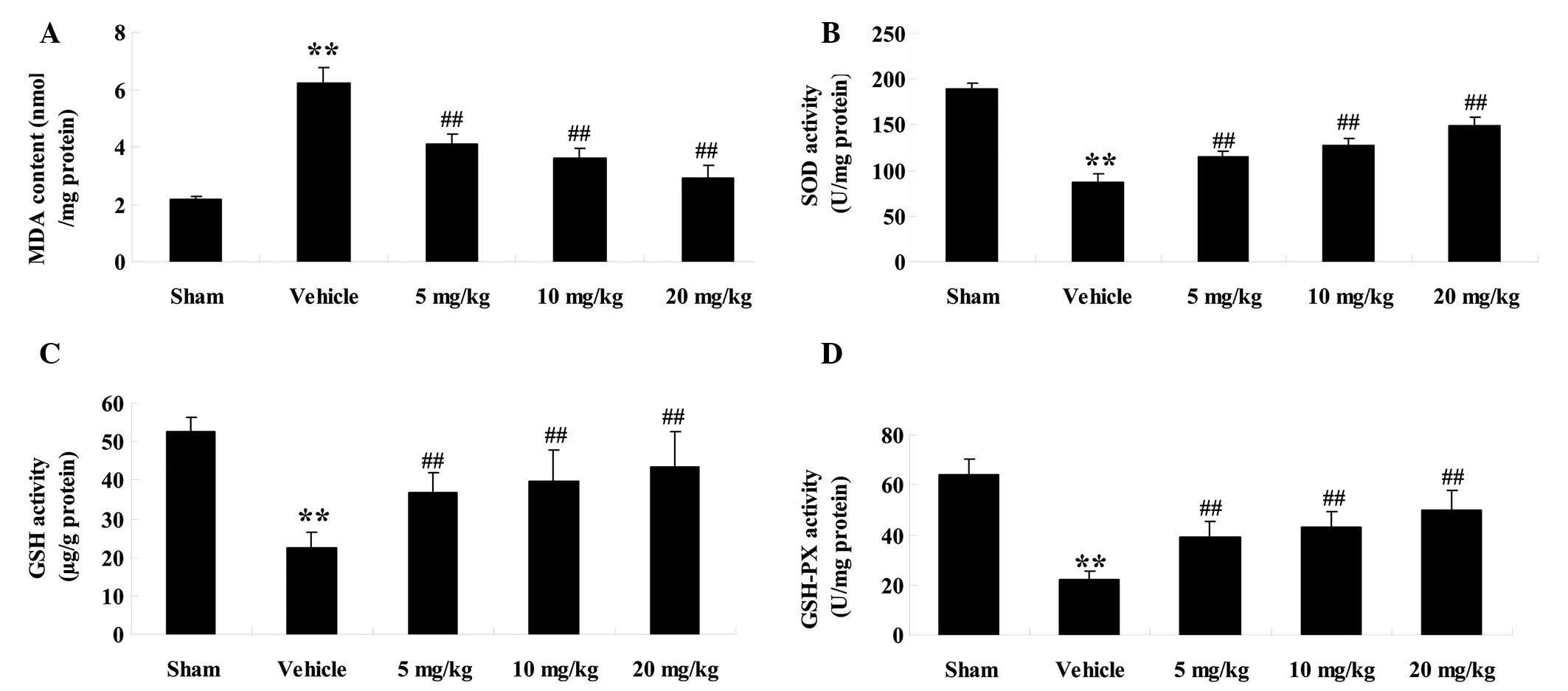 | Figure 3.Effects of paeoniflorin (PF) on the
levels of malondialdehyde (MDA), and the activity of superoxide
dismutase (SOD), glutathione (GSH) and glutatione-peroxidase
(GSH-PX) in hepatic tissue 6 h following hepatic
ischemia/reperfusion injury. The levels of (A) MDA, the activity of
(B) SOD, (C) GSH and (D) GSH-PX, were detected in the various
groups. Data are presented as the mean ± standard deviation (n=6).
**P<0.01 vs. the sham group; ##P<0.01 vs. the
vehicle group. Sham, sham-operated; Vehicle, vehicle-treated; 5
mg/kg, PF (5 mg/kg)-treated group, 10 mg/kg, PF (10 mg/kg)-treated
group and 20 mg/kg, PF (20 mg/kg)-treated group. |
Conversely, the activity of SOD in the vehicle group
was significantly reduced from 189.36±5.64 to 86.89±8.69 U/mg
protein (P<0.01, n=6) 6 h following HI/R, as compared with the
sham group (Fig. 3B). In the PF
treatment groups, the activity of SOD was significantly enhanced
from 86.89±8.69 to 114.86±6.53 (5 mg/kg treatment group, P<0.01,
n=6), 126.89±7.56 (10 mg/kg treatment group, P<0.01, n=6), and
149.26±9.56 U/mg protein (20 mg/kg treatment group, P<0.01,
n=6), as compared with the vehicle group (Fig. 3B).
The contents of GSH in the vehicle group were
significantly decreased from 52.63±3.56 to 22.56±4.01 µg/g protein
(P<0.01, n=6), as compared with the sham group, 6 h following
HI/R(Fig. 3C). Whereas, following PF
treatment, the levels of GSH significantly increased to 36.89±5.01
(5 mg/kg treatment group, P<0.01, n=6), 39.58±8.23 (10 mg/kg
treatment group, P<0.01, n=6) and 43.56±9.26 µg/g protein (20
mg/kg, treatment group, P<0.01, n=6) (Fig. 3C).
The activity of GSH-PX in the vehicle group declined
from 63.89±6.35 to 21.86±3.59 U/mg protein (P<0.01, n=6) as
compared with the sham group (Fig.
3D0 6 h following HI/R. In the PF treatment groups, the
activity of GSH-PX was significantly increased to 38.95±6.36 (5
mg/kg treatment group, P<0.01, n=6), 42.98±6.31 (10 mg/kg
treatment group, P<0.01, n=6) and 49.86±8.29 U/mg protein (20
mg/kg treatment group, P<0.01, n=6) (Fig. 3D).
Treatment with PF reduces the
expression levels of NF-κB, TNF-α, IL-1β and IL-6 following HI/R in
murine hepatic tissue
In order to investigate the effects of PF on
inflammatory mediators during HI/R injury in mice, the present
study evaluated the expression levels of NF-κB, TNF-α, IL-1β and
IL-6 in hepatic tissue (Fig. 4A–D).
Expression levels of NF-κB in the vehicle group significantly
increased from 9.63±1.23 to 45.26±3.65 ng/mg protein (P<0.01,
n=6) 6 h following HI/R, as compared with the sham group (Fig. 4A). In the PF treatment groups, the
expression of NF-κB significantly decreased from 45.26±3.65 to
29.86±2.56 (5 mg/kg treatment group, P<0.01, n=6), 22.59±3.01
(10 mg/kg treatment group, P<0.01, n=6), and 19.68±2.99 ng/mg
protein (20 mg/kg treatment group, P<0.01, n=6), as compared
with the vehicle group (Fig.
4A).
 | Figure 4.Effects of paeoniflorin (PF) on the
expression levels of nuclear factor (NF)-κB, tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-6 in hepatic tissues 6 h
following hepatic ischemia/reperfusion injury. The expression
levels of (A) NF-κB, (B) TNF-α, (C) IL-1β and (D) IL-6, were
detected in various groups. Data are presented as the mean ±
standard deviation (n=6). **P<0.01 vs. the sham group;
##P<0.01 vs. the vehicle group. Sham, sham-operated;
Vehicle, vehicle-treated; 5 mg/kg, PF (5 mg/kg)-treated group, 10
mg/kg, PF (10 mg/kg)-treated group, and 20 mg/kg, PF (20
mg/kg)-treated group. |
Similarly, the expression levels of TNF-α in the
vehicle group were increased from 76.56±6.59 to 289.65±8.56 pg/mg
protein (P<0.01, n=6) as compared with the sham group (Fig. 4B). Following treatment with PF, the
expression levels of TNF-α significantly decreased from 289.65±8.56
to 210.98±9.21 (5 mg/kg treatment group, P<0.01, n=6),
195.89±8.56 (10 mg/kg treatment group, P<0.01, n=6) and
168.56±6.89 pg/mg protein (20 mg/kg treatment group, P<0.01,
n=6), as compared with the vehicle group (Fig. 4B).
The expression levels of IL-1β in the vehicle group
were markedly increased from 2.65±0.25 to 5.26±0.59 pg/mg protein
(P<0.01, n=6), as compared with the sham group (Fig. 4C). In the PF treatment groups, the
expression levels of IL-1β were significantly reduced to 3.99±0.68
(5 mg/kg treatment group, P<0.01, n=6), 3.25±0.98 (10 mg/kg
treatment group, P<0.01, n=6) and 3.04±0.58 pg/mg protein (20
mg/kg treatment group, P<0.01, n=6) (Fig. 4C).
The expression levels of IL-6 in the vehicle group
were markedly increased from 1.65±0.26 to 5.01±0.35 mg/g protein
(P<0.01, n=6), as compared with the sham group (Fig. 4D). In the PF treatment groups, the
expression levels of IL-6 were significantly reduced to 3.14±0.31
(5 mg/kg treatment group, P<0.01, n=6), 2.98±0.36 (10 mg/kg
treatment group, P<0.01, n=6) and 2.56±0.33 pg/mg protein (20
mg/kg treatment group, P<0.01, n=6) (Fig. 4D).
Treatment with PF reduces caspase-3
activity
In order to confirm that PF was able to reduce
caspase-3 activity, a colorimetric analysis was conducted (Fig. 5). Caspase-3 activity in the vehicle
group was markedly increased to 6.58±0.16 (P<0.01, n=6), as
compared with the sham group. Whereas, in the PF treatment groups
(5, 10 and 20 mg/kg), there was a marked decrease in caspase-3
activity to 3.25±0.21 (P<0.01, n=6), 2.98±0.25 (P<0.01, n=6),
and 2.26±0.31 (P<0.01, n=6), respectively, as compared with the
vehicle group.
Discussion
PF, which is the main active ingredient of the
traditional Chinese medicine peony, has been shown to possess
numerous biological activities. Previous studies have demonstrated
that PF is able to increase SOD levels in ischemic brain tissue,
and reduce MDA content (27). In
addItion, it has been suggested that PF inhibits the production of
free radicals, improves SOD activity in the brain, and decreases
MDA content following cerebral ischemia; therefore protecting
secondary neurons from injury (15,28,29). PF
has a strong anti-inflammatory effect, which has been shown to
reduce the enhanced phagocytic function of peritoneal macrophages
in rheumatoid arthritis models, and lower levels of TNF-α, IL-1 and
IL-6 (30–32). Furthermore, PF may reduce the level
of lipid oxidation in liver homogenates, and increase the enzymatic
activities of SOD and GSH-PX (29).
The present study investigated whether PF could reduce HI/R in a
murine model. The results of the present study suggested that PF
may protect hepatic function from HI/R injury; and the
hepatoprotective effect of PF may be associated with its
anti-inflammatory and anti-oxidative properties.
HI/R injury is not only a predominant cause of poor
prognosis in patients with hepatic failure; it also causes
oxidative stress injury due to the over-production of ROS. ROS are
incredibly volatile and can destroy important structural and
functional proteins in cells, potentially causing cell and tissue
death. Furthermore, I/R injury may lead to high oxidative stress
and oxidative damage of brain tissue. The body responds to the
over-production of ROS by increasing the expression levels of
antioxidant enzymes, including SOD and catalase. Manipulation of
this strategy may become a novel measure for prevention or
alleviation of brain injury induced by I/R injury (33). In the present study, the activities
of SOD, GSH and GSH-PX in hepatic tissue were significantly higher
following treatment with PF, as compared with ischemic mice;
however, the levels of MDA were significantly lower. Therefore, the
present study demonstrated that PF may relieve HI/R injury via its
anti-oxidative activity.
When blood flow is restored, the tissue retrieves
oxygen, and this can activate the secretion of related humoral
inflammatory mediators in the body due to the stimulation of
apoptotic cells, including inflammatory mediators (NF-κB, TNF-α,
IL-1β and IL-6), ROS, lipid mediators and peptide media (7,10,34).
Activation and release of inflammatory factors is also a key
element of HI/R injury. Inflammatory cytokines activate endothelial
cells and are involved in the inflammatory process, which
stimulates the body to secrete vasoactive components and other
media. The media promote leukocyte infiltration into ischemic
tissue in order to enhance the production of ROS and other
cytotoxic factors; however, these also aggravate the damaged
tissue. Reducing the production of inflammatory factors in the HI/R
process may reduce injury from reperfusion and, concordantly,
inflammatory cells are becoming one of the hotspots in HI/R
research. In the present study, the expression levels of NF-κB,
TNF-α, IL-1β and IL-6 in hepatic tissue were markedly reduced
following treatment with PF, as compared with ischemic mice. The
present study demonstrated that PF may relieve HI/R injury via its
anti-inflammatory activity.
Caspase-3 is regarded as the ultimate mediator of
cell apoptosis in the caspase family. Activation of caspase-3 has
been reported to induce apoptosis via endoplasmic reticulum stress,
and HI/R may significantly enhance caspase-3 (35). The present study demonstrated the
ability of PF to reduce the HI/R injury-induced expression of
caspase-3 in hepatic tissue. Consistent with this finding, PF was
also shown to inhibit cellular apoptosis following renal HI/R
injury, suggesting that the therapeutic effect of PF may also be
associated with its anti-apoptotic action in ischemic mice, as well
as its anti-inflammatory activity.
In conclusion, the present data demonstrated that PF
was able to attenuate HI/R injury by minimizing oxidative and
inflammatory stress and by decreasing the expression of
apoptosis-associated proteins. Therefore, the hepatoprotective
effect of PF in a HI/R mouse model may be related to its
anti-oxidative, anti-inflammatory and/or anti-apoptotic
properties.
Acknowledgements
The present study was financially supported by the
first batch of the 2012 Liaoning Province Science and Technology
Plan Project (grant. no. 2012225020) and Dalian City Technology
Bureau in 2014 (grant. no. 2014E14SF188).
References
|
1
|
Grossini E, Pollesello P, Bellofatto K,
Sigaudo L, Farruggio S, Origlia V, Mombello C, Mary DA, Valente G
and Vacca G: Protective effects elicited by levosimendan against
liver ischemia/reperfusion injury in anesthetized rats. Liver
Transpl. 20:361–375. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Çekın AH, Gür G, Türkoğlu S, Aldemır D,
Yilmaz U, Gürsoy M, Taşkoparan M and Boyacioğlu S: The protective
effect of L-carnitine on hepatic ischemia-reperfusion injury in
rats. Turk J Gastroenterol. 24:51–56. 2013.PubMed/NCBI
|
|
3
|
Suzuki M, Takeuchi H, Kakita T, Unno M,
Katayose Y and Matsuno S: The involvement of the intracellular
superoxide production system in hepatic ischemia-reperfusion
injury. In vivo and in vitro experiments using transgenic mice
manifesting excessive CuZn-SOD activity. Free Radic Biol Med.
29:756–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng P, Wang F, Chen K, Shen M, Dai W, Xu
L, Zhang Y, Wang C, Li J, Yang J, et al: Hydrogen sulfide
ameliorates ischemia/reperfusion-induced hepatitis by inhibiting
apoptosis and autophagy pathways. Mediators Inflamm.
2014:9352512014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramachandran S, Liaw JM, Jia J, Glasgow
SC, Liu W, Csontos K, Upadhya GA, Mohanakumar T and Chapman WC:
Ischemia-reperfusion injury in rat steatotic liver is dependent on
NFkappaB P65 activation. Transpl Immunol. 26:201–206. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamo N, Ke B, Ghaffari AA, Shen XD,
Busuttil RW, Cheng G and Kupiec-Weglinski JW: ASC/caspase-1/IL-1β
signaling triggers inflammatory responses by promoting HMGB1
induction in liver ischemia/reperfusion injury. Hepatology.
58:351–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Dang S and Hua Z: Advanced
achievements about neuroprotective mechanisms of paeoniflorin.
Zhongguo Zhong Yao Za Zhi. 35:1490–1493. 2010.(In Chinese).
PubMed/NCBI
|
|
8
|
Ding MP, Feng F and Hu HT: Effects of
puerarin on expression of nuclear factor kappaB after cerebral
ischemia/reperfusion in rats. Zhongguo Zhong Yao Za Zhi.
32:2515–2518. 2007.(In Chinese). PubMed/NCBI
|
|
9
|
Hu W, Zhang Q, Yang X, Wang Y and Sun L:
Puerarin inhibits adhesion molecule expression in
tnf-alpha-stimulated human endothelial cells via modulation of the
nuclear factor kappaB pathway. Pharmacology. 85:27–35. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hino H, Takahashi H, Suzuki Y, Tanaka J,
Ishii E and Fukuda M: Anticonvulsive effect of paeoniflorin on
experimental febrile seizures in immature rats: Possible
application for febrile seizures in children. PLoS One.
7:e429202012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu DZ, Zhao FL, Liu J, Ji XQ, Ye Y and
Zhu XZ: Potentiation of adenosine A1 receptor agonist CPA-induced
antinociception by paeoniflorin in mice. Biol Pharm Bull.
29:1630–1633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen XD, Ke B, Zhai Y, Gao F, Anselmo D,
Lassman CR, Busuttil RW and Kupiec-Weglinski JW: Stat4 and Stat6
signaling in hepatic ischemia/reperfusion injury in mice: HO-1
dependence of Stat4 disruption-mediated cytoprotection. Hepatology.
37:296–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uchida Y, Ke B, Freitas MC, Ji H, Zhao D,
Benjamin ER, Najafian N, Yagita H, Akiba H, Busuttil RW and
Kupiec-Weglinski JW: The emerging role of T-cell immunoglobulin
mucin-1 in the mechanism of liver ischemia and reperfusion injury
in the mouse. Hepatology. 51:1363–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao C, He X, Wang W, Zhang L, Lin H and Du
L: Kinetic distribution of paeoniflorin in cortex of normal and
cerebral ischemia-reperfusion rats after intravenous administration
of Paeoniae Radix extract. Biomed Chromatogr. 20:1283–1288. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchida Y, Freitas MC, Zhao D, Busuttil RW
and Kupiec-Weglinski JW: The protective function of neutrophil
elastase inhibitor in liver ischemia/reperfusion injury.
Transplantation. 89:1050–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun R, Yi YP, Lv LL, Zhang ZP, Sun H and
Liu GQ: Effects of paeoniflorin on pathological changes in global
brain ischemia model rats. Zhongguo Zhong Yao Za Zhi. 32:2518–2522.
2007.(In Chinese). PubMed/NCBI
|
|
18
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA.hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Chen W, Yan X, Bi L, Guo S and
Zhan Z: Paeoniflorin protects cells from GalN/TNF-α-induced
apoptosis via ER stress and mitochondria-dependent pathways in
human L02 hepatocytes. Acta Biochim Biophys Sin (Shanghai).
46:357–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong P, Chi R, Zhang L, Wang N and Lu Y:
Effects of paeoniflorin on tumor necrosis factor-α-induced insulin
resistance and changes of adipokines in 3T3-L1 adipocytes.
Fitoterapia. 91:44–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng YQ, Wei W, Zhu L and Liu JX: Effects
and mechanisms of Paeoniflorin, a bioactive glucoside from paeony
root, on adjuvant arthritis in rats. Inflamm Res. 56:182–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He SQ, Zhang YH, Venugopal SK, Dicus CW,
Perez RV, Ramsamooj R, Nantz MH, Zern MA and Wu J: Delivery of
antioxidative enzyme genes protects against
ischemia/reperfusion-induced liver injury in mice. Liver Transpl.
12:1869–1879. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Kim HY and Lee SM: Protective
effects of geniposide and genipin against hepatic
ischemia/reperfusion injury in mice. Biomol Ther (Seoul).
21:132–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Underhill DM, Rossnagle E, Lowell CA and
Simmons RM: Dectin-1 activates Syk tyrosine kinase in a dynamic
subset of macrophages for reactive oxygen production. Blood.
106:2543–2550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun N, Kang JW and Lee SM: Protective
effects of chlorogenic acid against ischemia/reperfusion injury in
rat liver: Molecular evidence of its antioxidant and
anti-inflammatory properties. J Nutr Biochem. 23:1249–1255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zetzmann CP, Swamy OR, Loss GE Jr,
Bohorquez H and Cohen AJ: Improving Donor Livers by Inhibiting
TNF-α Production. Ochsner J. 10:250–255. 2010.PubMed/NCBI
|
|
27
|
Teoh N, Leclercq I, Pena AD and Farrell G:
Low-dose TNF-alpha protects against hepatic ischemia-reperfusion
injury in mice: Implications for preconditioning. Hepatology.
37:118–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan S, Liu L, Pan H, Ma Y, Wang D, Kang K,
Wang J, Sun B, Sun X and Jiang H: Protective effects of
hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol
Nutr Food Res. 57:1218–1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lan Z, Chen L, Fu Q, Ji W, Wang S, Liang
Z, Qu R, Kong L and Ma S: Paeoniflorin attenuates amyloid-beta
peptide-induced neurotoxicity by ameliorating oxidative stress and
regulating the NGF-mediated signaling in rats. Brain Res.
1498:9–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen T, Guo ZP, Jiao XY, Jia RZ, Zhang YH,
Li JY, Huang XL and Liu HJ: Peoniflorin suppresses tumor necrosis
factor-α induced chemokine production in human dermal microvascular
endothelial cells by blocking nuclear factor-κB and ERK pathway.
Arch Dermatol Res. 303:351–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen T, Guo ZP, Wang L, Qin S, Cao N, Li
MM, Jia RZ and Wang TT: Paeoniflorin suppresses vascular damage and
the expression of E-selectin and ICAM-1 in a mouse model of
cutaneous Arthus reaction. Exp Dermatol. 22:453–457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao ML, Tang M, He JY and Dong Z: Effects
of paeoniflorin on cerebral blood flow and the balance of PGI2/TXA2
of rats with focal cerebral ischemia-reperfusion injury. Yao Xue
Xue Bao. 49:55–60. 2014.(In Chinese). PubMed/NCBI
|
|
33
|
Gündüz E, Dursun R, Zengin Y, İçer M,
Durgun HM, Kanıcı A, Kaplan İ, Alabalık U, Gürbüz H and Güloğlu C:
Lycium barbarum extract provides effective protection against
paracetamol-induced acute hepatotoxicity in rats. Int J Clin Exp
Med. 8:7898–7905. 2015.PubMed/NCBI
|
|
34
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA.hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HY and Lee SM: Ferulic acid attenuates
ischemia/reperfusion-induced hepatocyte apoptosis via inhibition of
JNK activation. Eur J Pharm Sci. 45:708–715. 2012. View Article : Google Scholar : PubMed/NCBI
|