Introduction
Acute lung injury (ALI) is defined as a complex
syndrome associated with an intense pulmonary inflammation
(1). Clinically, ALI is
characterized by lung edema, neutrophil infiltration, hemorrhage,
bronchiole epithelial desquamation, and marked thickening of the
alveolar wall (2,3). Severe ALI may lead to enhanced
permeability and pulmonary edema, acute respiratory distress
syndrome, and eventual respiratory failure (4,5). Despite
marked advances in the treatment of ALI in recent years, ALI
remains a life-threatening disease with a high mortality rate of
30–40% (6). Therefore, it is crucial
that novel effective therapeutic strategies for the treatment of
ALI are developed.
Lipopolysaccharide (LPS) is the major constituent of
the outer membrane of Gram-negative bacteria, and is composed of a
polar lipid head group and a chain of repeating disaccharides. LPS
has been demonstrated to have an important role in the pathogenesis
of ALI (7–9). In vivo intratracheal
instillation of LPS, which causes pulmonary inflammation without
inducing systemic inflammation and multi-organ failure, has been
widely accepted as an ideal pharmacological tool for the in
vivo induction of ALI in model systems (10,11).
LPS-induced ALI leads to an inflammatory response cascade,
characterized by the release of various proinflammatory mediators
(12). Activated macrophages release
a broad spectrum of cytokines and inflammatory mediators, including
tumor necrosis factor (TNF)-α and interleukin (IL)-6, which not
only promote inflammatory injury, but also induce neutrophil influx
into the lung parenchyma (13).
Neutrophil activation induces the excessive production of reactive
oxygen species (ROS) and the release of granular enzymes, including
myeloperoxidase (MPO), which was associated with ALI in previous
studies (14,15). Furthermore, excessive ROS production
may induce impairment of DNA and membrane lipid damage, leading to
lipid peroxidation and the associated production of malondialdehyde
(MDA), which is a cell destruction-dependent index of oxidative
injury (16). Tissues are protected
from ROS-induced toxic damage by antioxidative enzymes, such as
superoxide dismutase (SOD) and glutathione peroxidase (GPx)
(17).
Pogostemon cablin (Blanco) Benth. (Lamiaceae)
is traditionally used in China to treat various illnesses,
including fever, common cold, diarrhea and nausea (18–20).
Previous studies have demonstrated that P. cablin also
exerts numerous bioactivities, including radical-scavenging,
anti-microbial, analgesic and anti-inflammatory activities
(21–23). Patchouli alcohol (PA; Fig. 1), which is a tricyclic sesquiterpene,
is the main active ingredient of P. cablin (24). The authors of the present study have
previously demonstrated that PA inhibits LPS-induced inflammatory
responses in LPS-stimulated RAW264.7 macrophages, and also
possesses potent anti-inflammatory activity in animal models of
inflammation (20,24). Furthermore, oral administration of PA
has been demonstrated to offer protection against influenza virus
infection in mice via the enhancement of host immune responses, and
the attenuation of systemic and pulmonary inflammatory responses
(18). In addition, it has been
reported that pretreatment with PA attenuates ROS generation
following Aβ25–35-induced toxicity (25). These findings indicated that PA
possesses anti-inflammatory and antioxidative activities. However,
to the best of our knowledge, the present study is the first to
report the effects of PA on LPS-induced ALI in a murine model.
Whether PA exerts protective effects on LPS-induced ALI in mice
remains unclear. Therefore, the aim of the present study was to
investigate these protective effects and the possible mechanism
offered by PA against LPS-induced ALI in mice.
Materials and methods
Plants
The aerial parts of P. cablin were obtained from
Guangzhou Zhixing Pharmaceutical Co., Ltd. (Guangzhou, China) and
authenticated by Professor Lai Xiaoping, an experienced
pharmacognosist, at the School of Chinese Materia Medica, Guangzhou
University of Chinese Medicine (Guangzhou, China). The voucher
specimen was deposited in the herbarium of the School of Chinese
Materia Medica, Guangzhou University of Chinese Medicine.
Isolation and purification of PA
PA was isolated from P. cablin according to methods
described in our previous studies (20,24,26). The
aerial parts of P. cablin, weighing 18 kg, were refluxed
with 95% v/v ethanol/aqueous (40 liters ×2; 60 min each time) and
the extract was evaporated under a vacuum in order to obtain a
residue. The residue was subsequently dissolved in acetone and
subjected to column chromatography over silica gel eluted with a
petroleum ether/ethyl acetate/0.1% formic acid (20:1:0.1, 9:1:0.1,
8:3:0.1 and 7:4:0.1, vol/vol/vol) gradient elution system with
increasing polarity, in order to produce a series of fractions.
Thin layer chromatography was performed to distinguish the
resulting fractions, and the fraction eluted with petroleum
ether/ethyl acetate/0.1% formic acid (9:1:0.1) was combined and
subsequently evaporated to yield a yellowish oily liquid. White
crystals of PA were obtained following crystallization from
n-hexane. The purity of PA was analyzed using analytical gas
chromatography (GC; Xcalibur 3.0; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the chemical structure was confirmed by
Fourier transform infrared spectroscopy (IRSolution 1.4; Thermo
Fisher Scientific, Inc.), mass spectrometry (Xcalibur 3.0) and
nuclear magnetic resonance spectroscopy (Topsin 3.2; Bruker
BioSpin, Zurich, Switzerland). GC analysis demonstrated the purity
of PA was >98% (26).
Animals
Male Kunming (KM) mice, 4–5 weeks old and weighing
20–22 g, were obtained from the Guangdong Provincial Medical Animal
Experimental Center (certificate no. SCXK2013-0002; Foshan, China).
The mice were maintained in microisolator cages with a regular
temperature (24±1°C), relative humidity (55±10%) and a 12-h
light/dark cycle. All the experimental protocols and schedules
involving animals were approved by the Animal Welfare Committee of
Guangzhou University of Chinese Medicine.
Reagents
Dexamethasone (DEX) was purchased from Guangdong
Huanan Pharmaceutical Group Co., Ltd. (Dongguan, China). LPS
(Escherichia coli O111:B4) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). TNF-α and IL-6 enzyme-linked immunosorbent
assay kits were obtained from eBioscience, Inc., (San Diego, CA,
USA). The MPO, GPx, MDA, SOD and Coomassie (Bradford) Protein Assay
kits were purchased from the Nanjing Jiancheng Bioengineering
Institute (Nanjing, China).
Survival studies
For the analysis of mortality rate, 100 mice were
randomly divided into five groups (n=20): Sham; LPS; and PA (10, 20
and 40 mg/kg) groups. Mice in the PA groups were intragastrically
administered 10, 20 and 40 mg/kg PA, whereas the sham and LPS
groups were administered 1% poloxamer 407 once a day for 7
consecutive days. The mice were anesthetized using 3% chloral
hydrate (Aladdin Reagent Co., Ltd., Shanghai, China) 1 h after the
final administration. Mice in the LPS and PA groups were
administered 20 mg/kg LPS via intratracheal instillation, whereas
mice in the sham group was administered an equal volume of
phosphate-buffered saline (PBS). The mortality rate was recorded
for 5 days.
Murine model of LPS-induced ALI
A total of 168 mice were randomly divided into six
groups: Sham, LPS, 5 mg/kg DEX, and 10, 20 and 40 mg/kg PA groups.
Mice from the sham and LPS groups were administered 1% poloxamer
407, whereas the PA groups were administered 10, 20 or 40 mg/kg PA
daily for 7 consecutive days. The DEX group was administered 5
mg/kg DEX daily for 5 consecutive days. The mice were anesthetized
1 h after the final administration. The LPS, PA and DEX groups were
administered 5 mg/kg LPS by intratracheal instillation, whereas the
sham group was administered an equal volume of PBS. All mice were
sacrificed by cervial dislocation after 24 h, and lung tissue and
bronchoalveolar lavage fluid (BALF) samples were harvested for
further study.
Measurement of protein content in
BALF
The mice lungs were lavaged with 1.5 ml PBS three
times and ~1.35 ml BALF was recovered with ~90±2% recovery rates.
The BALF samples were centrifuged at 800 × g for 10 min at 4°C and
the supernatants were collected in order to measure the protein
content using Coomassie (Bradford) Protein Assay kits (Thermo
Fisher Scientific, Inc.).
Measurement of lung edema
The lung wet/dry weight (W/D) ratios were determined
to evaluate the protective effects of PA on LPS-induced lung edema.
Upon completion of the experiments, lung tissues were excised and
immediately weighed to record the ‘wet’ weight, to obtain the ‘dry’
weight the tissues were weighed after being heated at 80°C for 48
h.
Histopathologic examination
Lung tissues were fixed in 4% paraformaldehyde,
embedded in paraffin and cut into 4 µm sections. The sections were
subsequently stained with hematoxylin and eosin according to the
manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute), examined, and images were captured using a TE2000-S
inverted microscope (Nikon Corporation, Tokyo, Japan).
Measurement of MPO, SOD, GPx and
MDA
Lung tissues were homogenized using PBS and
centrifuged at 14,167 × g for 10 min at 4°C. Subsequently, the
supernatants were collected and the levels of MPO, SOD, GPx and MDA
in the lung tissue were examined by the respective assay kits
(Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's protocol.
Measurement of proinflammatory
cytokines in BALF
The expression levels of the proinflammatory
cytokines TNF-α and IL-6 were examined in the BALF using
enzyme-linked immunosorbent assay kits (eBioscience, CA, USA),
according to the manufacturer's protocols.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Experimental values were analyzed by one-way analysis of
variance using SPSS 17.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA). Mortality was presented as Kaplan-Meier curves
and differences were assessed by the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
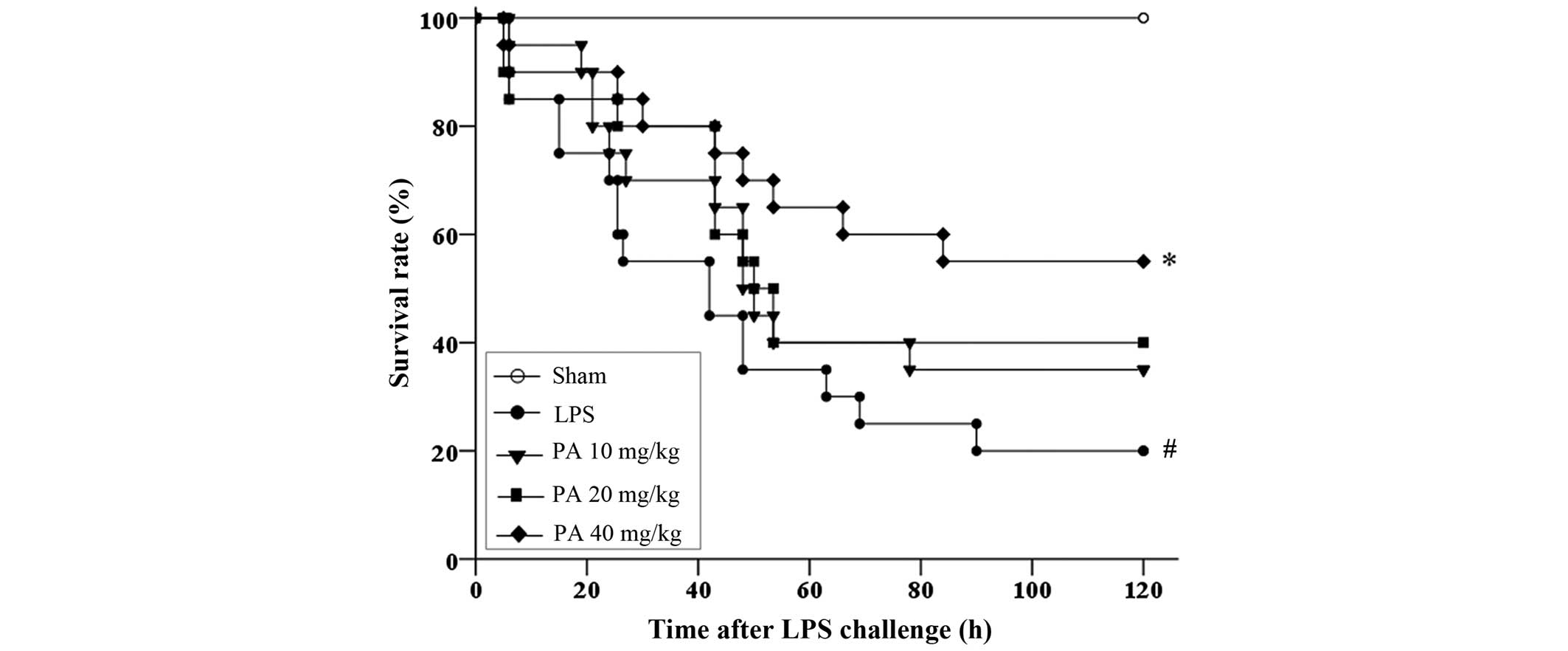
PA improved the survival rate of
LPS-induced ALI in mice
As compared with the sham group, which had a
survival rate of 100%, the survival rate of the LPS group was
significantly decreased (20%; P<0.01) (Fig. 2). The 5-day survival rates of the 10,
20 and 40 mg/kg PA pretreatment groups were 35, 40 and 55%
(P<0.05 vs. the LPS group), respectively. The Kaplan-Meier
survival analysis demonstrated that pretreatment with PA protected
mice with ALI from mortality in a dose-dependent manner.
Effects of PA on the protein content
in BALF
Protein content in the BALF corresponds to the
vascular permeability of the lungs. The BALF protein content in the
LPS group was significantly increased (P<0.01), as compared with
the sham group (Fig. 3A).
Conversely, the BALF protein content was significantly decreased in
the 20 and 40 mg/kg PA (P<0.05) and 5 mg/kg DEX (P<0.01)
groups, as compared with the LPS group. These results suggest that
PA may improve LPS-induced pulmonary vascular permeability in
mice.
Effects of PA on lung edema
The W/D ratios of the mice with ALI were evaluated,
in order to assess the severity of pulmonary edema. The lung W/D
weight ratio significantly increased (P<0.01) following LPS
stimulation, as compared with the sham group (Fig. 3B). However, pretreatment with 20 and
40 mg/kg PA and 5 mg/kg DEX significantly suppressed (P<0.05)
lung W/D weight ratio, as compared with the LPS group.
Effects of PA on histopathologic
alterations
In the sham group, the structure of the alveolar
wall was normal and minimal inflammation was detected (Fig. 4). Characteristic histopathologic
alterations in the lung tissue were observed following LPS
challenge, including lung edema, neutrophil infiltration,
hemorrhage and marked thickening of the alveolar wall. Pretreatment
with 20 and 40 mg/kg PA and 5 mg/kg DEX markedly attenuated these
histopathologic changes.
Effects of PA on MPO, MDA, SOD and GPx
levels
In the LPS group, a significant increase in MPO
(Fig. 5A) activity and MDA levels
(Fig. 5B), and a significant
decrease (P<0.01) in SOD (Fig.
5C) and GPx (Fig. 5D) activities
were detected in the pulmonary homogenate, as compared with the
sham group. However, the mice in the groups pre-treated with PA
(10, 20 and 40 mg/kg) or DEX (5 mg/kg) demonstrated significantly
decreased MDA levels (P<0.05), as compared with the LPS group
(Fig. 5B). Furthermore, treatment
with 20 and 40 mg/kg PA and DEX significantly inhibited the
activities of MPO (Fig. 5A), and
increased SOD (Fig. 5C) and GPx
(Fig. 5D) activities in the
pulmonary homogenate of mice with ALI (P<0.05), as compared with
the LPS group.
Effects of PA on the expression of
inflammatory cytokines in BALF
The expression levels of TNF-α (Fig. 6A) and IL-6 (Fig. 6B) proinflammatory cytokines were
significantly increased in the BALF of the LPS group (P<0.01),
as compared with the sham group. Conversely, the expression levels
of TNF-α and IL-6 in the BALF of the 20 and 40 mg/kg PA and 5 mg/kg
DEX groups were significantly suppressed (P<0.05), as compared
with the LPS group.
Discussion
Numerous plant-derived natural products, including
polyphenols, chlorogenic acid, ethyl gallate, rutin, magnolol and
shikonin (10,13,27–29);
saponins, esculentoside A, ruscogenin and diosgenin (30–32); and
alkaloids, isotetrandrine, oxymatrine and matrine (33–35),
have been demonstrated to exert anti-inflammatory effects in animal
models of ALI. However, few studies have investigated the
protective actions of terpenes on ALI. PA is a tricyclic
sesquiterpene hydrocarbon, which has an oral median lethal dose
value of 4,693 mg/kg in mice (20).
The present study demonstrated that pretreatment with PA improved
the survival rate of mice with LPS-induced ALI. In addition, the
beneficial effects of PA pretreatment included attenuation of lung
pathological alterations, reduced W/D ratio and protein leakage,
reduced elevated proinflammatory cytokine expression, suppression
of lipid peroxidation and MPO levels, and enhanced antioxidant
enzyme activities. These results indicated that PA may effectively
prevent LPS-induced ALI.
Pulmonary edema, which is a typical symptom of ALI,
is usually assessed by measuring the W/D ratio (36). Pulmonary edema is characterized by
diffuse alveolar damage, marked increases in the permeability of
the alveolar-capillary membrane, and accumulation of protein-rich
fluid in the interstitial spaces and alveoli (37). In order to quantify the severity of
pulmonary edema in the present study, the lung W/D ratio was
examined. Pretreatment with PA significantly decreased the lung W/D
ratio induced by the LPS challenge. As another index of ALI
following LPS exposure, the total protein concentration in the BALF
was determined, which indicates endothelial permeability and
pulmonary edema (38,39). As hypothesized, intratracheal
instillation of LPS induced a significant increase in BALF protein
levels. Conversely, pretreatment with PA reduced the total protein
content in the BALF. These results indicated that PA may prevent
the leakage of protein-rich fluid into the lung tissue, thus
attenuating the development of pulmonary edema. Furthermore,
histopathologic analysis 24 h following LPS challenge demonstrated
significant infiltration of inflammatory cells, extensive
thickening of the alveolar wall, demolished structure of pulmonary
alveoli, and hemorrhage. PA pretreatment may attenuate these
LPS-induced pathological changes in the lung.
MPO is an enzyme predominantly stored in the primary
granules of neutrophils, therefore MPO activity in the parenchyma
reflects neutrophil adhesion and margination in the lungs (40). Predominantly released by activated
neutrophils, MPO is characterized by powerful pro-oxidative and
proinflammatory properties (41). In
addition to supporting the host defense mechanisms against
infective microbes, MPO also contributes to the initiation and
propagation of acute and chronic inflammatory reactions (42). However, when released, MPO may
catalyze hydrogen peroxide and chloride anions to form hypochlorous
acid, which may lead to tissue injury (28). Notably, LPS-induced ALI is
characterized by the infiltration of neutrophils into the lung,
resulting in increased MPO activity levels (43,44). In
the present study, pretreatment with PA significantly decreased the
activity of MPO, which was consistent with reduced neutrophil
infiltration in the lung tissue, thus suggesting that PA may exert
anti-neutrophil influx effects in LPS-induced ALI.
Previous experimental and clinical studies have
demonstrated that LPS-induced ALI may lead to a rapid
overproduction of proinflammatory cytokines, including TNF-α and
IL-6, which are characteristic cytokines associated with the
inflammatory process of ALI (45–47).
TNF-α, which is predominantly produced by activated
monocytes/macrophages, is capable of amplifying the inflammatory
cascade, which may damage vascular endothelial cells (48). Furthermore, TNF-α is capable of
inducing the production of other inflammatory cytokines, including
IL-6, which stimulates the migration and adherence of neutrophils
to endothelial cells (49). IL-6 is
a principal cytokine mediator of the acute phase response, and a
previous study suggested that IL-6 may act as a marker for
predicting the severity of ALI (50). PA was previously reported to suppress
the inflammatory response by inhibiting the production of
proinflammatory cytokines, including TNF-α and IL-6 (20,24). The
present study demonstrated that the expression levels of TNF-α and
IL-6 were increased following LPS challenge, whereas pretreatment
with PA significantly decreased the production of TNF-α and IL-6 in
the BALF. These results suggested that the protective effects of PA
on ALI may, at least in part, be attributed to the inhibition of
inflammatory factors.
Another possible mechanism for the anti-inflammatory
effects induced by PA may be associated with its antioxidant
activity. Inflammatory stimuli promote the generation of ROS,
generated by activated inflammatory cells and circulating enzymatic
generators, which contribute to lung pathophysiology (51). When cellular production of ROS
overwhelms antioxidant capacity, cellular macromolecules, including
lipids, proteins and DNA, may be damaged (52). It has previously been suggested that
such a state of ‘oxidative stress’ may contribute to the
pathogenesis of numerous human diseases, including those of the
lung (52). MDA is the breakdown
product of polyunsaturated fatty acids following oxidation in the
chain reaction of lipid peroxidation; therefore, the levels of MDA
are often used as an index of oxidative stress (53,54). The
increase in MDA caused by lipid peroxidation may lead to the
destruction of biological membranes (55). In the present study, pretreatment
with PA significantly inhibited the production of MDA in a
dose-dependent manner, which alleviated oxidative stress in the
lung tissues of mice with ALI. Furthermore, tissues may escape
ROS-induced toxic damage via ROS scavenging enzymes, including SOD
and GPx, which are the first-line cellular defense against
oxidative injury (17,56). The equilibrium between these enzymes
and ROS is important for the effective removal of oxidative stress
from intracellular organelles (57).
Superoxide anions are converted to hydrogen peroxide by SOD, which
is then metabolized to water by GPx (28). In the present study, PA
administration significantly increased the activities of SOD and
GPx in the lungs, as compared with the LPS group; SOD and GPx
activities were markedly reduced following LPS administration.
Conversely, MDA levels were significantly decreased; therefore, the
results of the present study suggested that the suppression of MDA
production was associated with the increase in SOD and GPx
activities. Collectively, these findings suggested that PA may
effectively reduce the effects of oxidative stress in ALI.
It has previously been reported that PA has a short
elimination half-life (t1/2β), with values of 21.51±8.46
(10 mg/kg, i.g.), 17.81±7.52 (30 mg/kg, i.g.) and 17.82±9.29 h (100
mg/kg, i.g.) detected in rats (58).
PA is metabolized in the liver and kidney, and two hydroxylated
metabolites have been demonstrated in the liver of rabbits
(59), and one carboxylate
metabolite of PA has been identified in rat urine (60). The present study demonstrated that
intragastric administration of PA has a potent protective effect
against LPS-induced ALI in mice. The mechanism may be related to
the anti-inflammatory and antioxidative activities of PA and/or its
metabolites. Although our previous in vitro study
demonstrated that PA has direct anti-inflammatory activity in
RAW264.7 macrophages (24), further
investigation is required to clarify this.
In conclusion, the results of the present study
demonstrated that pretreatment with PA improved the survival rate
of mice with LPS-induced ALI, and effectively attenuated
LPS-induced ALI by inhibiting pulmonary histopathologic
alterations. The mechanisms underlying this protective effect
included (i) reduced W/D ratio and protein leakage; (ii) reduced
MPO activity levels; (iii) reduced lipid peroxidation and MDA
formation; (iv) elevated activity of antioxidative enzymes,
including SOD and GPx; and (v) decreased secretion of
proinflammatory cytokines, including TNF-α and IL-6 (Fig. 7). These results suggested that PA may
be a potential therapeutic agent for the prevention of ALI.
Acknowledgements
The present study was supported by grants from: The
National Natural Science Foundation of China (grant nos. 81303200
and 81403169); the Natural Science Foundation of Guangdong Province
(grant no. S2013010016627); the Administration of Traditional
Chinese Medicine Project of Guangdong Province (grant no.
20132142); the Medical Scientific Research Foundation of Guangdong
Province (grant no. A2013232); the Combined Program of Ministry of
Education of Guangdong Province (grant no. 2012B090600007); the
Science and Technology Cooperation Project of Hong Kong, Macao, and
Taiwan (grant no. 2014DFH30010); and the Special Funds from Central
Finance of China in Support of the Development of Local Colleges
and University [grant no. 276 (2014)].
References
|
1
|
Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M and
Liu Z: The effects of the combination of sodium ferulate and
oxymatrine on lipopolysaccharide-induced acute lung injury in mice.
Inflammation. 35:1161–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu WS, Chou MT, Chao CM, Chang CK, Lin MT
and Chang CP: Melatonin reduces acute lung inflammation, edema and
hemorrhage in heatstroke rats. Acta Pharmacol Sin. 33:775–782.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuyama H, Amaya F and Hashimoto S, Ueno
H, Beppu S, Mizuta M, Shime N, Ishizaka A and Hashimoto S: Acute
lung inflammation and ventilator-induced lung injury caused by ATP
via the P2Y receptors, an experimental study. Respir Res. 9:792008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wyncoll DL and Evans TW: Acute respiratory
distress syndrome. Lancet. 354:497–501. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abraham E: Neutrophils and acute lung
injury. Critical Care Medicine. 31:S195–S199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atabai K and Matthay MA: The pulmonary
physician in critical care. Histopathology. Thorax. 57:452–458.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Diane P, Martin DP, Neff M, Stern EJ and Hudson LD:
Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knight PR, Druskovich G, Tait AR and
Johnson KJ: The role of neutrophils, oxidants and proteases in the
pathogenesis of acid pulmonary injury. Anesthesiology. 77:772–778.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yunhe F, Bo L, Xiaosheng F, Fengyang L,
Dejie L, Zhicheng L, Depeng L, Yongguo C, Xichen Z, Naisheng Z and
Zhengtao Y: The effect of magnolol on the Toll-like receptor
4/nuclear factor kappaB signaling pathway in
lipopolysaccharide-induced acute lung injury in mice. Eur J
Pharmacol. 689:255–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szarka RJ, Wang N, Gordon L, Nation PN and
Smith RH: A murine model of pulmonary damage induced by
lipopolysaccharide via intranasal instillation. J Immunol Methods.
202:49–57. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulich TR, Yin S, Remick DG, Russell D,
Eisenberg SP and Kohno T: Intratracheal administration of endotoxin
and cytokines IV. Histopathology. Am J Pathol. 142:1335–1338.
1993.PubMed/NCBI
|
|
13
|
Zhang X, Huang H, Yang T, Ye Y, Shan J,
Yin Z and Luo L: Chlorogenic acid protects mice against
lipopolysaccharide-induced acute lung injury. Injury. 41:746–752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sibille Y and Reynolds HY: Macrophages and
polymorphonuclear neutrophils in lung defense and injury. Am Rev
Respir Dis. 141:471–501. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: Four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edem VF, Kosoko A, Akinyoola SB, Owoeye O,
Rahamon SK and Arinola OG: Plasma antioxidant enzymes, lipid
peroxidation and hydrogen peroxide in wistar rats exposed to
Dichlorvos insecticide. Archives of Applied Science Research.
4:1778–1781. 2012.
|
|
17
|
Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ
and Kuan YH: Ginkgo biloba leaves extract (EGb 761) attenuates
lipopolysaccharide-induced acute lung injury via inhibition of
oxidative stress and NF-kappaB-dependent matrix metalloproteinase-9
pathway. Phytomedicine. 20:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YC, Peng SZ, Chen HM, Zhang FX, Xu PP,
Xie JH, He JJ, Chen JN, Lai XP and Su ZR: Oral administration of
patchouli alcohol isolated from Pogostemonis Herba augments
protection against influenza viral infection in mice. Int
Immunopharmacol. 12:294–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CW, Wu XL, Zhao XN, Su ZQ, Chen HM,
Wang XF, Zhang XJ, Zeng HF, Chen JN, Li YC and Su ZR:
Anti-inflammatory property of the ethanol extract of the root and
rhizome of Pogostemon cablin (Blanco) Benth.
ScientificWorldJournal. 2013:Article ID 434151. 2013.
|
|
20
|
Li YC, Xian YF, Ip SP, Su ZR, Su JY, He
JJ, Xie QF, Lai XP and Lin ZX: Anti-inflammatory activity of
patchouli alcohol isolated from Pogostemonis Herba in animal
models. Fitoterapia. 82:1295–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HW, Cho SJ, Kim BY, Cho SI and Kim YK:
Pogostemon cablin as ROS Scavenger in Oxidant-induced Cell
death of human neuroglioma cells. Evid Based Complement Alternat
Med. 7:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Fan R, Zhang Y and Zhu M: Study on
antimicrobial activities of extracts from Pogostemon cablin
(Blanco) Benth. Food Sci Technol. 34:220–227. 2009.
|
|
23
|
Lu TC, Liao JC, Huang TH, Lin YC, Liu CY,
Chiu YJ and Peng WH: Analgesic and anti-inflammatory activities of
the methanol extract from Pogostemon cablin. Evid Based
Complement Alternat Med. 2011:6717412011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xian YF, Li YC, Ip SP, Lin ZX, Lai XP and
Su ZR: Anti-inflammatory effect of patchouli alcohol isolated from
Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. Exp Ther
Med. 2:545–550. 2011.PubMed/NCBI
|
|
25
|
Huang XW, Bai L, Xu FH and Wu YJ:
Inhibitory activities of patchouli alcohol on neurotoxicity of
β-amyloid peptide. Jie Fang Jun Yi Xue Za Zhi. 24:338–340.
2008.
|
|
26
|
Liao JB, Wu DW, Peng SZ, Xie JH, Li YC, Su
JY, Chen JN and Su ZR: Immunomodulatory Potential of patchouli
alcohol isolated from Pogostemon cablin (Blanco) Benth
(Lamiaceae) in mice. Tropical Journal of Pharmaceutical Research.
12:559–565. 2013.
|
|
27
|
Mehla K, Balwani S, Agrawal A and Ghosh B:
Ethyl gallate attenuates acute lung injury through Nrf2 signaling.
Biochimie. 95:2404–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeh CH, Yang JJ, Yang ML, Li YC and Kuan
YH: Rutin decreases lipopolysaccharide-induced acute lung injury
via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free
Radic Biol Med. 69:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai GZ, Yu HT, Ni YF, Li XF, Zhang ZP, Su
K, Lei J, Liu BY, Ke CK, Zhong DX, et al: Shikonin attenuates
lipopolysaccharide-induced acute lung injury in mice. J Surg Res.
182:303–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong WT, Jiang LX, Wei JY, Qiao AN, Wei
MM, Soromou LW, Xie XX, Zhou X, Ci XX and Wang DC: Protective
effect of esculentoside A on lipopolysaccharide-induced acute lung
injury in mice. J Surg Res. 185:364–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Q, Chen L, Gao M, Jiang W, Shao F, Li
J, Wang J, Kou J and Yu B: Ruscogenin inhibits
lipopolysaccharide-induced acute lung injury in mice, involvement
of tissue factor, inducible NO synthase and nuclear factor
(NF)-kappaB. Int Immunopharmacol. 12:88–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao M, Chen L, Yu H, Sun Q, Kou J and Yu
B: Diosgenin down-regulates NF-kappaB p65/p50 and p38MAPK pathways
and attenuates acute lung injury induced by lipopolysaccharide in
mice. Int Immunopharmacol. 15:240–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang XM, Guo GF, Huang XH, Duan WL and
Zeng ZL: Isotetrandrine protects against lipopolysaccharide-induced
acute lung injury by suppression of mitogen-activated protein
kinase and nuclear factor-kappa B. J Surg Res. 187:596–604. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ
and Yu SQ: Attenuation of acute lung injury in mice by oxymatrine
is associated with inhibition of phosphorylated p38
mitogen-activated protein kinase. J Ethnopharmacol. 98:177–183.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Faffe DS, Seidl VR, Chagas PS, Gonçalves
de Moraes VL, Capelozzi VL, Rocco PR and Zin WA: Respiratory
effects of lipopolysaccharide-induced inflammatory lung injury in
mice. Eur Respir J. 15:85–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perina DG: Noncardiogenic pulmonary edema.
Emerg Med Clin North Am. 21:385–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beck BD, Brain JD and Bohannon DE: An in
vivo hamster bioassay to assess the toxicity of particulates for
the lungs. Toxicol Appl Pharmacol. 66:9–29. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Song K, Xiong H, Li H, Chu X and
Deng X: Protective effect of florfenicol on acute lung injury
induced by lipopolysaccharide in mice. Int Immunopharmacol.
9:1525–1529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klebanoff SJ: Myeloperoxidase: friend and
foe. J Leukoc Biol. 77:598–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Loria V, Dato I, Graziani F and Biasucci
LM: Myeloperoxidase: A new biomarker of inflammation in ischemic
heart disease and acute coronary syndromes. Mediators Inflamm.
2008:1356252008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alfakry H, Sinisalo J, Paju S, Nieminen
MS, Valtonen V, Tervahartiala T, Pussinen PJ and Sorsa T: The
association of serum neutrophil markers and acute coronary
syndrome. Scand J Immunol. 76:181–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moraes TJ, Zurawska JH and Downey GP:
Neutrophil granule contents in the pathogenesis of lung injury.
Curr Opin Hematol. 13:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yao HY, Zhang LH, Shen J, Shen HJ, Jia YL,
Yan XF and Xie QM: Cyptoporus polysaccharide prevents
lipopolysaccharide-induced acute lung injury associated with
down-regulating Toll-like receptor 2 expression. J Ethnopharmacol.
137:1267–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cribbs SK, Matthay MA and Martin GS: Stem
cells in sepsis and acute lung injury. Crit Care Med. 38:2379–2385.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Giebelen IA, van Westerloo DJ, LaRosa GJ,
de Vos AF and van der Poll T: Local stimulation of alpha7
cholinergic receptors inhibits LPS-induced TNF-alpha release in the
mouse lung. Shock. 28:700–703. 2007.PubMed/NCBI
|
|
49
|
Liang X, Wang RS, Wang F, Liu S, Guo F,
Sun L, Wang YJ, Sun YX and Chen XL: Sodium butyrate protects
against severe burn-induced remote acute lung injury in rats. PLoS
One. 8:e687862013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leser HG, Gross V, Scheibenbogen C,
Heinisch A, Salm R, Lausen M, Rückauer K, Andreesen R, Farthmann EH
and Schölmerich J: Elevation of serum interleukin-6 concentration
precedes acute-phase response and reflects severity in acute
pancreatitis. Gastroenterology. 101:782–785. 1991.PubMed/NCBI
|
|
51
|
Gow AJ, Farkouh CR, Munson DA, Posencheg
MA and Ischiropoulos H: Biological significance of nitric
oxide-mediated protein modifications. Am J Physiol Lung Cell Mol
Physiol. 287:L262–268. 2004. View Article : Google Scholar
|
|
52
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–1028. 2000.
|
|
53
|
Chevalier G, Ricard AC and Manca D:
Age-related variations of lipid peroxidation in cadmium-treated
rats. Toxicol Ind Health. 10:43–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Manca D, Ricard AC, Trottier B and
Chevalier G: Studies on lipid peroxidation in rat tissues following
administration of low and moderate doses of cadmium chloride.
Toxicology. 67:303–323. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Draper H and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vijayaraj P, Muthukumar K, Sabarirajan J
and Nachiappan V: Antihyperlipidemic activity of Cassia auriculata
flowers in triton WR 1339 induced hyperlipidemic rats. Exp Toxicol
Pathol. 65:135–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kareem MA, Gadhamsetty SK, Shaik AH,
Prasad EM and Kodidhela LD: Protective effect of nutmeg aqueous
extract against experimentally-induced hepatotoxicity and oxidative
stress in rats. J Ayurveda Integr Med. 4:216–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang R, Yan P, Li Y, Xiong L, Gong X and
Peng C: A pharmacokinetic study of patchouli alcohol after a single
oral administration of patchouli alcohol or patchouli oil in rats.
Eur J Drug Metab Pharmacokinet March. 10:2015.(Epub ahead of
print).
|
|
59
|
Bang L, Ourisson G and Teisseire P:
Hydroxylation of patchoulol by rabbits. Histopathology. Tetrahedron
Letters. 26:2211–2214. 1975. View Article : Google Scholar
|
|
60
|
Bang L, Ourisson G and Teisseire P:
Hydroxylation of patchoulol by rabbits. Histopathology. Tetrahedron
Letters. 16:2211–2214. 1975. View Article : Google Scholar
|