Introduction
Although radiotherapy remains a key treatment of
central nervous system (CNS) tumors in both children and adults,
secondary malignancies have been documented as potential rare
long-term adverse effects of radiation, the risk of which has not
been reduced by newer protocols and stereotactic radiosurgery
(1). According to diagnostic
criteria extrapolated from Cahan's definition of
radiotherapy-related systemic neoplasm, a diagnosis of secondary
brain malignancies is possible when: i) tumors are located in a
prior radiation field, ii) an adequate latency period, usually
years, passes between the radiation therapy and the onset of the
secondary tumor, iii) the new neoplasm differs histopathologically
from the primary disease, and iv) any carcinogenic disease such as
tuberous sclerosis and neurofibromatosis has been excluded
(2). Since the first reported cases
of radiation-induced CNS tumors in the early 1950s, meningiomas
have been the most frequently observed tumor type, but low-or
high-grade gliomas and sarcomas have been also been described
(1,3). Anaplastic ependymoma is a rare subtype
of ependymoma, particularly in adults, that frequently has an
extraventricular and ectopic supratentorial location (4,5). By
contrast, intra- and extraventricular ependymomas of the posterior
fossa most often arise in children (5,6). A total
of 76 cases of ectopic ependymoma have previously been reported, of
which 53 were intraspinal tumors and 23 were intracranial
supratentorial tumors; 9/23 cases of ectopic supratentorial
ependymoma exhibited anaplastic characteristics (4–6). To the
best of our knowledge, only one case of post-irradiation anaplastic
ependymoma has previously been described in the literature, with a
remarkable clinical response to temozolomide (7). The present study reports an unusual
case of a radiation-induced ectopic anaplastic subependymoma
mimicking a skull base meningioma.
Case report
A 63-year-old woman was admitted from the Emergency
Room to the Department of Clinical Neurosciences at the
Neurological Centre of Latium (Rome, Italy) with a drug-resistant
fronto-temporal headache and dizziness associated with incoercible
vomiting without nausea. Neurological examination showed static and
dynamic axial ataxia associated with dysphonia. The clinical
history of the patient reported a previous neurosurgical
intervention for the subtotal removal of a right cerebellar
low-grade glioma 15 years earlier at a different institution. A
ventricular-peritoneal shunt was positioned at the same time
because of hydrocephalus. Subsequently, the patient had been
treated with conventional 60-Gy radiotherapy.
Acutely performed no-contrast computed tomography
scanning of the brain revealed a mass-occupying solid lesion in
left side of the lower clivus. The patient rapidly underwent
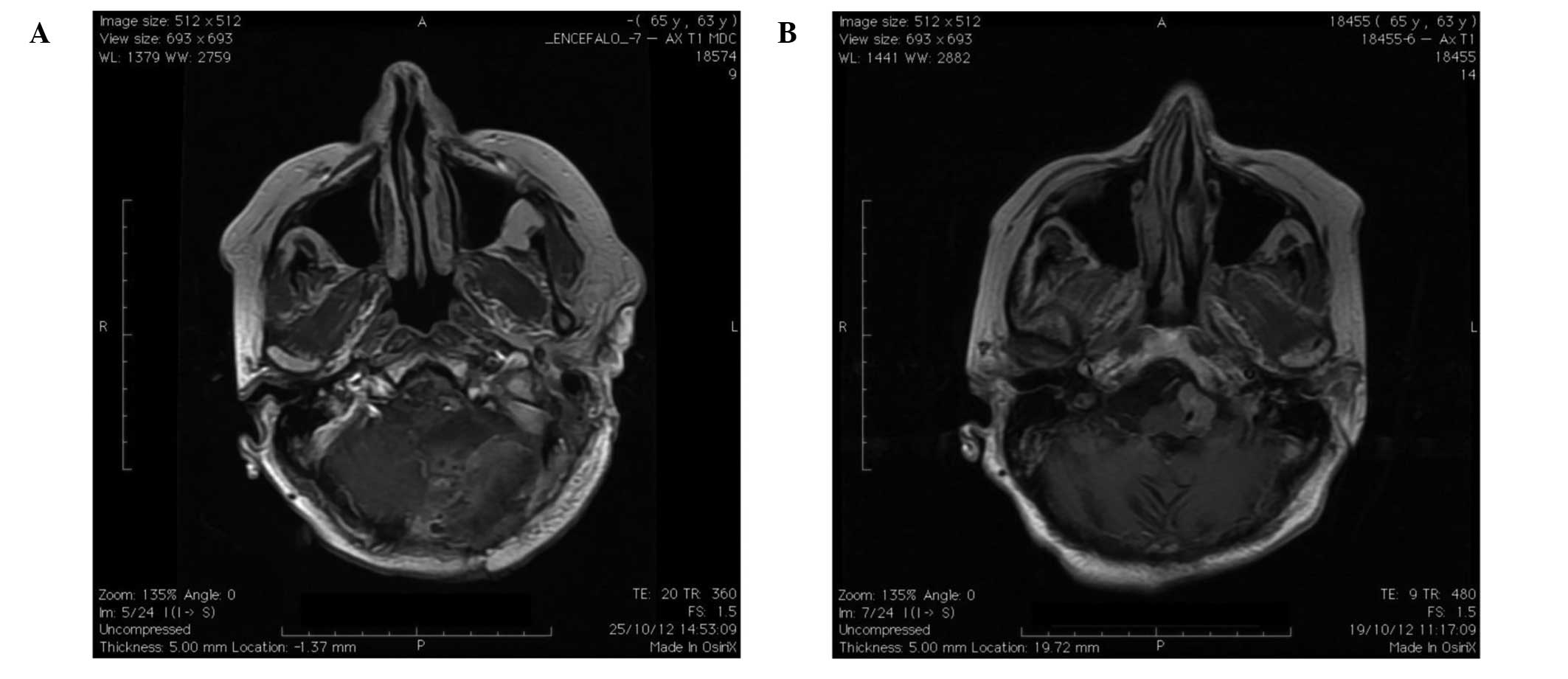
contrast-enhanced magnetic resonance imaging (MRI) of the brain,
which showed an ovoid T2-hyperintense and T1-hypointense mass of
dimensions 25×20×13 mm in the left pre-bulbar cistern, apparently
arising from the left side of the lower clivus and the foramen
magnum (Fig. 1A). The lesion reached
the left anterior-lateral portion of the lower brainstem, which was
shifted controlaterally and showed a homogeneous enhancement after
contrast injection. The left vertebral artery was entrapped in the
mass, however without any narrowing of the lumen. In addition, the
ventricular shunt appeared correctly placed in the frontal horn of
the right lateral ventricle. Radiological features and the clinical
history of the patient suggested a post-irradiation meningioma of
the lower clivus. The patient underwent microsurgical resection via
a far lateral approach to the foramen magnum by means of a limited
condylar resection and displacement of the extradural vertebral
artery that allowed an excellent exposure of the tumor.
Intraoperatively, the macroscopic aspect of the lesion confirmed
the suspicion of a meningioma; however, a dural attachment was not
found in spite of the neuroradiological appearance. The dissection
was challenging because of the close relationship to the lower
cranial nerves and the firm adhesion to the vertebral artery.
However, an almost gross total resection was possible, with the
exception of only a very small fragment, closely adherent to the
arterial wall. Nevertheless, post-operative contrast-enhanced MRI
of the brain showed an apparently complete removal of the lesion
(Fig. 1B). Notably,
histopathological examination showed a high cellularity with
typical perivascular pseudorosettes. Immunochemical analysis
revealed increased nuclear atypia and vascular proliferation, and a
Ki67 level of >20%. These microscopic features resulted in a
diagnosis of anaplastic ependymoma (Fig.
2).
The postoperative course of the patient was
characterised by a progressive improvement of ataxia without any
other neurological signs and symptoms. Since radiation therapy was
not recommended, the patient underwent chemotherapy with
temozolomide. At the 6-month follow-up, the patient was free from
clinical and radiological recurrence. Written informed consent was
obtained from the patient for publication of this case report and
the accompanying images.
Discussion
Although rare, a secondary brain tumor has to be
considered in a long-term follow up of patients treated with
radiotherapy (1,8). In the present case, the observed lesion
fulfilled the extrapolated Cahan's criteria for a radiation-induced
brain tumor: Site location in the previously irradiated area; a
sufficiently long time interval; a hystologically different nature
from the primary one; and the absence of pathologies favoring the
development of tumors such as von Recklinghausen's disease
(2). The main features that make the
present case unusual are the morphological characteristics of the
lesion at neuroimaging and the unusual location of the histological
subtype for a patient of this age. In particular, the extra-assial
location of the tumor in the left pre-bulbar cistern, apparently
arising from the left side of the lower clivus and the foramen
magnum, suggested more intuitively a diagnosis of post-radiation
meningioma. According to the literature, secondary meningiomas have
a strong tendency to an aggressive biological behavior and a higher
recurrence rate than spontaneous meningiomas, with a slight male
predominance (1,3,9,10). Such an association with occlusion of
the larger intracranial arteries has been reported as a result of
extracranial radiation. By contrast, gliomas following irradiation,
as post-traumatic gliomas (11,12) have
been more rarely described and have a higher incidence in younger
patients (60–75% of cases) (1). In a
previous study it was demonstrated that, following radiotherapy,
gliomas developed within both the full-dose prescription volume and
in the lower-dose periphery, suggesting a non linear relationship
between radiation dose and risk of secondary glioma and a more
relevant role of dose volume in risk definition (8). Radiation therapy for brain tumors and
leukemia in childhood has been significantly associated with the
occurrence of gliomas with a markedly longer latency period, as
compared with meningiomas (6).
Furthermore, radiation-induced high-grade gliomas and secondary
glioblastomas more often occur in the supratentorial location and
the cerebellum than primitive ones (13). To our knowledge, only a few cases of
post-radiation ependymoma have been reported. Among them only one
case was an anaplastic subtype (7).
This occurred in a middle-aged patient with a right caudate
anaplastic ependymoma associated with a right temporal meningioma
and a left frontal cavernous malformation following radiation
treatment for a pituitary macroadenoma (14). All other cases are relatively young
with a diagnosis of the first neoplasm in childhood or in
adolescence. A radiation-induced anaplastic ependymoma has also
been described in a 25-year-old woman with a marked clinical
response to temozolomide (7).
Ependymomas are relatively uncommon tumors of the CNS with a
prevalence of 1.2–7.8% of all intracranial neoplasms and 2–6% of
all gliomas (5). They represent the
most frequent primary brain tumors after pilocytic astrocytomas and
medulloblastomas in children, but are particularly rare in adults
(5,6). Usually they arise from the cells lining
the ventricular system and central canal in the spinal cord, whilst
the ectopic variant starting from brain parenchyma has been
sporadically observed in various locations (4,8). The
malignant form, namely anaplastic ependymoma, is a high-grade
glioma with ependymal differentiated cells and high mitotic
activity. A typical microvascular proliferation and necrosis with
perivascular pseudorosettes has frequently been reported (13,14).
In the present case, the high cellularity,
perivascular pseudorosettes and high mitotic index confirmed a
histological diagnosis of anaplastic ependymoma. In contrast with
previous reports, the present patient was not young and the lesion
was atypically located in the posterior fossa. Supratentorial and
infratentorial anaplastic ependymomas exhibit different
histopathological behaviours; the former are more frequently
high-grade tumors (50–60% of cases) than the latter (20–40% of
cases) and show a typical age-related distribution (1,4,5). Supratentorial and intra-medullary
ependymomas are more frequent in adults whilst the posterior fossa
location is typical of childhood (4–6).
Moreover, an ectopic location is more frequent in supratentorial
than in infratentorial ependymomas (5).
Although the risk/benefit ratio is more than
acceptable, the likelihood of developing a secondary tumor in an
intracranial regions previously irradiated for therapeutic purposes
requires consideration, although it is relatively rare in
comparison with other long-term complications of radiotherapy, such
as recurrence of the primary lesion and necrosis of the irradiated
tissue. Earlier reports have suggested that low-dose ionising
irradiation increases the risk of meningiomas, while
radiation-induced gliomas are uncommon. The present case is
exceptional for several reasons. First, the lesion closely mimicked
a skull base meningioma; secondly, it required the same difficult
intraoperative dissection as would have a real meningioma, but was
later identified to be an ependymoma; and finally, it occurred in a
location and in an age range quite different from the usual ones
for similar histological subtypes. For irradiation-induced
malignant tumors, the therapeutic options following neurosurgery
are limited to chemotherapy due to the prior exposure to
radiotherapy. The results of treatment in the present case were
very good, although the follow-up is very short and recurrence is
possible in the future.
References
|
1
|
Ecemis GC, Atmaca A and Meydan D:
Radiation-associated secondary brain tumors after conventional
radiotherapy and radiosurgery. Expert Rev Neurother. 13:557–565.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cahan WG and Woodard HQ: Sarcoma arising
in irradiated bone; report of 11 cases. Cancer. 1:3–29. 1948.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waga S and Handa H: Radiation-induced
meningioma: With review of literature. Surg Neurol. 5:215–229.
1976.PubMed/NCBI
|
|
4
|
Schwartz TH, Kim S, Glick RS, Bagiella E,
Balmaceda C, Fetell MR, Stein BM, Sisti MB and Bruce JN:
Supratentorial ependymomas in adult patients. Neurosurgery.
44:721–731. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mork SJ and Loken AC: A follow-up study of
101 cases. Cancer. 40:907–915. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coulon RA and Till K: Intracranial
ependymomas in children: A review of 43 cases. Childs Brain.
3:154–164. 1977.PubMed/NCBI
|
|
7
|
Khoo HM, Kishima H, Kinoshita M, Goto Y,
Kagawa N, Hashimoto N, Maruno M and Yoshimine T: Radiation-induced
anaplastic ependymoma with a remarkable clinical response to
temozolomide, A case report. Br J Neurosurg. 27:259–261. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salvati M, Frati A, Russo N, Caroli E,
Polli FM, Minniti G and Delfini R: Radiation-induced gliomas,
Report of 10 cases and review of the literature. Surg Neurol.
60:60–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spallone A, Gagliardi FM and Vagnozzi R:
Intracranil meningiomas related to external cranial radiation. Surg
Neurol. 12:153–159. 1979.PubMed/NCBI
|
|
10
|
Umansky F, Shoshan Y, Rosenthal G,
Fraifeld S and Spektor S: Radiation-induced meningioma. Neurosurg
Focus. 24:E72008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spallone A, Izzo C and Orlandi A: Post
traumatic glioma, Report of a case. Case Rep Oncol. 7:403–409.
2013.
|
|
12
|
Spallone A, Neroni M and Giuffrè R:
Multiple skull base meningioma Case report. Surg Neurol.
51:274–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paulino AC, Mai WY, Chintagumpala M, Taher
A and Teh BS: Radiation-induced malignant gliomas, Is there a role
for reirradiation? Int J Radiat Oncol Biol Phys. 71:1381–1387.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alexander MJ, DeSalles AA and Tomiyasu U:
Multiple radiation-induced intracranial lesions after treatment for
pituitary adenoma. Histopathology. J Neurosurg. 88:111–115. 1998.
View Article : Google Scholar : PubMed/NCBI
|