Introduction
Thrombolysis is an approved, effective treatment for
acute ischemic stroke if administered within the first 4.5 h after
the onset of symptoms; however, thrombolysis increases the risk of
symptomatic intracranial hemorrhage, which occurs in 5–10% of
patients (1–4). In addition, due to the limits of the
thrombolysis time window, <10% of patients with acute ischemic
stroke receive recombinant plasminogen activator (rt-PA)
thrombolytic therapy, particularly in developing countries
(5,6). Furthermore, the combined thrombolytic
recanalization rate of the cerebral artery and veins remains
insufficient (7). Therefore, there
is an urgent requirement for a novel therapy that is able to extend
the treatment time window for stroke and decrease bleeding
complications.
Recently, experimental studies that investigated the
efficacy of a platelet glycoprotein IIb/IIIa receptor antagonist in
the treatment of stroke, demonstrated that the treatment was able
to reduce the cerebral infarct volume even if it was not
administered immediately (8,9). Tirofiban is a non-peptide platelet
IIb/IIIa receptor antagonist, and is able to dose-dependently
inhibit the platelet aggregation induced by various stimuli and
eliminate newly formed thrombus, including thrombus containing
fibrin (10). Thus, tirofiban has
been frequently used in endovascular interventional therapy to
prevent the occurrence of vascular endothelial damage-induced
thromboembolic complications and early re-occlusion (11,12).
The aims of the present study were to investigate
the feasibility, safety and efficacy of tirofiban, alone and in
combination with urokinase, in the treatment of intra-arterial
thrombolysis, and to provide a reference for the clinical treatment
of acute ischemic stroke.
Materials and methods
Thrombosis preparation
A total of 40 male New Zealand white rabbits (Wuhan
Wanqianjiahe Experimental Animal Breeding Co., Ltd., Wuhan, China),
weighing 2.5–3.0 kg, were obtained for the present study. This
study was conducted in strict accordance with the recommendations
of the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (1993). The animal use protocol has
been reviewed and approved by the Institutional Animal Care and Use
Committee of Jining No. 1 People's Hospital (Jining, China).
Rabbits were anesthetized via an intravenous
injection of 3% pentobarbital (Shanghai Research Biological
Technology Co., Ltd., Shanghai, China) through the ear vein. Then
the rabbit was fixed in place, and the bilateral ears' dorsal
surface was disinfected with iodine. An improved lumbar puncture
needle (Changzhou Shixing Medical Equipment Co., Ltd., Changzhou,
China) was used to puncture and scrape the artery intima of
bilateral ears. After ~2 cm of the artery intima had been abraded,
the proximal end of the rabbit ear artery was ligated with a suture
to reduce the blood flow velocity and increase the possibility of
embolus formation. Rabbits were subsequently returned to the cage
and fed. After 24 h, the rabbit was re-anesthetized and fixed in
place in order to remove the scraped ear artery. The intravascular
thrombus was then removed under magnification. Following
extraction, the thrombus was cut into 0.5×0.4-mm pieces using an
ophthalmic scalpel and stored in sterile saline for future use.
Establishment of the embolism
model
Rabbits that had been subjected to ear artery
abrasion and thrombosis removal were fixed supinely on the
operating table and routinely disinfected. The interior skin of the
right femur was incised, the right femoral artery was separated,
hung with a thin thread and prepared for intubation and
thrombolysis. The distal end of the right femoral artery was
ligated, and the proximal end was temporarily occluded using an
arterial clip. Next, half of the right femoral artery was cut at a
45° angle using ophthalmic scissors. A 4F sheath catheter was
placed into the right femoral artery, followed by an Echelon-10
microcatheter (ev3 Neurovascular, Irvine, CA, USA) under TV
guidance. Under road-map guidance, the right or left common carotid
artery was selected for insertion; the catheter tip reached
approximately the lateral side of the inferior edge of the second
cervical vertebrae and was set as the pathway. At this site, the
internal carotid artery was visible as a branch of the common
carotid artery, which expanded backwards and upwards, and with an
ampulla-like swelling at the internal carotid artery as a marker.
The catheter was inserted into the proximal end of the internal
carotid artery, passed the occipital artery and created an opening
for normal and lateral angiography to determine the dilation of the
blood vessels. After no significant regurgitation was confirmed
using a contrast agent bolus administered manually, a 1-ml syringe
was used to inject three thrombus strips into the internal carotid
artery. The catheter was then withdrawn after the injection of
contrast agent had confirmed the occlusion of the middle cerebral
artery. Following the embolization, the animals were housed at
~37°C, and the vascular recanalization was reconfirmed at 2 and 5 h
using angiography. The catheter was flushed with heparin saline
throughout the surgery (4).
Grouping and treatment
The successful establishment of a model of embolic
stroke in New Zealand white rabbits was confirmed by digital
subtraction angiography using an OEC 9800 workstation (GE
Healthcare, Milwaukee, WI, USA). The model rabbits were allocated
at random into four treatment groups (n=10 per group): Tirofiban (T
group), Urokinase (UK group), tirofiban + urokinase group (T + UK
group) and the control group (C group). Each group was subjected to
1.5T magnetic resonance imaging (MRI) T1-weighted imaging (T1WI),
T2WI and diffusion (D)WI examination 2 h following modeling, using
a SIGNA high-speed MRI scanner (GE Healthcare).
At 2 h after infarction, the T group was slowly
infused with 20 ml saline + tirofiban (5 µg/kg; Wuhan Yuancheng
Technology Development Co., Ltd., Wuhan, China) using an
internal-carotid-artery microcatheter, which was completed within
10 min. The UK group received 20 ml saline + urokinase (25,000
U/kg; Wuhan Yuancheng Technology Development Co., Ltd.) within 10
min. The T + UK group was slowly treated with 10 ml saline +
urokinase (15,000 U/kg) within 10 min, followed by 10 ml saline +
tirofiban (3 µg/kg) within 10 min. Finally, the C group was slowly
given 20 ml saline.
Detection methods and observation
outcomes
Recanalization rate
The rabbits in each group were bolus-injected with 2
ml iopromide (Ultravist, 300 g/l, diluted to 150 g/l; Schering
Pharmaceutical Co., Ltd., Guangzhou, China) using a high-pressure
syringe and an Echelon-10 microcatheter. The microcatheter was
detained in the internal carotid artery at 30 min and 1 h after the
treatment for digital subtraction angiography (pressure, 50 Pa;
rate, 0.5 ml/sec) to observe the degree of recanalization.
Recanalization rate (%) = Number of rabbits with MCA thrombolytic
recanalization/total number of experimental rabbits × 100.
MRI examination
The animals were immediately subjected to DWI and
T2WI examination after 2 h of successful modeling and 2 h following
the thrombolysis. The SIGNA highspeed MRI scanner was used, with a
small joint coil. DWI was performed using the following parameters:
Echo planar imaging (EPI) sequence; time of repetition (TR), 5,000
msec; time of echo (TE), 10 msec; slice thickness, 3 mm; spacing, 1
mm; field of view (Fov), 16×16 cm; matrix, 128×128; and scan time,
40 sec. T2WI parameters were as follows: TR, 2,500 msec; TE, 99
msec; slice thickness, 3 mm; spacing, 1 mm; Fov, 24×24 cm; and
matrix, 128×128. Finally, T1WI parameters were as follows: TR, 564
msec; TE, 15 msec; slice thickness, 3 mm; spacing, 1 mm; Fov, 24×24
cm; and matrix, 128×128 cm. The FuncTool 2000 software package (GE
Healthcare) was used to conduct the DWI and T2WI examinations. The
apparent diffusion coefficient (ADC) was measured, and the
contralateral hemisphere, which was at the corresponding position
and not subjected to embolization, was set as the reference. The
ratio of these two values was the relative apparent diffusion
coefficient (rADC). rADC = ADC value of infarct area/ADC value of
corresponding normal contralateral area. The rADC values of
different groups were compared prior to and following
treatment.
Neurological impairment (NI) assessment
Using Bederson's 5-point scale (13), scoring was performed within 24 h
after the animals had recovered from the anesthesia to determine
the neurological function deficit score (NFDS). The 5-point scoring
criteria were as follows: 0 point, no NI symptom; 1 point, rabbit
could not fully extend the contralateral paw; 2 points, circled
towards the contralateral side; 3 points, leant towards the
contralateral side; and 4 points, was unable to walk spontaneously
and lost consciousness. The higher the NFDS score, the more severe
the NI.
Pathological examination
The differences in animal behavior were examined 24
h after the thrombosis. Then, the animals were sacrificed via
carotid artery exsanguination. The animals were fixed on a console
and the brain tissues from the medulla oblongata were completely
transected using olecranon pliers, knife, hacksaw and other tools.
The brain was then rapidly removed and frozen at approximately
−20°C for 20 min, then separated into 5 mm-thick brain slices and
incubated in 2% 2,3,5-triphenyl-2H-tetrazolium chloride solution at
37°C for 30 min. Slices were fixed in phosphate buffer
solution-prepared formalin, and the ischemic range was determined.
Then, the slices were subjected to hematoxylin-eosin (H&E)
staining and embedded in paraffin for pathological observation
under a light microscope (Olympus BX41; Olympus, Shanghai, China)
(Changzhou Shixing Medical Equipment Co., Ltd.). The infarct area
was determined using Image-Pro Plus Analysis software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical processing
SPSS software, version 13.0 was used for data
analysis (SPSS Inc., Chicago, IL, USA). Measurement data were
expressed as the mean ± standard deviation. Multi-group comparisons
were performed using one-way analysis of variance, and the further
pairwise comparison used the least significant difference t-test.
Counting data are expressed as ratio (%), the intergroup comparison
used the χ2 test and exact probabilities, with P<0.05
considered to indicate a statistically significant difference.
Results
Revascularization rate
Statistically significant differences were detected
in the recanalization rates among the groups. The T + UK group
exhibited the highest revascularization rate, while the C group
presented the lowest rate (Table
I).
 | Table I.Recanalization rates among different
treatment groups and intergroup comparisons (n=10). |
Table I.
Recanalization rates among different
treatment groups and intergroup comparisons (n=10).
|
|
|
| Statistical
analysis |
|---|
|
|
|
|
|
|---|
| Group | Complete
recanalization | Partial
recanalization or non-recanalization | Intergroup
comparison | P-value |
|---|
| T (1) | 3 | 7 | (1):(2) | 0.070 |
| T + UK (2) | 8 | 2 | (1):(3) | 0.065 |
| UK (3) | 5 | 5 | (1):(4) | 0.211 |
| C (4) | 0 | 10 | (2):(3) | 0.350 |
| χ2 | 17.753a |
| (2):(4) | 0.001 |
|
|
|
| (3):(4) | 0.033 |
ADC comparison
Statistically significant differences were detected
in the rADC among the four groups. The rADC of the T + UK group was
the largest, and the rADCs of each group were increased compared
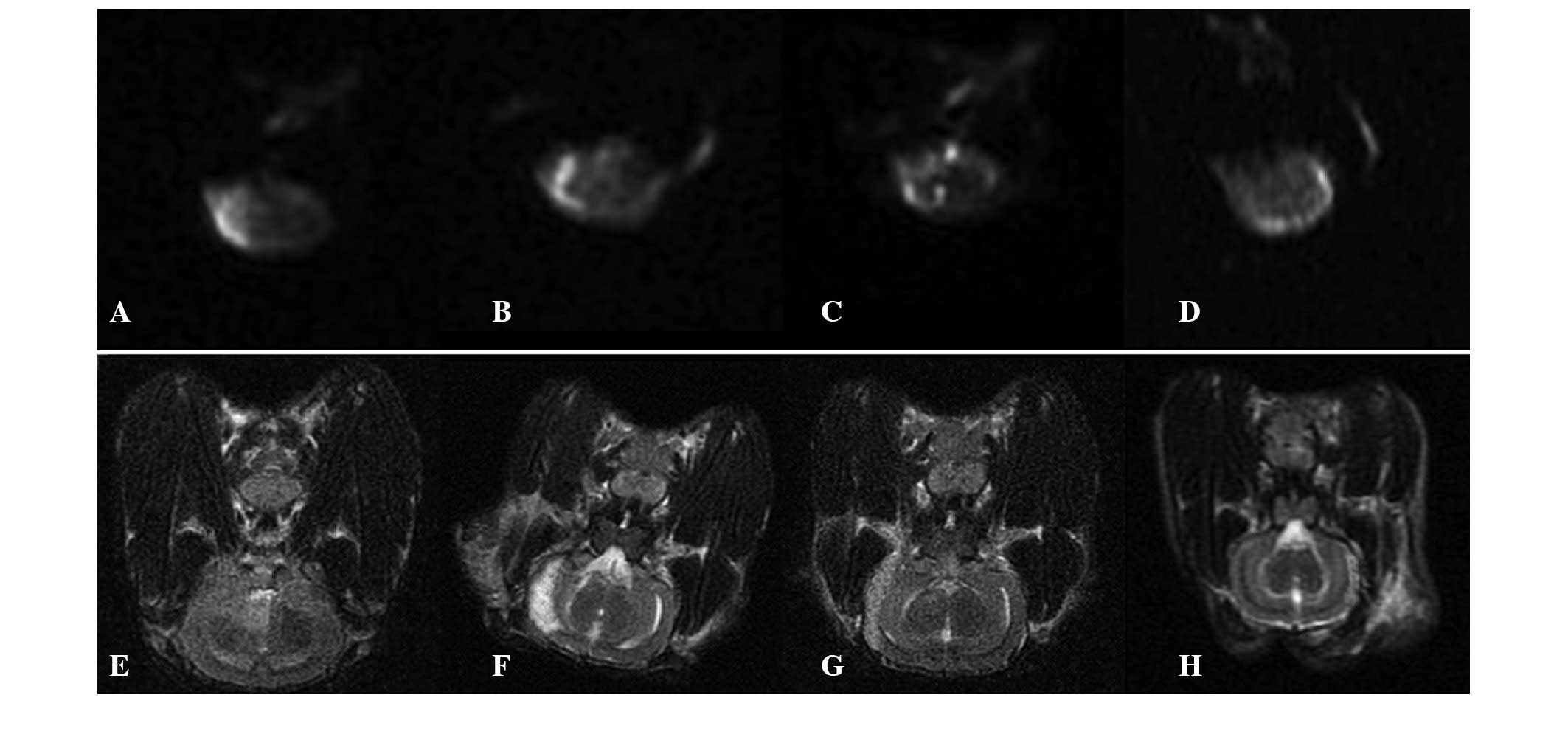
with those before the treatment (Fig.
1 and Table II) .
 | Table II.rADC of the groups before and after
treatment and intergroup comparisons (mean ± standard deviation,
n=10). |
Table II.
rADC of the groups before and after
treatment and intergroup comparisons (mean ± standard deviation,
n=10).
|
|
|
| Statistical
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Group | rADC before | rADC after | Intergroup
comparison | P-value | t |
|---|
| T (1) | 0.65±0.03 | 0.72±0.06 | (1):(2) |
0.012 |
3.300a |
| T + UK (2) | 0.67±0.02 | 0.88±0.03 | (1):(3) |
0.054 | 18.418a |
| UK (3) | 0.65±0.02 | 0.75±0.08 | (1):(4) |
0.001 |
3.835a |
| C (4) | 0.65±0.03 | 0.58±0.04 | (2):(3) |
0.021 |
4.427a |
| F | 1.641 | 12.546a | (2):(4) | <0.001 |
|
|
|
|
| (3):(4) |
0.014 |
|
NFDS
The NFDS value of the T + UK group was the lowest
among the four groups. Intergroup comparison revealed significant
differences between the groups, although not between the T and UK
groups, and the T + UK and UK groups (Table III).
 | Table III.NFDS comparison among the groups
(mean ± standard deviation, n=10). |
Table III.
NFDS comparison among the groups
(mean ± standard deviation, n=10).
|
|
| Statistical
analysis |
|---|
|
|
|
|
|---|
| Group | NFDS | Intergroup
comparison | P-value |
|---|
| T (1) | 1.80±0.63 | (1):(2) |
0.009 |
| T + UK (2) | 0.90+0.74 | (1):(3) |
0.139 |
| UK (3) | 1.40+0.52 | (1):(4) | <0.001 |
| C (4) | 3.20+0.63 | (2):(3) |
0.098 |
| F | 23.143a | (2):(4) | <0.001 |
|
|
| (3):(4) | <0.001 |
Percentage of cerebral infarct
area
The T + UK group exhibited the smallest cerebral
infarct area, while the C group presented the largest. Comparisons
among the four groups revealed that intergroup differences between
pairs of groups were statistically significant (P<0.01), with
the exception of the T and UK groups (Fig. 2 and Table
IV).
 | Table IV.Comparison of infarcted area
percentage of each group (mean ± standard deviation, n=10). |
Table IV.
Comparison of infarcted area
percentage of each group (mean ± standard deviation, n=10).
|
|
| Statistical
analysis |
|---|
|
|
|
|
|---|
| Group | Infarct area,
% | Intergroup
comparison | P-value |
|---|
| T (1) | 31.39±4.75 | (1):(2) | <0.001 |
| T + UK (2) | 20.07+4.03 | (1):(3) |
0.211 |
| UK (3) | 27.79+5.38 | (1):(4) |
0.001 |
| C (4) | 38.67+7.03 | (2):(3) |
0.003 |
| F | 15.241a | (2):(4) |
0.002 |
|
|
| (3):(4) |
0.001 |
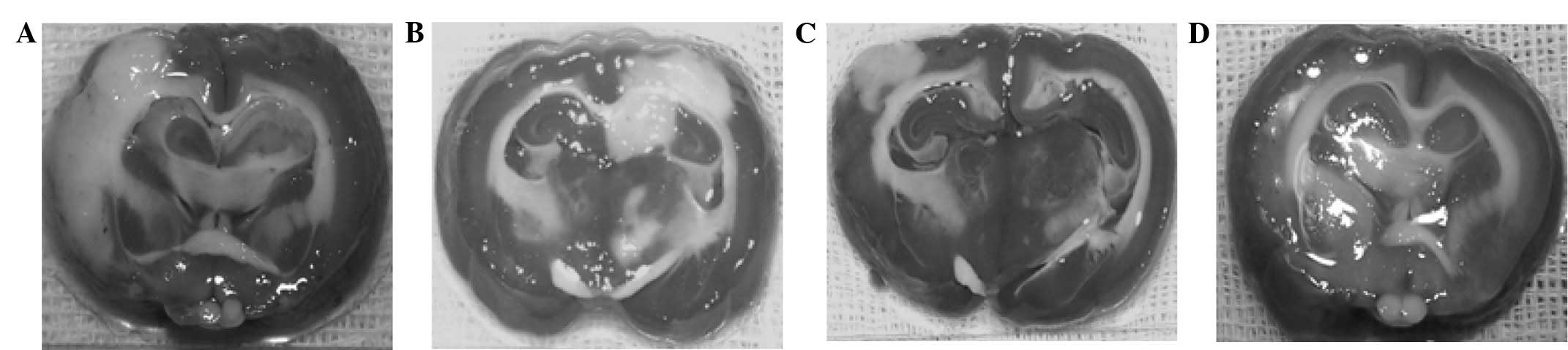
H&E staining
Observation of the C group under an optical
microscope revealed neuronal karyopyknosis, dissolution of nuclear
chromatin, marked eosin staining inside the cytoplasm and the
degradation of cellular structure. In addition, the C group
exhibited evident brain edema, with numerous neurons exhibiting
vacuolar degeneration and mesh-like cells that exhibited severe
damage. By contrast, the T + UK group exhibited almost normal
histology, with a limited number of neurons with vacuolar
degeneration that exhibited mild damage. The T group cells
exhibited edema, and numerous cells with vacuolar degeneration
exhibited moderate changes. The UK group exhibited limited neuronal
degeneration, and necrosis was rare (Fig. 3).
Discussion
The present study investigated the feasibility,
safety and efficacy of combined intra-arterial therapy with
low-dose tirofiban and urokinase in a rabbit model of acute
ischemic stroke. The early recanalization rate following
intravenous rt-PA use is low, with M1 and M2 MCA recanalization
rates of ~25 and 30%, respectively, for acute cerebral arterial
thromboembolism (2) and 4–68% for
occluded macro-vessels (14,15). In a previous study, the average
vascular recanalization rate was reported to be 46% (16), and in another study, the incidence of
symptomatic intracerebral hemorrhage was found to be 4–6% (17). Consistent with the results of the
present study, a previous study that used an autologous thrombus to
block the MCA revealed that the simple application of a GP IIb/IIIa
receptor antagonist was able to induce the recanalization of 33% of
the completely occluded vessels, while a combination of rt-PA (low
dose, 10 mg/kg) and high dose thrombolytic therapy (20 mg/kg)
resulted in a recanalization rate of ~66% (18). Furthermore, Jeon et al
(19) observed that the
intra-arterial administration of tirofiban during the endovascular
treatment of intracranial aneurysms resulted in recanalizations of
myocardial infarction (TIMI) grade III in 3 out of 4 patients with
total occlusion and 5 out of 6 patients with partial occlusion. The
results of the present study indicate that combined intra-arterial
therapy with low-dose tirofiban and urokinase reduces MCA
thrombosis and cerebral ischemia without provoking intracranial
hemorrhage in a rabbit model of acute ischemic stroke.
The activation, adhesion and aggregation of
platelets is a critical factor in the development of arterial
thrombosis. The platelet glycoprotein IIb/IIIa receptor is the
final common pathway in the induction of platelet aggregation
(20). The combined application of
antiplatelet agents enhanced the thrombolytic effects. The
thrombolytic therapy restored cerebral blood flow and improved the
blood supply towards the ischemic area, while the antiplatelet
agent prevented post-thrombolytic restenosis and inhibited the
formation of thrombosis in the microcirculatory system. Therefore,
the combined application of the two treatments described for the
thrombolytic treatment of acute cerebral infarction may be a
feasible and effective intervention. The results of the present
study indicate that the recanalization rate of tirofiban-urokinase
combination in the treatment of acute cerebral infarction was
notably increased compared with the application of tirofiban or
urokinase alone, and further increased compared with the control
group. It has previously been considered that tirofiban functions
only as an anticoagulant (21).
However, the experimental results of the current study indicate
that tirofiban additionally exhibits thrombolytic effects, and that
urokinase is able to enhance the thrombolytic efficacy of
tirofiban. Thus, tirofiban and urokinase may be co-applied in order
to achieve clinical anticoagulation and thrombolysis for the
treatment of cerebral infarction. This combination may enhance the
anticoagulatory and thrombolytic efficacies of the thrombolytic
drugs.
In the present study, rADC was used as an indicator
of the extent of the cerebral infarction. Following the combination
therapy, the rADC of the T + UK group was elevated compared with
the other groups, and the histological observation indicated that
the size of the infarct area was significantly reduced.
Furthermore, after the drugs were injected into the animals via
intracerebral arteries, no new intracerebral hemorrhage was
detected. Recently, the application of ultraselective
intra-arterial tirofiban thrombolysis has been reported to be safe
and effective for the treatment of acute thrombotic events
occurring during the treatment procedure of rupture or non-ruptured
aneurysm embolization, and the incidence of intracranial hemorrhage
was reported to be low (20,22–25). The
ultraselective intra-arterial injection of tirofiban may enable the
dosage to be reduced while improving the local drug concentration
at the thrombotic site and reducing the thrombolysis-induced
systemic fibrinolysis and the incidence of intracranial hemorrhage
(10,20,26).
These results indicate that the combined therapy exhibited good
therapeutic value for the recovery of post-embolic brain tissues.
As the cerebral intra-arterial injection method was safe and
effective, the application of low-dose tirofiban and urokinase may
be a feasible intervention. NFSD was used as an effective indicator
of neurological recovery in the experimental animals; the lower the
NFDS value, the better the functional recovery (27). Observations using light microscopy
indicated that the T + UK group exhibited normal histology, cells
with neuronal vacuolar degeneration occasionally exhibited mild
changes, and neuronal injury was minor. The T group exhibited brain
edema, with numerous neurons with vacuolar degeneration that
exhibited moderate changes. The UK group exhibited partial neuronal
degeneration but necrosis was rare, indicating that the brain
tissues were ischemic without evident necrosis; however,
irreversible necrosis may have subsequently developed if the
ischemia and hypoxia did not improve. The C group neurons exhibited
karyopyknosis, dissolution of the nuclear chromatin, marked eosin
staining inside the cytoplasm and degradation of the cellular
structure. The brain tissues exhibited obvious edema, with numerous
neurons with vacuolar degeneration and mesh-like cells that
exhibited severe alteration; the brain tissues were in a state of
irreversible necrosis. These results suggest that the selective
intra-arterial administration of combined tirofiban and urokinase
possesses therapeutic value for the recovery of post-embolic brain
tissues, as no new cerebral hemorrhage was observed. The present
results showed that the combination of tirofiban and urokinase
induced improved nerve-function recovery compared with that in the
other groups. A possible mechanism may be that tirofiban improves
the microcirculation of the infarcted brain tissues, inhibiting the
formation of thrombus inside the microcirculatory system, and
indirectly increasing microcirculatory perfusion. Furthermore, the
combined treatment exhibited certain neuroprotective effects, and
thus was able to enhance the restoration of neurological
function.
Due to the overlapping and complementary effects of
tirofiban and urokinase in the treatment of acute cerebral
embolism, the combined treatment was more effective than the
individual drug therapies. The present study demonstrated that an
MCA injection of tirofiban prevents cerebral arterial thrombosis
and induces early reperfusion, which reduces brain infarct area
without inducing intracerebral bleeding. This provides a basis for
the development of treatments involving the intra-arterial
administration method of tirofiban. However, the current
experiments used a small sample population of animals, and the
universality and clinical value of the combined treatment remains
unclear. Therefore, future studies are required with increased
sample sizes and limited clinical application of the combined
treatment, with the aim of providing an improved reference for the
treatment of clinical acute cerebral infarction.
References
|
1
|
Gaillard N, Schmidt C, Costalat V, et al:
Hemorrhagic risk of recent silent cerebral infarct on
prethrombolysis MR imaging in acute stroke. AJNR Am J Neuroradiol.
33:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung SM, Lee TH, Cho HJ, Sol YL, Park KH,
Jung DS and Kim CW: Recanalization with Wingspan stent for acute
middle cerebral artery occlusion in failure or contraindication to
intravenous thrombolysis: A feasibility study. AJNR Am J
Neuroradiol. 33:1156–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kellert L, Hametner C, Rohde S, Bendszus
M, Hacke W, Ringleb P and Stampfl S: Endovascular stroke therapy
tirofiban is associated with risk of fatal intracerebral hemorrhage
and poor outcome. Stroke. 44:1453–1455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mullen MT, Pisapia JM, Tilwa S, Messé SR
and Stein SC: Systematic review of outcome after ischemic stroke
due to anterior circulation occlusion treated with intravenous,
intra-arterial, or combined intravenous+intra-arterial
thrombolysis. Stroke. 43:2350–2355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XQ, Zu QQ, Lu SS, et al: Use of FLAIR
imaging to identify onset time of cerebral ischemia in a canine
model. ANJR Am J Neuroradiol. 35:311–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siebler M, Hennerici MG, Schneider D, et
al: Safety of Tirofiban in acute ischemic Stroke: The SaTIS trial.
Stroke. 42:2388–2392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Georgiadis AL, Memon MZ, Shah QA, Vazquez
G, Suri MF, Lakshminarayan K and Qureshi AI: Comparison of partial
(6 mg/kg) versus full-dose (9 mg/kg) intravenous recombinant tissue
plasminogen activator followed by endovascular treatment for acute
ischemic stroke: A meta-analysis. J Neuroimaging. 21:113–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Zhang ZG, Zhang R, Morris D, Lu
M, Coller BS and Chopp M: Adjuvant treatment with a glycoprotein
IIb/IIIa receptor inhibitor increases the therapeutic window for
low-dose tissue plasminogen activator administration in a rat model
of embolic stroke. Circulation. 107:2837–2843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Junghans U, Seitz R, Ritzl A, Wittsack HJ,
Fink GR, Freund HJ and Siebler M: Ischemic brain tissue salvaged
from infarction by the GP II b/III a platelet antagonist tirofiban.
Neurology. 58:474–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baik SK, Oh SJ, Park KP and Lee JH:
Intra-arterial tirofiban infusion for partial recanalization with
stagnant flow in hyperacute cerebral ischemic stroke. Interv
Neuroradiol. 17:442–451. 2011.PubMed/NCBI
|
|
11
|
Ottani F, La Vecchia L, De Vita M,
Catapano O, Tarantino F and Galvani M: Comparison by meta-analysis
of eptifibatide and tirofiban to abciximab in patients with
ST-elevation myocardial infarction treated with primary
percutaneous coronary intervention. Am J Cardiol. 106:167–174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiariti M, Saladini A, Cuturello D,
Missiroli B and Puddu PE: Long-term efficacy of high-dose tirofiban
versus double-bolus eptifibatide in patients undergoing
percutaneous coronary intervention. J Cardiovasc Med (Hagerstown).
12:29–36. 2011.PubMed/NCBI
|
|
13
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowksi H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatia R, Hill MD, Shobha N, et al: Low
rates of acute recanalization with intravenous recombinant tissue
plasminogen activator in ischemic stroke: Real-world experience and
a call for action. Stroke. 41:2254–2258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zangerle A, Kiechl S, Spiegel M, et al:
Recanalization after thrombolysis in stroke patients: Predictors
and prognostic implications. Neurology. 68:39–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rha JH and Saver JL: The impact of
recanalization on ischemic stroke outcome: A meta-analysis. Stroke.
38:967–973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangiafico S, Cellerini M, Nencini P,
Gensini G and Inzitari D: Intravenous glycoprotein IIb/IIIa
inhibitor (tirofiban) followed by intra-arterial urokinase and
mechanical thrombolysis in stroke. AJNR Am J Neuroradiol.
26:2595–2601. 2005.PubMed/NCBI
|
|
18
|
Shuaib A, Yang Y, Nakada MT, Li Q and Yang
T: Glycoprotein IIb/IIIa antagonist, murine 7E3 F (ab') 2 and
tissue plasminogen activator in focal ischemia: Evaluation of
efficacy and risk of hemorrhage with combination therapy. J Cereb
Blood Flow Metab. 22:215–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeon JS, Sheen SH, Hwang G, Kang SH, Heo
DH and Cho YJ: Intraarterial tirofiban thrombolysis for
thromboembolisms during coil embolization for ruptured intracranial
aneurysms. J Cerebrovasc Endovasc Neurosurg. 14:5–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong HW and Jin SC: Intra-arterial
infusion of a glycoprotein IIb/IIIa antagonist for the treatment of
thromboembolism during coil embolization of intracranial aneurysm:
A comparison of abciximab and tirofiban. AJNR Am J Neuroradiol.
34:1621–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen MH, Wu SL, Lo MC, Chen WL and Wang
WF: Basilar artery occlusion in a teenaged boy treated with
intra-arterial thrombolysis. Acta Neurol Taiwan. 19:281–286.
2010.PubMed/NCBI
|
|
22
|
Kwon BJ, Seo DH, Ha YS and Lee KC:
Endovascular treatment of wide-necked cerebral aneurysms with an
acute angle branch incorporated into the Sac: Novel methods of
branch access in 8 aneurysms. Neurointervention. 7:93–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rho MH, Kim BM, Suh SH, Kim DJ and Kim DI:
Initial experience with the new double-lumen scepter balloon
catheter for treatment of wide-necked aneurysms. Korean J Radiol.
14:832–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho YD, Lee JY, Seo JH, Kang HS, Kim JE,
Jung KH and Han MH: Intra-arterial tirofiban infusion for
thromboembolic complication during coil embolization of ruptured
intracranial aneurysms. Eur J Radiol. 81:2833–2838. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Won YS, Rho MH, Kim BM, Park HJ, Kwag HJ
and Chung EC: Various techniques of stent-assisted coil
embolization of wide-necked or fusiform middle cerebral artery
aneurysms: initial and mid-term results. J Korean Neurosurg Soc.
53:274–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang SH, Manning N, Armstrong N, Misso K,
Allen A, Di Nisio M and Kleijnen J: Treatment with tirofiban for
acute coronary syndrome (ACS): A systematic review and network
analysis. Curr Med Res Opin. 28:351–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Syfret DA, Mitchell P, Dowling R and Yan
B: Does intra-arterial thrombolysis have a role as first-line
intervention in acute ischaemic stroke. Intern Med J. 41:220–226.
2011. View Article : Google Scholar : PubMed/NCBI
|