Introduction
Malignant glioma is a type of primary brain tumor of
the central nervous system (1–3). Gliomas
are the most common type of intracranial tumors, accounting for
~50% of intracranial tumors (4,5). Glioma
tumor cells grow infiltratively via diffusion, therefore there are
no clear boundaries, leading to unlimited proliferation and high
invasiveness (6). Glioma incidence
is higher in male patients, as compared with females, and is most
prevalent in adults aged 30–40 years (7,8).
Although significant progress has been made in the diagnosis and
treatment of malignant gliomas, including surgery, radiotherapy and
chemotherapy (9–11), there have been no significant
improvements in patient survival and the efficacy of treatments
against malignant glioma remain poor (12,13).
Therefore, it is necessary to identify more effective therapeutic
strategies and to investigate the mechanisms associated with the
development and progression of gliomas.
The Wnt/β-catenin pathway is a key regulatory
mechanism that controls developmental processes and homeostasis
(14–16). Under normal circumstances, β-catenin
interacts with the glycogen synthase kinase (GSK)-3β, adenomatous
polyposis (APC) and axis suppression proteins, such as Axin, to
form a complex (16,17). Excess β-catenin is phosphorylated by
GSK-3β at the amino-terminal end and is subsequently degraded by
the ubiquitin proteasome system. However, activation abnormal of
the Wnt/β-catenin pathway frequently induces various types of
cancer (18). As previous studies
have demonstrated, Wnts interact with Frizzled (Fz) receptors to
activate the Wnt/β-catenin pathway, which stabilizes β-catenin,
resulting in accumulation in the cytoplasm (19–21). The
stable β-catenin is subsequently translocated into the nucleus and
forms a complex with transcription factors, including T cell
factor/lymphocyte enhancer factor (TCF/LEF); inducing the
activation and expression of cell proliferation-related genes,
including c-Myc and cyclin D1 (17,22).
In the present study, small interfering (si)RNA was
used to investigate whether silencing of β-catenin could inhibit
the proliferation of human glioma U251 cells. The apoptosis rates
of U251 cells transfected with β-catenin siRNA were also
investigated, with the aim of identifying novel therapeutic options
for the treatment of human glioma.
Materials and methods
Cell lines, agents and antibodies
Human glioma U251 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium supplemented with 10% fetal
calf serum (both Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) at 37°C in an atmosphere containing 5% CO2.
Methylthiazolyl-tetrazolium bromide (MTT) reagent was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Three pairs of β-catenin siRNA
were designed by Jima Corporation (Shanghai, China) using
Invitrogen Lipofectamine® transfection agent obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA) to transfect U251 cells.
Anti-β-catenin antibody was obtained from Abcam (Cambridge, UK).
β-catenin shRNA(h) lentiviral particles (sc-29209) and control
shRNA lentiviral particles-A (sc-108080) were obtained from Santa
Cruz Biotechnology Inc for the animal experiments.
MTT assay
MTT assay was performed as previously described
(23,24). Briefly, U251 human glioma cells
(2×103 cells/well) were seeded in 48-well plates.
Following 24 h, cells were transfected with β-catenin siRNA and
negative control siRNA, and cultured for 48, 72 or 96 h.
Subsequently, the plate was supplemented with 20 µl MTT agent (5
mg/ml) and cultured for an additional 4 h. Finally, 200 µl dimethyl
sulfoxide (Sigma-Aldrich) was added and the cells were incubated
for a further 15 min by gently siphoning off the medium. Data were
tested and analyzed.
Cell transfection
Human glioma U251 cells (2×105
cells/well) were cultured in a 24-well plate and, after 24 h, were
transfected with three pairs of siRNA specific for β-catenin, as
designed by Jima Corporation. β-catenin siRNA was transfected using
Lipofectamine® according to the manufacturer's protocol. All
laboratory supplies were lacking ribonucleases. siRNAs were
transfected for 48 h and cell lysates were subsequently prepared
for western blot analysis. Endogenous levels of β-catenin were
determined by western blot analysis and the most effective siRNA
was chosen for interference.
Western blot analysis
Total protein was extracted using cell protein
extraction reagent (AR0103; Boster Biological Technology, Ltd.,
Wuhan, China). Samples (15 ng in each well) were loaded and the
proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 80 V for 15 min,
followed by 120 V at 1 h, and were subsequently transferred onto a
nitrocellulose membrane (Beijing Biodee Biotechnology Co., Ltd.,
Beijing, China) for protein transfer at 400 mA for 1 h. Following
this, the membrane was blocked with Tris buffered saline with Tween
20 (TBST) supplemented with 5% bovine serum albumin for 40 min
prior to incubation with rabbit anti-β-catenin (1:5,000; ab32572)
and mouse immunoglobulin (Ig)G1 anti-β-actin (1:5,000;
sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
monoclonal antibodies in TBST containing 5% bovine serum albumin at
4°C overnight. The membrane was subsequently washed three times
with TBST and incubated with the horseradish peroxidase-conjugated
goat anti-mouse IgG (1:2,000; ab6789) and goat anti-rabbit IgG
(1:2,000; ab6721; both Abcam) secondary antibodies, respectively,
for 1 h at room temperature. Following this, the membranes were
washed three times with TBST and the bands were detected in a dark
room using chemiluminescence techniques. Images were captured using
a ChemiDoc MP gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Scrambled siRNA was used as the negative
control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In order to further investigate the effects of the
three pairs of siRNAs specific for β-catenin, RT-qPCR was
performed. Scrambled siRNA was used as the negative control. Total
RNA was extracted from β-catenin siRNA-transfected U251 glioma
cancer cells and the negative control siRNA-transfected U251 cells
using TRIzol® (Takara Biotechnology Co., Ltd., Dalian, China). RNA
samples were reverse transcribed using MLV-reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) with random primers in
a 20 µl final reaction volume containing 500 ng RNA, 0.5 µl
PrimeScript® RT Enzyme mix, 4 µl 5X PrimeScript® buffer and 1 µl
random primer. qPCR was performed to a final reaction volume of 20
µl containing 1µl template, 10 µl SYBR® Green PCR master mix (2X;
Invitrogen; Thermo Fisher Scientific, Inc.), 1 µl forward and
reverse specific primers (10 µm) and 7 µl water. Primer sequences
were as follows: β-actin, forward 5′-CCTGTACGCCAACACAGTGC-3′ and
reverse 5′-ATACTCCTGCTTGCTGATCC-3′; and β-catenin, forward
5′-AAAATGGCAGTGCGTTTAG-3′ and reverse 5′-TTTGAAGGCAGTCTGTCGTA-3′.
Thermal cycling was performed on an ABI Prism 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) as
follows: 40 cycles of 95°C for 30 sec and 60°C for 1 min.
Animals
A total of 30 male BALB/c (nu/nu) mice, aged 6–8
weeks old and weighing 18–20 g, were purchased from Biomed Science
and Technology Co., Ltd. (Wuhan, China) and maintained in specific
pathogen-free conditions. Mice were randomly divided into three
equal groups: Control, β-catenin short hairpin (sh)RNA and control
shRNA groups. As previously described, U251 cells were transfected
with lentiviral β-catenin shRNA and control shRNA for 48 h, and the
expression levels of β-catenin were subsequently detected via
western blotting and RT-qPCR. The stable cell lines were screened
using a blind screening method. Briefly, the transfected cells were
plated into a 96-well plate containing diluted single cell solution
(3–4 cells were cultured in every well). Cells were cultured for
two weeks at 37°C in an atmosphere containing 5% CO2.
Once the clone had formed, western blotting was used to detect the
levels of β-catenin. Cell lines with effective interfering effects
were collected and used for mice injection. Control group mice were
injected with untreated cells. Each group was subcutaneously
injected with 6×105 cells per mouse into the flank area.
Survival duration was determined as follows: Survival rate: 100% ×
(number of survivors)/(number of challenged mice) and the data were
analyzed using GraphPad 5.0. software (GraphPad software, Inc., La
Jolla, CA, USA). All protocols were approved by the Institutional
Animal Care and Use Committee of Renmin Hospital (Wuhan, China) in
accordance with the Declaration of Helsinki outlined by the World
Medical Association.
Flow cytometry
Apoptosis rates of β-catenin siRNA-transfected U251
glioma cancer cells and negative control-siRNA transfected U251
cells were analyzed using Annexin V-propidium iodide (PI) staining,
according to the manufacturer's protocol (Santa Cruz Biotechnology,
Inc.). Briefly, the cells in each group were washed with
phosphate-buffered saline and resuspended in binding buffer
containing 10 mM HEPES-NaOH (pH 7.4), 25 mM CaCl2 and
144 mM NaCl. Subsequently, the cells were incubated with 0.1 µg/µl
Annexin V and 0.05 µg/µl PI stain in the dark for 30 min on ice. In
every sample, >10,000 cells were detected. The experiment was
performed ≥3 times.
Scratch assay
U251 cells were treated with negative control siRNA
or β-catenin siRNA2 and were subsequently plated into a multi-well
assay plate and allowed to attach, spread and form a confluent
monolayer. Subsequently, a pipette tip was used to scratch the
confluent monolayer in order to remove cells from a discrete area
to form a cell-free zone into which cells at the edges of the wound
could migrate. Cells in each group were cultured and images
documenting cell migration were captured at 0 and 24 h following
scratching using an Olympus CKX31/41 microscope (Olympus
Corporation, Tokyo, Japan). Images were subsequently analyzed using
ImageJ software (National Institutes of Health, Bethesda, MA,
USA).
Statistical analysis
Experiments were performed in triplicate and the
results were analyzed via one-way analysis of variance using SPSS
20.0 statistical software (IBM SPSS, Armonk, NY, USA). Data from
the assay of U251 cell proliferation and mice survival were
analyzed using GraphPad 5.0 software. Data were presented as the
mean ± standard error of the mean. P<0.01 was considered to
indicate a statistically significant difference.
Results
siRNA silencing of β-catenin
In order to knockdown the expression of β-catenin in
glioma cancer cells, three pairs of siRNA specific for β-catenin
were designed and their effects investigated using western blot
analysis and RT-qPCR. siRNA2 was demonstrated to be the most
effective siRNA at silencing the β-catenin gene, as detected by
western blot analysis (Fig. 1A).
This was consistent with the results of RT-qPCR analysis (Fig. 1B). Scrambled siRNA was used as the
negative control siRNA for RT-qPCR.
β-catenin siRNA2 inhibits the
proliferation of human U251 glioma cells
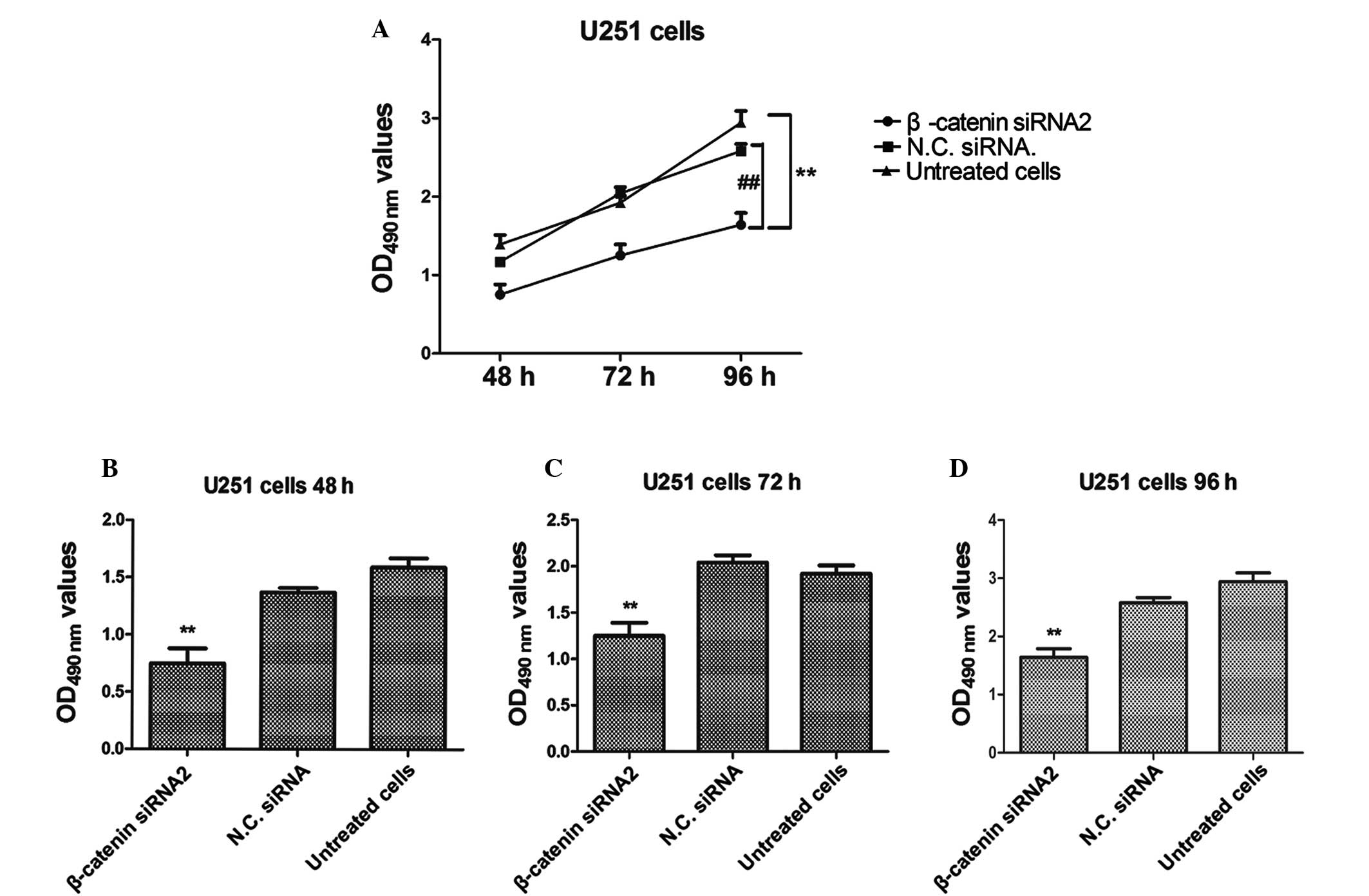
An MTT assay was performed in order to investigate
the proliferation rate of U251 cells transfected with β-catenin
siRNA2. The proliferation of glioma cancer U251 cells was
significantly inhibited in the β-catenin siRNA2-transfected group,
as compared with the negative control group (P<0.01; Fig. 2). Furthermore, the results
demonstrated that the proliferation rate of U251 cells was markedly
reduced in the β-catenin siRNA2-transfected group, as compared with
the control group, in a time-dependent manner. Untreated cells and
cells transfected with negative control siRNA were used as negative
controls.
Silencing of β-catenin with siRNA2
promotes apoptosis of U251 glioma cells
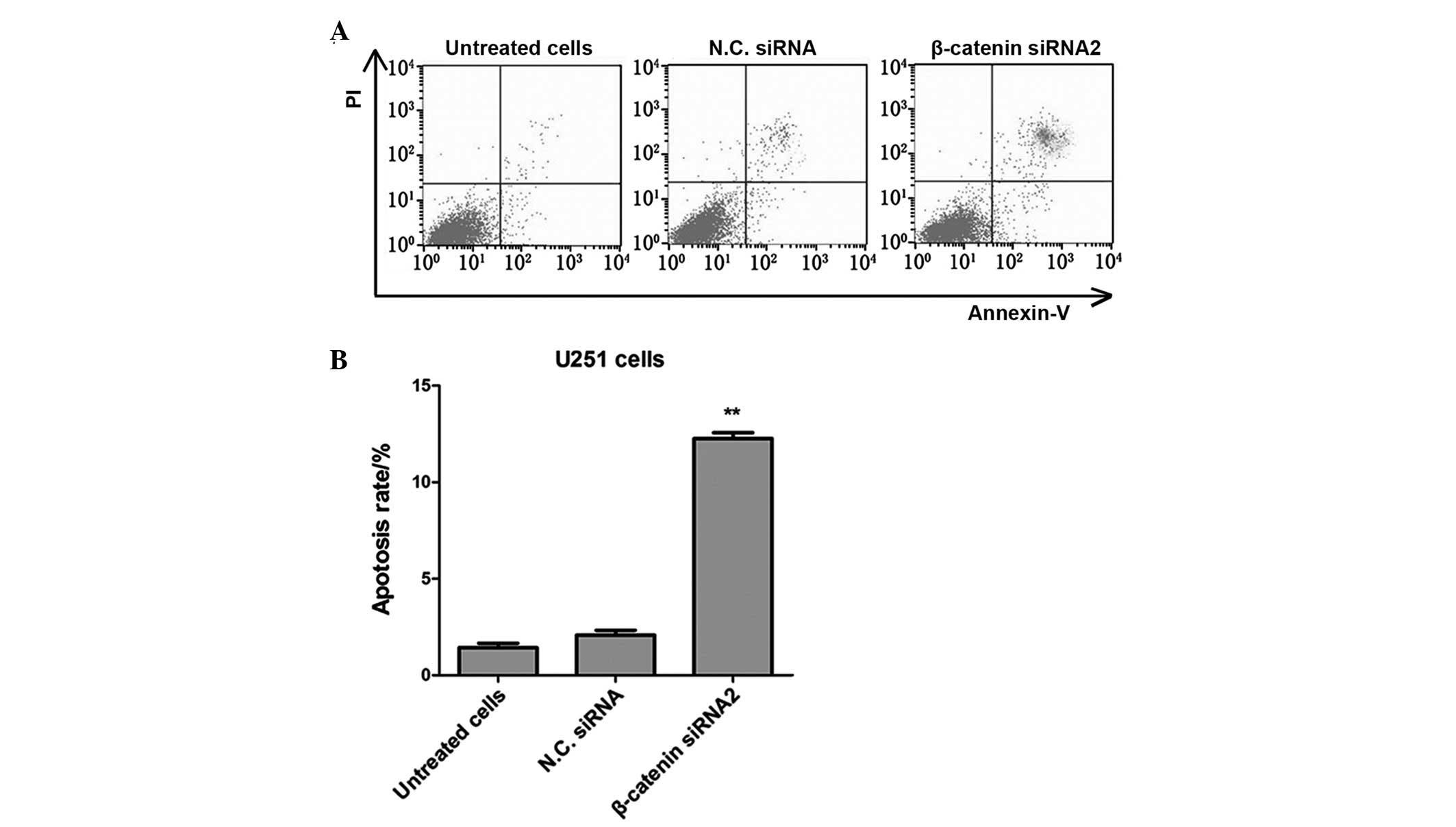
Apoptosis rates of U251 glioma cancer cells in the
β-catenin siRNA transfected group were analyzed using flow
cytometry with Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) dual labeling. The apoptosis rates of
U251 cells were significantly elevated in the β-catenin transfected
group, as compared with the untreated cells and the negative
control siRNA group (P<0.01; Fig.
3). These results demonstrated that transfection with
β-catenin-siRNA2 may promote the apoptosis of human glioma cancer
cells.
β-catenin siRNA2 inhibits cell
invasion in U251 human glioma cells
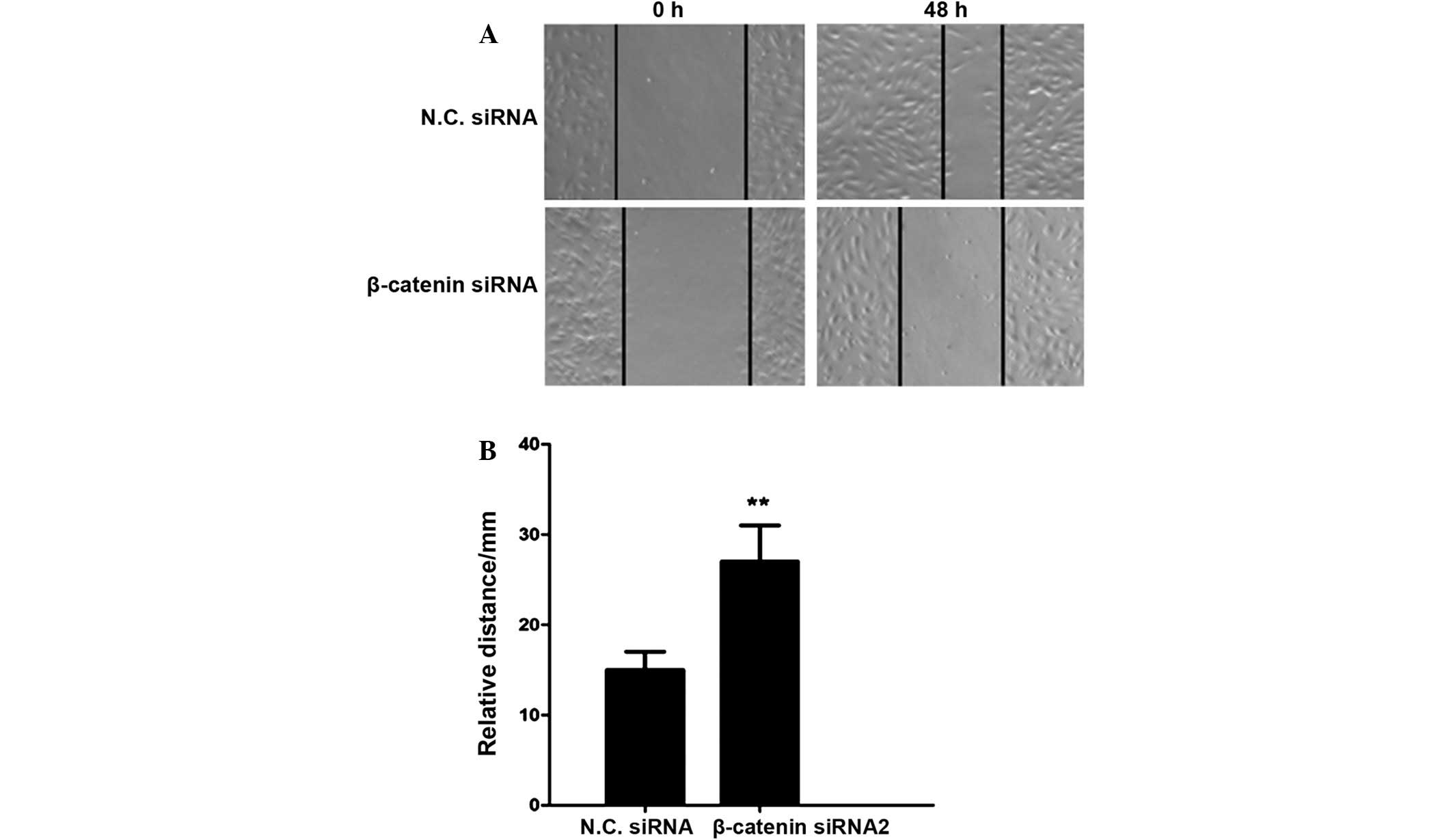
As glioma is type of malignant tumor capable of
invasion and metastasis, a scratch assay was performed to determine
the effects of β-catenin siRNA on cell invasion. The relative
migratory distance of glioma cells was significantly reduced in the
β-catenin silencing group, as compared with the negative control
group (P<0.01; Fig. 4). These
results demonstrated that β-catenin siRNA may suppress the invasive
activity of U251 human glioma cells.
β-catenin silencing increases survival
rates in a nude mice model
In order to further define the potential efficacy of
β-catenin, a lentiviral vector of β-catenin shRNA was used to
evaluate its activity against the proliferation and metastasis of
glioma cancer cells in a nude mice model. U251 cells were
transfected with β-catenin shRNA using a lentiviral vector, and
β-catenin expression levels were significantly inhibited, as
determined by western blot analysis and RT-qPCR (Fig. 5). Notably, the survival rates of mice
in the β-catenin knockdown group were significantly increased, as
compared with the control shRNA group and control groups
(P<0.01). Therefore, these results demonstrated that knockdown
of β-catenin expression significantly inhibited the proliferation
of glioma cancer cells in vivo.
Discussion
Gliomas are the most common and aggressive type of
brain tumors, accounting for ~50% of intracranial tumors (4,5). In
order to elucidate whether the expression of β-catenin affects the
proliferation, apoptosis and invasion of glioma cells, the U251
human glioma cancer cell line was used to clarify the molecular
mechanisms of human glioma cancer. Thus, we aimed to identify novel
and effective methods for the prevention and treatment of advanced
glioma cancer. Firstly, three pairs of siRNAs specific to β-catenin
were designed and screened, and the most effective siRNA was to
silence the endogenous expression of β-catenin in U251 human glioma
cells. The results of the present study demonstrated that β-catenin
silencing may inhibit the proliferation of U251 human glioma cells.
Furthermore, the results of the in vitro scratch assay
demonstrated that the proliferation of U251 cells was significantly
suppressed in the cells which were transfected with β-catenin
siRNA2. Therefore, the present data suggested that siRNA knockdown
of β-catenin may inhibit the proliferation and migration of human
glioma cancer cells.
In order to clarify the underlying mechanisms of
U251 cell proliferation, Annexin V-FITC/PI staining analysis was
used to investigate the apoptosis rate of β-catenin silenced cells.
As hypothesized, β-catenin knockdown suppressed the proliferation
of U251 cells, as compared with the control group. These data were
consistent in vivo as tumorigenicity experiments
demonstrated that β-catenin silencing significantly increased the
survival rates of nude mice. Therefore, these results suggested
that β-catenin knockdown may lead to apoptosis and death of glioma
cancer cells.
In conclusion, the results of the present study
clarified the role of β-catenin in the progression of human glioma
cancer, which may inform novel therapeutic strategies for the
treatment of malignant glioma cancer in humans.
References
|
1
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glass LR, Canoll P, Lignelli A, Ligon AH
and Kazim M: Optic nerve glioma: Case series with review of
clinical, radiologic, molecular, and histopathologic
characteristics. Ophthal Plast Reconstr Surg. 30:372–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diamond EL, Corner GW, De Rosa A,
Breitbart W and Applebaum AJ: Prognostic awareness and
communication of prognostic information in malignant glioma: A
systematic review. J Neurooncol. 119:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A “state of the
science” review. Neurooncol. 16:896–913. 2014.
|
|
6
|
Englot DJ, Berger MS, Chang EF and Garcia
PA: Characteristics and treatment of seizures in patients with
high-grade glioma: A review. Neurosurg Clin N Am. 23:227–235,
vii-viii. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexander H, Irwin C, Purdie G and Hunn M:
Incidence and management of high grade glioma in Mãori and
non-Mãori patients. J Clin Neurosci. 17:1144–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hess KR, Broglio KR and Bondy ML: Adult
glioma incidence trends in the United States, 1977–2000. Cancer.
101:2293–2299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuijlen JM, Bremer E, Mooij JJ, den Dunnen
WF and Helfrich W: Review: On TRAIL for malignant glioma therapy?
Neuropathol Appl Neurobiol. 36:168–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walter F, la Fougère C, Belka C and Niyazi
M: Technical Issues of [(18)F]FET-PET Imaging for Radiation Therapy
Planning in Malignant Glioma Patients - A Review. Front Oncol.
2:1302012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stylli SS and Kaye AH: Photodynamic
therapy of cerebral glioma - a review. Part II - clinical studies.
J Clin Neurosci. 13:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tobias A, Ahmed A, Moon KS and Lesniak MS:
The art of gene therapy for glioma: A review of the challenging
road to the bedside. J Neurol Neurosurg Psychiatry. 84:213–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carpentier AF, Auf G and Delattre JY:
CpG-oligonucleotides for cancer immunotherapy: Review of the
literature and potential applications in malignant glioma. Front
Biosci. 8:e115–127. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barker N: The canonical Wnt/beta-catenin
signalling pathway. Methods Mol Biol. 468:5–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lugli A, Zlobec I, Minoo P, Baker K,
Tornillo L, Terracciano L and Jass JR: Prognostic significance of
the wnt signalling pathway molecules APC, beta-catenin and
E-cadherin in colorectal cancer: A tissue microarray-based
analysis. Histopathology. 50:453–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu L, Zhang C, Zhang LY, Dong SS, Lu LH,
Chen J, Dai Y, Li Y, Kong KL, Kwong DL, et al: Wnt2 secreted by
tumour fibroblasts promotes tumour progression in oesophageal
cancer by activation of the Wnt/β-catenin signalling pathway. Gut.
60:1635–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matono H, Tamiya S, Yokoyama R, Saito T,
Iwamoto Y, Tsuneyoshi M and Oda Y: Abnormalities of the
Wnt/β-catenin signalling pathway induce tumour progression in
sporadic desmoid tumours: Correlation between β-catenin widespread
nuclear expression and VEGF overexpression. Histopathology.
59:368–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caspi M, Zilberberg A, Eldar-Finkelman H
and Rosin-Arbesfeld R: Nuclear GSK-3beta inhibits the canonical Wnt
signalling pathway in a beta-catenin phosphorylation-independent
manner. Oncogene. 27:3546–3555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng X, Lin P, Liu F, Chen J, Li H, Huang
L, Zhen C, Xu H, Liu X, Ye H and Li X: Achyranthes bidentata
polysaccharides activate the Wnt/β-catenin signaling pathway to
promote chondrocyte proliferation. Int J Mol Med. 34:1045–1050.
2014.PubMed/NCBI
|
|
20
|
Petersen CP and Reddien PW:
Smed-betacatenin-1 is required for anteroposterior blastema
polarity in planarian regeneration. Science. 319:327–330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torday JS and Rehan VK: Up-regulation of
fetal rat lung parathyroid hormone-related protein gene regulatory
network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene
regulatory network. Pediatr Res. 60:382–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du R, Xia L, Sun S, Lian Z, Zou X, Gao J,
Xie H, Fan R, Song J, Li X, et al: URG11 promotes gastric cancer
growth and invasion by activation of beta-catenin signalling
pathway. J Cell Mol Med. 14:621–635. 2010.PubMed/NCBI
|
|
23
|
Bilmin K, Kopczyńska B and Grieb P:
Influence of serum and albumin on the in vitro anandamide
cytotoxicity toward C6 glioma cells assessed by the MTT cell
viability assay: Implications for the methodology of the MTT tests.
Folia Neuropathol. 51:44–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cookson MR, Mead C, Austwick SM and
Pentreath VW: Use of the MTT assay for estimating toxicity in
primary astrocyte and C6 glioma cell cultures. Toxicol In Vitro.
9:39–48. 1995. View Article : Google Scholar : PubMed/NCBI
|