Introduction
Coronary artery bypass (CAB) grafting is the main
surgical treatment for coronary heart disease, which increases
oxygen supply by coronary revascularization. Novel surgical
techniques have been developed, such as off-pump CAB (OPCAB)
surgery, which is currently widely used. OPCAB has a reduced effect
on the physiological circulation (1); however, myocardial ischemia-reperfusion
injury (MIRI) remains inevitable. Local and systemic inflammatory
responses are considered to serve an important role in MIRI, but
its development is also associated with the release of free
radicals, calcium overload and ATP reduction. MIRI leads to
decreased myocardial contractility, apoptosis of myocytes,
subendocardial hemorrhage and myocardial infarction (2,3).
Therefore, it is important to mitigate MIRI and protect the
myocardium during the CAB surgery.
Sevoflurane is an inhaled anesthetic frequently used
in cardiac surgery, since it has been reported to provide cardiac
protection against MIRI by pre- or post-conditioning (4–9).
Pre-conditioning by sevoflurane may improve the long-term outcomes
of patients, decrease their mortality and morbidity rates, and
prolong their survival (10,11). A previous meta-analysis included 22
studies on sevoflurane, involving 1,922 patients undergoing cardiac
surgery (12). The study showed that
desflurane and sevoflurane have cardioprotective effects that
result in decreased morbidity and mortality. It was also shown that
the selection of an anesthetic regimen based on halogenated
anesthetics was associated with an improved outcome subsequent to
cardiac surgery (12). The majority
of previous clinical studies have selected to use inhaled
anesthetics in combination with intravenous administration of
propofol (12).
Cardiac troponins have been introduced as cardiac
markers (13,14). Cardiac troponin I (cTnI)
determination has been shown to have a potentially high diagnostic
efficacy for cardiac ischemic injury (13). In addition, the nonspecific marker
lactate dehydrogenase (LDH) and the cardiac-specific enzyme
creatine kinase-MB (CK-MB) are frequently-used markers of cardiac
infarction (15). However, these
biomarkers are not consistently sensitive following ischemia onset,
requiring repetitive measurements (16).
MicroRNAs (miRNAs) are short, non-coding small RNAs
that regulate the expression of proteins by translational
repression or degradation of mRNAs (17). In the last decade, studies revealed
that miRNAs serve a crucial role in cardiac development and
homeostasis, and that their expression is altered in the patients
with heart diseases (18,19). It was also demonstrated that
circulating miRNAs may be potential novel early biomarkers of
cardiac injury and may help improve the management of suspected
acute coronary syndrome (ACS) patients (20). In particular, miR-208b and miR-499
levels can provide a reasonable prognosis of left ventricular
dysfunction (21).
In the present study, sevoflurane inhalation was
used to perform volatile induction and maintenance of anesthesia
(VIMA), and its effect was compared with that of total intravenous
anesthesia (TIVA) with propofol. In addition, the cardioprotection
induced by sevoflurane compared with propofol in off-pump CABG was
evaluated using miR-208b and miR-499 as diagnostic biomarkers.
Patients and methods
Patient selection
In the present single-center randomized controlled
study, 36 patients diagnosed with coronary artery disease and
scheduled to undergo OPCAB were enrolled between January 2010 and
December 2012. The patient characteristics were as follows: Age,
34–70 years; American Society of Anesthesiology physical status
classification II–III; New York Heart Association (NYHA) functional
classification II–III; and a body-mass index (BMI) of <30
kg/m2. The study was approved by the Ethical Committee
of Xiangya Hospital, Central South University (Changsha, China).
All ongoing and associated trials for this intervention were
registered. Written informed consent was obtained from all the
patients. The exclusion criteria included emergency surgery, left
ventricular ejection fraction of <40%, diabetes, previous
administration of K-ATP channel antagonists or agonists, previous
myocardial infarction, history of allergy to anesthetics and
abnormal coagulate function. All patients were administered 25–100
mg metoprolol (AstraZeneca plc, London, UK) and 10–20 mg lovastatin
(Yangzijiang Pharmaceutical Group, Wuhan, China) per day as
preoperative medications. Coronary artery revascularization by
OPCAB grafting was performed by one surgeon using two or three
coronary vessel grafts per patient and the Octopus 4.3 Tissue
Stabilizer (Medtronic, Inc, Minneapolis, MN, USA).
Anesthetic technique
The 36 patients undergoing OPCAB were randomly
divided into two groups, namely the sevoflurane and propofol groups
(n=18 each). For the random allocation of patients into the groups,
a computer-generated list of random numbers was used. Two
additional participants were excluded from each group due to a
change to the surgical method. In total, 10 mg morphine (Renfu
Pharmaceutical Co., Ltd., Yichang, China) and 0.3 mg scopolamine
(Hefeng Pharmaceutical, Co., Ltd., Shanghai, China) were
administrated 30 min before the anesthesia. The electrocardiogram
(ECG), blood pressure (BP), heart rate (HR) and pulse oxygen
saturation were continuously monitored using the CARESCAPE Monitor
B850 (GE Healthcare, Dallas, TX, USA), and the bispectral index
(BIS) value (BIS VISTA™ monitoring system; Aspect Medical Systems,
Inc., Norwood, MA, US) was continuously recorded. Invasive
monitoring lines, including a radial artery catheter (Edwards
Lifesciences Corp., Irvine, CA, USA), a jugular vein catheter (B.
Braun Melsungen AG, Melsungen, Germany) and a Swan-Ganz catheter
(Edwards Lifesciences Corp.), were inserted under local anesthesia.
Anesthesia was induced with 1–8% sevoflurane inhalation (Maruishi
Pharmaceutical Co., Ltd., Osaka, Japan) in the sevoflurane group,
or with 2–4 mg/kg propofol injection (AstraZeneca plc) in the
propofol group, until the BIS reached 45. Subsequent to
administration of 1.0 µg/kg sufentanil (Renfu Pharmaceutical Co.,
Ltd.) and 0.15 mg/kg vecuronium (Enhua Pharmaceutical Group Co.,
Ltd., Xuzhou, China), the patient was intubated and mechanically
ventilated, and the ventilator parameters were adjusted to maintain
the end-tidal CO2 level at 40–45 mmHg. All patients
received an intravenous infusion of 10–15 ng/kg/min sufentanil and
1.0 µg/kg/min vecuronium during the surgery. In addition,
anesthesia was maintained with a 0.6–1.5 minimum alveolar
concentration (MAC) of sevoflurane in the sevoflurane group or 4–8
mg/kg/h propofol in the propofol group, in order to maintain a BIS
value of 40–50. Patients received routine monitoring for OPCAB
using the Vigilance II Monitor (Edwards Lifesciences Corp.) to
monitor the mean arterial pressure (MAP), pulmonary artery mean
pressure (PAMP), pulmonary artery wedge pressure (PAWP), central
vein pressure and core temperature. Nitroglycerin (Yimin
Pharmaceutical Co., Ltd., Beijing, China), esmolol (Qilu
Pharmaceutical Co., Ltd., Jinan, China) and phenylephrine (Hefeng
Pharmaceutical, Co., Ltd.) were administered, according to
hemodynamic parameters.
Hemodynamic parameters, including systolic BP,
diastolic BP, MAP and PAMP, were monitored automatically and
recorded prior to anesthesia, intraoperatively and postoperatively.
In addition, the central venous pressure (CVP), cardiac output (CO)
and stroke volume (SV) were recorded using pulmonary artery
catheters. Furthermore, blood samples were obtained subsequent to
anesthesia induction and surgery, and were immediately centrifuged
at 3,000 × g for 10 min at 4°C. The serum was stored at −70°C for
measurement of cTnI, CK-MB, LDH, miR-499 and miR-208b expression
levels. Laboratory technicians were blinded to the sample
groups.
Determination of quantitative cTnI,
CK-MB and LDH cardiac marker expression levels using enzyme-linked
immunosorbent assay (ELISA)
The details of the series were unknown to any of the
investigators who performed the laboratory tests. Circulating cTnI
protein concentration was measured by an ELISA. The method was
based on a single-step sandwich principle (22), performed according to the
instructions of the human cTnI ELISA kit (cat. no. GWB-83A61F;
GenWay Biotech Inc., San Diego, CA, US). cTnI measurements were
performed in duplicate and recorded by a batch ELISA analyzer
(iMark 680 microplate absorbance reader; Bio-Rad Laboratories,
Inc., Richmond, CA, US). In addition, the cardiac isoenzymes CK-MB
and LDH were detected using commercially available kits (cat. nos.
04525299190 and 11644793001, respectively; Roche Diagnostics,
Indianapolis, IN, USA) using an autoanalyzer system (Cobas 4000;
Roche Diagnostics).
Determination of miR-208b and miR-499
cardiac marker expression levels using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The serum, which had been stored at −70°C in
RNAse-free tubes, was processed for miRNA analysis by RT-qPCR.
Total RNA was isolated using TRIzol LS reagent (cat. no. 15596-026;
Thermo Fisher Scientific Inc., Carlsbad, CA, USA). Genomic DNA
contamination was eliminated using a DNAse I kit (cat. no. 89836;
Fermentas; Thermo Fisher Scientific Inc., Vilnius, Lithuania) and
RNA concentration was quantified with a spectrophotometer (NanoDrop
ND-1000; Thermo Fisher Scientific Inc., Wilmington, DE, USA). The
RNA integrity of small RNAs was determined using small RNA Chip
analysis kit (cat. no. 5067-1548) along with an Agilent 2100
Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, US). A
total of 2 µg DNA-free RNA was reverse transcribed into cDNA using
the TaqMan miRNA Reverse Transcription kit (cat. no. 4366596;
Thermo Fisher Scientific Inc., Carlsbad, CA, USA). qPCR was
performed on a QuantStudio™ 7 Flex Real Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the TaqMan Fast
Advanced Master Mix (cat. no. 4444556; Thermo Fisher Scientific,
Inc.) and miRNA-specific primers as follows: miR-208b forward,
5′-CCATAAGACGAACAAAAGGT-3′, and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
miR-499 forward, 5′-ACAGACTTGCTGTGATG-3′, and reverse,
5′-GTGCAGGGTCCGAGGT-3′; and RNU6 forward, 5′-CTCGCTTCGGCAGCACA-3′,
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ (Applied Biosystems; Thermo
Fisher Scientific Inc.). The PCR conditions were as follows: 95°C
for 15 min, followed by 35 cycles at 94°C for 15 sec, 55°C for 30
sec and 72°C for 30 sec. RT-qPCR amplification was performed in
duplicate using 1:5 diluted RT products for miR-208b and miR-499.
All RT-qPCR data were normalized to RNU6, and the RNU6 levels did
not differ between the two groups. After normalization for RNU6,
the relative gene expression was calculated by the
2−∆∆Cq method (23).
Statistical analysis
All data for variables are expressed as the mean ±
standard deviation. For comparison between the two groups, the
Mann-Whitney test was used, while categorical data were analyzed
using χ2-test. The hemodynamic and serological data were analyzed
using repeated measures analysis of variance (ANOVA), followed by a
Bonferroni post-hoc correction or non-parametric test in cases
where the ANOVA prerequisite was not met. A P-value of <0.05 was
considered to indicate statistically significant differences.
GraphPad Prism-5 statistical software (GraphPad Software, Inc., La
Jolla, CA, USA) was used for data analysis.
Results
Patient characteristics
The basic characteristics of the patients in the two
groups, including gender, age, BMI, NYHA level, the number of
grafts and surgery duration, showed no significant differences
between the two groups (P>0.05; Table
I). In addition, the dosages of sufentanil, vecuronium,
nitroglycerin, esmolol, and phenylephrine used in surgery were not
significantly different between the two groups (P>0.05). None of
these patients required further surgery.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Parameter | Sevoflurane group
(n=18) | Propofol group
(n=18) |
|---|
| Gender,
male/female | 11/7 | 13/5 |
| Age (year) | 63.3±7.2 | 61.8±10.8 |
| Body mass index
(kg/m2) | 26.5±3.2 | 25.8±2.7 |
| NYHA level,
II/III | 7/11 | 8/10 |
| Number of grafts,
2/3 | 8/10 | 8/10 |
| Surgery duration
(min) | 247.8±31.5 | 253.3±33.7 |
Hemodynamic and cardiac function
measurements
Table II lists the
preoperative and postoperative values of various hemodynamic and
cardiac function measurements in the two groups. The results
demonstrated no statistically significant differences in the
preoperative hemodynamic variables, including HR, MAP, CVP, PAWP
and PAMP, between the sevoflurane and propofol groups (P>0.05).
By contrast, the CO and SV measured by pulmonary artery catheters
showed a significant difference between the levels prior to surgery
and subsequent to surgery in the sevoflurane group (P<0.05).
 | Table II.Intraoperative hemodynamic and
cardiac function measurements. |
Table II.
Intraoperative hemodynamic and
cardiac function measurements.
|
| Sevoflurane
group | Propofol group |
|---|
|
|
|
|
|---|
| Measurement | Preoperative | Postoperative | P-value | Preoperative | Postoperative | P-value |
|---|
| HR (beats/min) |
71.7±12.5 |
77.3±10.4 | 0.163 |
69.3±11.5 |
75.6±10.5 | 0.106 |
| MAP (mmHg) |
95.3±14.3 |
84.1±12.5 | 0.027 |
92.1±17.6 |
78.8±13.4 | 0.029 |
| CVP (mmHg) |
11.4±3.4 |
12.5±2.7 | 0.381 |
10.8±3.0 |
12.4±2.5 | 0.067 |
| PAMP (mmHg) |
23.0±5.6 |
25.0±5.7 | 0.288 |
22.8±5.1 |
26.2±6.3 | 0.092 |
| PAWP (mmHg) |
15.7±3.7 |
16.2±3.4 | 0.588 |
15.6±3.1 |
16.5±3.9 | 0.482 |
| CO (l/min) |
4.5±0.9 |
5.5±0.7 | 0.002 |
4.6±1.1 |
5.1±1.1 | 0.121 |
| SV (ml) |
62.8±10.7 |
72.3±12.7 | 0.034 |
66.8±12.9 |
69.1±11.6 | 0.476 |
Cardiac marker expression
The expression of cTnI, CK-MB and LDH cardiac
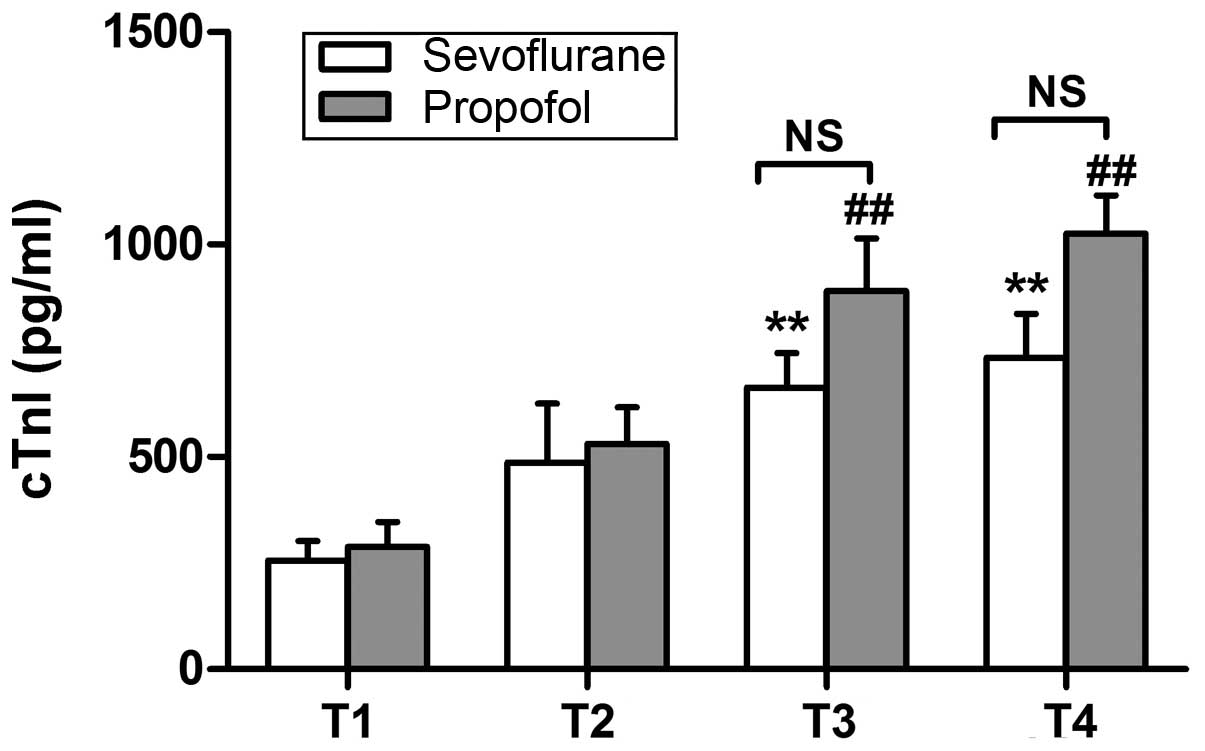
markers was determined using ELISA. As shown in Fig. 1, the protein expression of cTnI at 12
h after surgery was significantly increased in both groups, when
compared with the corresponding preoperative values (P<0.01).
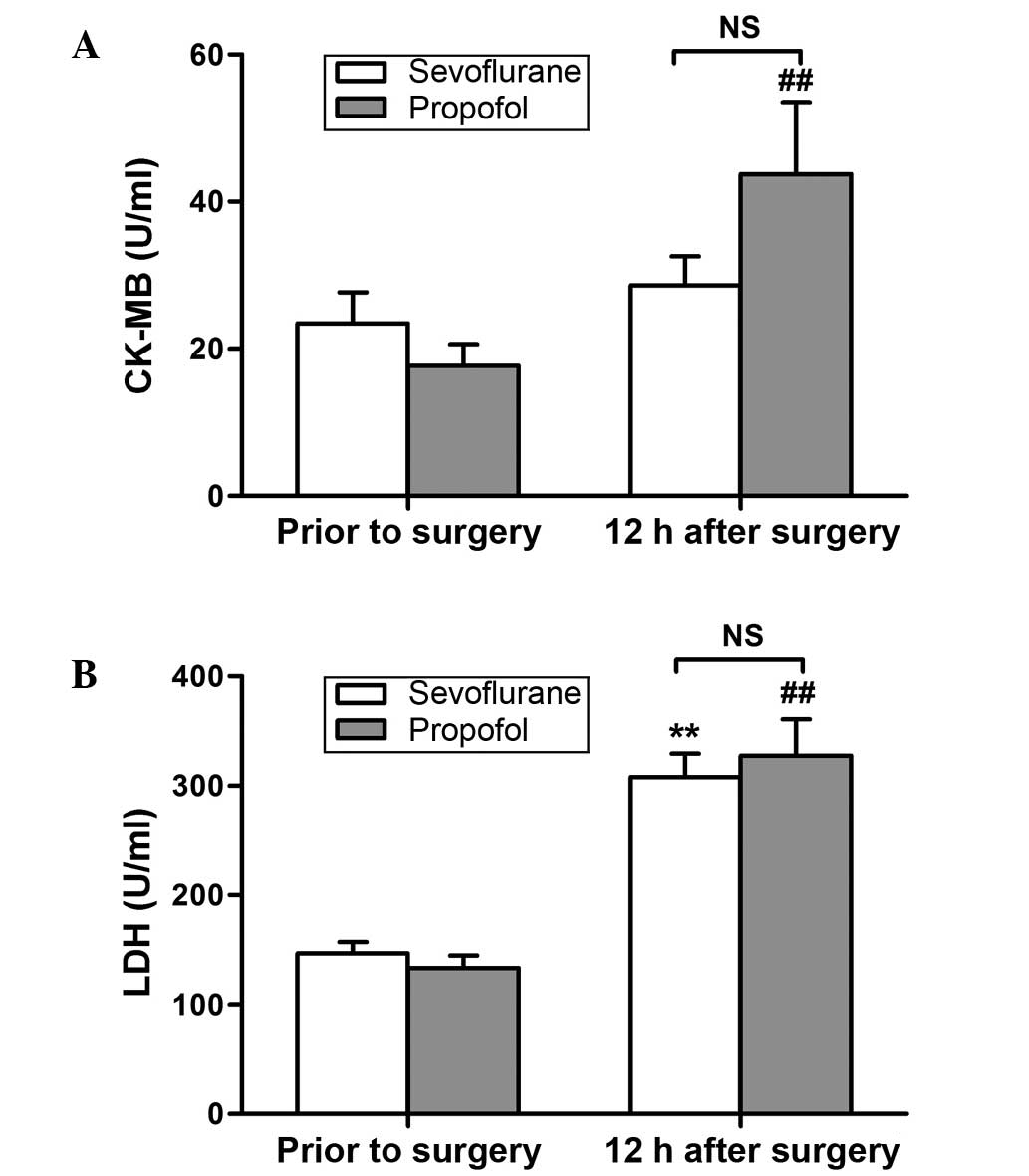
The CK-MB levels were found to be significantly increased at 12 h
after surgery in the propofol group only (P<0.01; Fig. 2A), whereas significantly increased
levels of LDH were observed at 12 h after surgery in both groups
(P<0.01; Fig. 2B). However, no
significant differences were detected between the two groups in the
expression of these variables (Figs.
1 and 2).
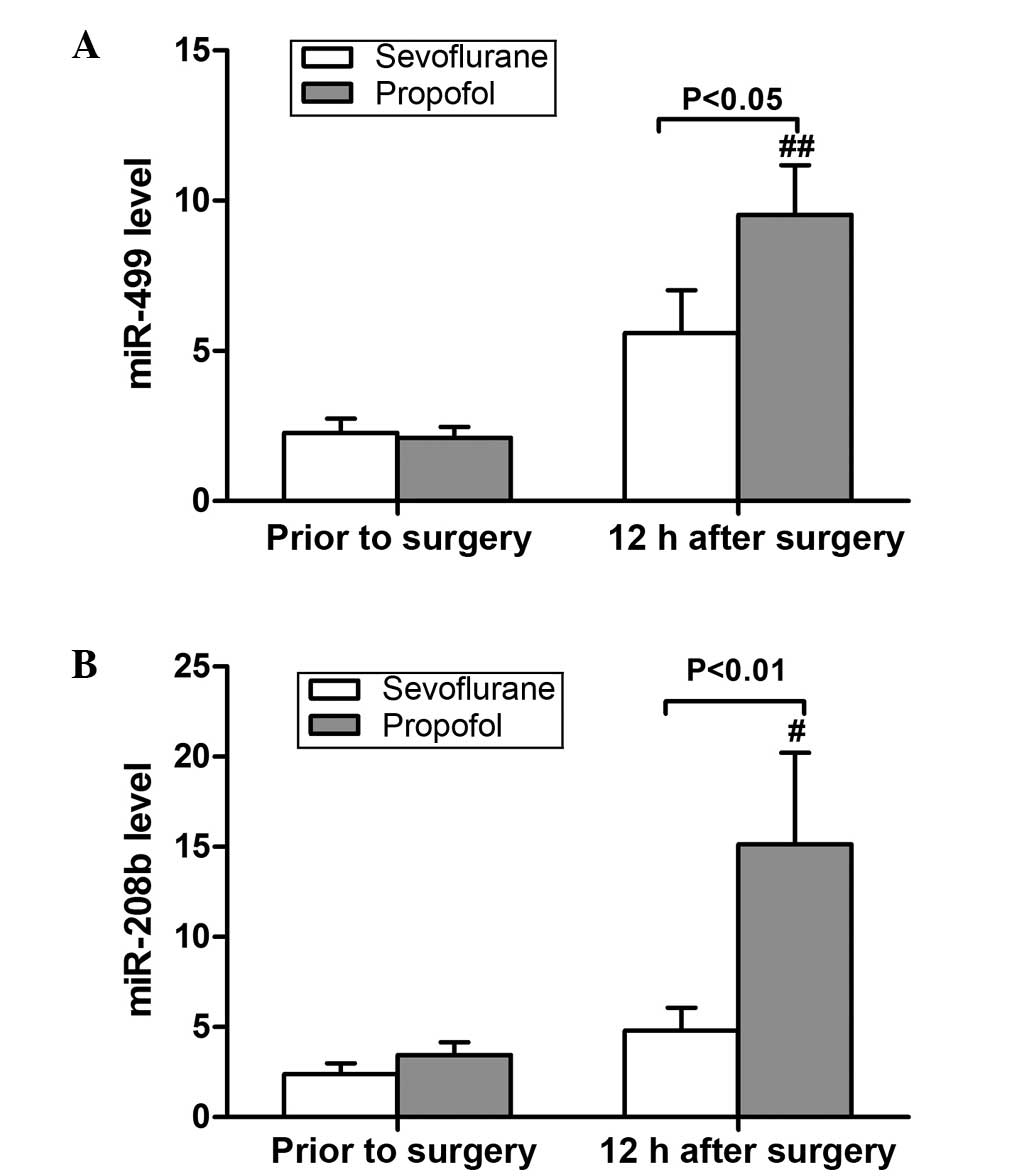
The expression levels of miR-499 and miR-208b were
detected using RT-qPCR, and were found to be increased following
surgery in both anesthesia groups, although the difference was only
significant in the propofol group (P<0.05; Fig. 3). There was no significant difference
between the two groups prior to surgery (P>0.05; Fig. 3). However, at 12 h after surgery, the
levels of the two miRNA markers were significantly reduced in the
sevoflurane group when compared with the propofol group (P<0.05;
Fig. 3).
Discussion
Previous studies have suggested that sevoflurane
exerts a cardioprotective effect, mitigating myocardial damage,
reducing the levels of CK-MB and cTnI, reducing the infarction
area, and decreasing the incidence of arrhythmia and cardiac
dysfunction (6–9). A recent study demonstrated that rat
heart infarct size was significantly reduced in the sevoflurane
preconditioned group compared with that in the ischemia/reperfusion
group (24). The underlying
mechanisms may include nuclear factor-κB activation, upregulation
of autophagy, and the attenuation of tumor necrosis factor-α,
interleukin-1β and caspase-3 expression (25–27).
Another study found that sevoflurane preconditioning mediates
against MIRI via caveolin-3-dependent cyclooxygenase-2 inhibition
and antioxidative effects (28). In
addition, sevoflurane postconditioning for 5 min was sufficient to
activate protein kinase B and exert maximal cardioprotection
against MIRI in isolated rat hearts (29). However, these studies used
sevoflurane pre- or post-conditioning, along with propofol for
induction and maintenance. Zaugg et al (30) found that propofol was able to cancel
the cardioprotective effect of sevoflurane in a
concentration-dependent manner. By contrast, certain other studies
suggested the same protective effect for propofol (31,32).
Therefore, it is not certain which of the two drugs is mainly
responsible for cardioprotection. The results of Jovic et al
(33) indicated that sevoflurane and
propofol led to cardiac protection via different
mitochondrially-associated molecular mechanisms. It appears that
sevoflurane acts by regulating cytochrome c oxidase and ATP
synthase, while the effects of propofol occur through regulation of
cytochrome c, connexin 43, mtDNA transcription and
uncoupling protein 2 (33). Other
studies also showed the superiority of sevoflurane over propofol
anesthesia. For instance, Ballester et al (34) compared the influence of sevoflurane
and propofol on the levels of myocardial oxidative stress markers
(F2-isoprostanes and nitrates/nitrites) in coronary sinus blood
samples from patients undergoing OPCAB. Their data suggested that
sevoflurane showed improved antioxidative properties compared with
propofol (34).
In the present study, sevoflurane inhalation for
VIMA without propofol was used to observe its clinical effect in
comparison with propofol TIVA. Our data showed that there was no
significant difference between the sevoflurane group and propofol
group in hemodynamic variables and cardiac function, which is
similar to the results of previous studies (35). The CO and SV of patients were higher
subsequent to surgery in the sevoflurane group. Although the
difference between the two group following surgery was not
significant, sevoflurane may potentially benefit the clinical
outcome.
Myocardial damage leads to the release of specific
enzymes from myocardial cells into the blood. The most common
enzymes include cTnI, CK-MB and LDH. cTnI has a high degree of
myocardial specificity and sensitivity and is considered as a ‘gold
marker’ of myocardial infarction (36). Numerous previous studies have
investigated the influence of sevoflurane on myocardial enzyme
levels in patients undergoing CABG, and the results have been
contradictory (37–40). Law-Koune et al (37) and Hemmerling et al (38) found no difference between sevoflurane
and propofol in their effects on cTnI and CK-MB in patients
undergoing OPCAB (37,38). However, two other studies showed the
advantage of sevoflurane over propofol by reducing the levels of
cardiac markers (39,40). In the current study, the increase in
the levels of cTnI following surgery was less in the sevoflurane
group compared with the propofol group; however, the difference was
not statistically significant. The influence of sevoflurane on cTnI
may be associated with its inhaled concentration. Exposure to 2.8
vol% sevoflurane provides a better functional return and a lower
percentage of infarction on reperfusion compared with 1.4 vol%
sevoflurane (40). In the current
study, the depth of anesthesia was adjusted by BIS (value, 40–50),
so that only a low inhaled concentration of sevoflurane (1.35±0.79
vol%) was required for the majority of time, with the exception of
the induction period (8 vol%). Piriou et al (41) also found that inhaled sevoflurane at
1MAC for 15 min had limited impact on cTnI. The CK-MB levels
significantly increased in the sevoflurane and propofol groups
following surgery. However, compared with the propofol group, CK-MB
expression was significantly lower in the sevoflurane group after
the surgery. Vikenes et al (42) identified that CK-MB was superior to
the cardiac troponins in predicting long-term event-free survival
subsequent to elective cardiac surgery in low-risk patients with
stable symptoms undergoing CAB grafting. Furthermore, LDH is a
nonspecific marker of myocardial injury, and LDH activity in the
serum can be released from various types of damaged cells (43). In the present study, LDH expression
increased following surgery in both groups; however, this was not
significantly different between the two groups.
miRNAs are short non-coding RNAs, approximately
18–25 nucleotides long, which act as negative or positive
regulators, primarily post-transcriptionally. Besides their
intracellular function, recent studies have demonstrated that
miRNAs can be exported or released by cells and circulate within
the blood in a remarkably stable form (44,45). The
circulating miRNA patterns may be used as biomarkers for
cardiovascular diseases. miR-499 and miR-208b are specifically
expressed in myocardial tissue (46,47).
Recent animal experiments and clinical studies revealed that
miR-499 and miR-208b increased subsequent to myocardial injury, and
the degree of increase was positively correlated with the increase
in cTnI levels (48,49). Their diagnostic accuracy was found to
be superior to other traditional biomarkers. Gidlof et al
(50) collected plasma samples from
424 patients with suspected ACS treated in a coronary care unit, in
order to measure cardio-enriched miRNAs. The authors found that
miR-208b and miR-499 expression levels were higher in myocardial
infarction patients, and established the association of increased
miRNA levels with reduced systolic function subsequent to
myocardial infarction and the risk of mortality or heart failure
(50). The level of miR-499 in the
plasma is very low in healthy individuals. Within 1–3 h after
myocardial damage, this level begins to increase and reaches a peak
at 3–12 h, and then decreases after 12–24 h (51). By contrast, miR-208b cannot be
detected in healthy individuals, but appears in the plasma 1 h
after myocardial injury, reaching a peak level at 3 and then
decreasing after 12 h (46). A study
by Ishikawa et al (52)
showed that anesthetics cause numerous miRNA expression changes,
and that the miRNA expression pattern was particular for each
anesthetic, such as sevoflurane or propofol. Tanaka et al
(53) found dynamic changes in miRNA
expression caused by sevoflurane anesthesia on rats suffering from
lung diseases.
The results of the present study showed that miR-499
and miR-208b were higher subsequent to surgery compared with those
prior to surgery in both anesthesia groups. In addition, the two
miRNA biomarkers were much lower in the sevoflurane group compared
with the propofol group subsequent to surgery. This confirmed that
the cardioprotective effect of sevoflurane on patients undergoing
OPCAB is superior to that of propofol. Furthermore, the current
study established an association between sevoflurane and miRNAs in
patients undergoing OPCAB for the first time. The small number of
patients included in the study limits our conclusion, and thus
larger numbers should be used to further assess the
cardioprotective effect of sevoflurane. Whether the results can be
extended to other cardiac patients requires further research.
In conclusion, the present study demonstrated that
VIMA with sevoflurane in patients undergoing OPCAB resulted in
improved cardiac protection compared with propofol TIVA, and this
effect was indicated based on the levels of two sensitive
biomarkers, circulating miR-499 and miR-208b.
References
|
1
|
Parissis H, Mbarushimana S, Ramesh BC,
Parissis M, Lampridis S, Mhandu P and Al-Alao B: The impact of
off-pump surgery in end-organ function: Practical end-points. J
Cardiothorac Surg. 10:1582015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonenc A, Hacişevki A, Griffiths HR, Torun
M, Bakkaloglu B and Simsek B: Free radical reaction products and
antioxidant capacity in beating coronary artery surgery compared to
conventional bypass. Biochemistry (Mosc). 76:677–685. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leslie JB: Incidence and aetiology of
perioperative hypertension. Acta Anaesthesiol Scand Suppl. 99:5–9.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao YT, Fang NX, Shi CX and Li LH:
Sevoflurane postconditioning protects isolated rat hearts against
ischemia-reperfusion injury. Chin Med J (Engl). 123:1320–1328.
2010.PubMed/NCBI
|
|
5
|
Coetzee JF, Roux PJ, Genade S and Lonchner
A: Reduction of postischemic contractile dysfunction of the
isolated rat heart by sevoflurane: Comparison with halothane.
Anesth Analg. 90:1089–1097. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Hert SG, Broecke PW, Mertens E, Van
Sommeren EW, De Blier IG, Stockman BA and Rodrigus IE: Sevoflurane
but not propofol preserves myocardial function in coronary surgery
patients. Anesthesiology. 97:42–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Hert SG, Van der Linden PJ, Cromheecke
S, Meeus R, Nelis A, Van Reeth V, ten Broecke PW, De Blier IG,
Stockman BA and Rodrigus IE: Cardioprotective properties of
sevoflurane in patients undergoing coronary surgery with
cardiopulmonary bypass are related to the modalities of its
administration. Anesthesiology. 101:299–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haroun-Bizri S, Khoury SS, Chehab IR,
Kassas CM and Baraka A: Does isoflurane optimize myocardial
protection during cardiopulmonary bypass? J Cardiothorac Vasc
Anesth. 15:418–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Julier K, da Silva R, Garcia C, Bestmann
L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von
Segesser LK, et al: Preconditioning by sevoflurane decreases
biochemical markers for myocardial and renal dysfunction in
coronary artery bypass graft surgery: A double-blinded,
placebo-controlled, multicenter study. Anesthesiology.
98:1315–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fisher LA, Beckman JA, Brown KA, Calkins
H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK,
Kersten JR, et al: ACC/AHA 2007 guidelines on perioperative
cardiovascular evaluation and care for noncardiac surgery: A report
of the American college of cardiology/American heart association
task force on practice guidelines. Circulation. 116:e418–e499.
2007.PubMed/NCBI
|
|
11
|
Landoni G, Bignami E, Oliviero F and
Zangrillo A: Halogenated anaedthetics and cardiac protection in
cardiac and non-cardiac anesthesia. Ann Card Anesth. 12:4–9. 2009.
View Article : Google Scholar
|
|
12
|
Landoni G, Biondi-Zoccai GG, Zangrillo A,
Bignami E, D'Avolio S, Marchetti C, Calabrò MG, Fochi O, Guarracino
F, Tritapepe L, et al: Desflurane and sevoflurane in cardiac
surgery: A meta-analysis of randomized clinical trials. J
Cardiothorac Vasc Anesth. 21:502–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamm CW, Goldmann BU, Heeschen C, Kreymann
G, Berger J and Meinertz T: Emergency room triage of patients with
acute chest based on rapid testing for troponin T or troponin I. N
Engl J Med. 337:1648–1653. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davies E, Gawad Y, Takahashi M, Shi Q, Lam
P, Styba G, Lau A, Heeschen C, Usategui M and Jackowski G:
Analytical performance and clinical utility of a sensitive
immunoassay for determination of human cardiac troponin I. Clin
Biochem. 30:479–490. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewandrowski K, Chen A and Januzzi J:
Cardiac markers for myocardial infarction. Am J Clin Pathol.
118(Suppl): S93–S99. 2002.PubMed/NCBI
|
|
16
|
Dekker MS, Mosterd A, van't Hof AW and
Hoes AW: Novel biochemical markers in suspected acute coronary
syndrome: Systematic review and critical appraisal. Heart.
96:1001–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sluijter JP, van Mil A, van Vliet P, Metz
CH, Liu J, Doevendans PA and Goumans MJ: MicroRNA-1 and −499
regulate differentiation and proliferation in human-derived
cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol.
30:859–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA.
103:18255–18260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oerlemans MI, Mosterd A, Dekker MS, de
Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW and
Sluijter JP: Early assessment of acute coronary syndromes in the
emergency department: The potential diagnostic value of circulating
microRNAs. EMBO Mol Med. 4:1176–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devaux Y, Vausort M, Goretti E, Nazarov
PV, Azuaje F, Gilson G, Corsten MF, Schroen B, Lair ML and Heymans
S: Use of circulating microRNAs to diagnose acute myocardial
infarction. Clin Chem. 58:559–567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lequin RM: Enzyme immunoassay
(EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem.
51:2415–2418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiao S, Xie H, Wang C, Wu X, Liu H and Liu
C: Delayed anesthetic preconditioning protects against myocardial
infarction via activation of nuclear factor-κB and upregulation of
autophagy. J Anesth. 27:251–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torina AG, Reichert K, Lima F, de Souza
Vilarinho KA, de Oliveira PP, do Carmo HR, de Carvalho DD, Saad MJ,
Sposito AC and Petrucci O: Diacerein improves left ventricular
remodeling and cardiac function by reducing the inflammatory
response after myocardial infarction. PLoS One. 10:e01218422015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, He L, Chen F, He X, Cai Y, Zhang G,
Yi Q, He M and Luo J: Impaired autophagy contributes to adverse
cardiac remodeling in acute myocardial infarction. PLoS One.
9:e1128912014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q: Lentivirus mediated interference of
Caspase-3 expression ameliorates the heart function on rats with
acute myocardial infarction. Eur Rev Med Pharmacol Sci.
18:1852–1858. 2014.PubMed/NCBI
|
|
28
|
Zhao J, Wang F, Zhang Y, Jiao L, Lau WB,
Wang L, Liu B, Gao E, Koch WJ and Ma XL: Sevoflurane
preconditioning attenuates myocardial ischemia/reperfusion injury
via caveolin-3-dependent cyclooxygenase-2 inhibition. Circulation.
128(11 Suppl 1): S121–S129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao YY, Zhu MH, Zhang FJ, Wen CY, Ma LL,
Wang WN, Wang CC, Liu XB, Yu LN, Qian LB, et al: Activation of Akt
and cardioprotection against reperfusion injury are maximal with
only five minutes of sevoflurane post-conditioning in isolated rat
hearts. J Zhejiang Univ Sci B. 14:511–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaugg M, Wang L, Zhang L, Lou PH,
Lucchinetti E and Clanachan AS: Choice of anesthetic combination
determines Ca2+ leak after ischemia-reperfusion injury in the
working rat heart: Favorable versus adverse combinations.
Anesthesiology. 116:648–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Samir A, Gandreti N, Madhere M, Khan A,
Brown M and Loomba V: Anti-inflammatory effects of propofol during
cardiopulmonary bypass: A pilot study. Ann Card Anaesth.
18:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia WF, Liu Y, Zhou QS, Tang QZ and Zou
HD: Protective effect of propofol and its relation to postoperation
recovery in children undergoing cardiac surgery with
cardiopulmonary bypass. Pediatr Cardiol. 32:940–946. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jovic M, Stancic A, Nenadic D, Cekic O,
Nezic D, Milojevic P, Micovic S, Buzadzic B, Korac A, Otasevic V,
et al: Mitochondrial molecular basis of sevoflurane and propofol
cardioprotection in patients undergoing aortic valve replacement
with cardiopulmonary bypass. Cell Physiol Biochem. 29:131–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ballester M, Llorens J,
Garcia-de-la-Asuncion J, Perez-Griera J, Tebar E, Martinez-Leon J,
Belda J and Juez M: Myocardial oxidative stress protection by
sevoflurane vs. propofol: A randomised controlled study in patients
undergoing off-pump coronary artery bypass graft surgery. Eur J
Anaesthesiol. 28:874–881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El Azab SR, Scheffer GJ, Rosseel PM and De
Lange JJ: Induction and maintenance of anaesthesia with sevoflurane
in comparison to high dose opioid during coronary artery bypass
surgery. Eur J Anaesthesiol. 17:336–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Futterman LG and Lemberg L: SGOT, LDH,
HBD, CPK, CK-MB, MB1MB2, cTnT, cTnC, cTnI. Am J Crit Care.
6:333–338. 1997.PubMed/NCBI
|
|
37
|
Law-Koune JD, Raynaud C, Liu N, Dubois C,
Romano M and Fischler M: Sevoflurane-remifentanyl versus
propofol-remifentanyl anesthesia at a similar bispectral level for
off-pump coronary artery surgery: No evidence of reduced myocardial
ischemia. J Cardiothorac Vasc Anesth. 20:484–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hemmerling T, Olivier J, Le N, Prieto I
and Bracco D: Myocardial protection by isoflurane vs. sevoflurane
in ultra-fast-track anaesthesia for off-pump aortocoronary bypass
grafting. Eur J Anaesthesiol. 25:230–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Conzen PF, Fischer S, Detter C and Peter
K: Sevoflurane provides greater protection of the myocardium than
propofol in patients undergoing off-pump coronary artery bypass
surgery. Anesthesiology. 99:826–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riess ML, Kevin LG, Camara AK, Heisner JS
and Stowe DF: Dual exposure to sevoflurane improves anesthetic
preconditioning in intact hearts. Anesthesiology. 100:569–574.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Piriou V, Mantz J, Goldfarb G, Kitakaze M,
Chiari P, Paquin S, Cornu C, Lecharny JB, Aussage P, Vicaut E, et
al: Sevoflurane preconditioning at 1 MAC only provides limited
protection in patients undergoing coronary artery bypass surgery: A
randomized bi-centre trial. Br J Anaesth. 99:624–631. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vikenes K, Andersen KS, Melberg T, Farstad
M and Nordrehaug JE: Long-term prognostic value of cardiac troponin
I and T versus creatine kinase-MB mass after cardiac surgery in
low-risk patients with stable symptoms. Am J Cardiol. 106:780–786.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ekaney ML, Otto GP, Sossdorf M, Sponholz
C, Boehringer M, Loesche W, Rittirsch D, Wilharm A, Kurzai O, Bauer
M and Claus RA: Impact of plasma histones in human sepsis and their
contribution to cellular injury and inflammation. Crit Care.
18:5432014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Rosa S and Indolfi C: Circulating
microRNAs as Biomarkers in Cardiovascular Diseases. EXS.
106:139–149. 2015.PubMed/NCBI
|
|
45
|
Schulte C, Molz S, Appelbaum S, Karakas M,
Ojeda F, Lau DM, Hartmann T, Lackner KJ, Westermann D, Schnabel RB,
et al: miRNA-197 and miRNA-223 Predict Cardiovascular Death in a
Cohort of Patients with Symptomatic Coronary Artery Disease. PLoS
One. 10:e01459302015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ji X, Takahashi R, Hiura Y, Hirokawa G,
Fukushima Y and Iwai N: Plasma miR-208 as a biomarker of myocardial
injury. Clin Chem. 55:1944–1949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reddy A, Zheng Y, Jagadeeswaran G, Macmil
SL, Graham WB, Roe BA, Desilva U, Zhang W and Sunkar R: Cloning,
characterization and expression analysis of porcine microRNAs. BMC
genomics. 10:652009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gidlöf O, Andersson P, van der Pals J,
Götberg M and Erlinge D: Cardiospecific microRNA plasa levels
correlate with troponin and cardiac function in patients with ST
elevation myocardial infarction, are selectively dependent on renal
elimination and can be detected in urine samples. Cardiology.
118:217–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Olivieri F, Antonicelli R, Lorenzi M,
D'Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La
Sala L, Galeazzi R, et al: Diagnostic potential of circulating
miR-499-5p in elderly patients with acute non ST-elevation
myocardial infarction. Int J Cardiol. 167:531–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gidlof O, Smith JG, Miyazu K, Gilje P,
Spencer A, Blomquist S and Erlinge D: Circulating cardio-enriched
microRNAs are associated with long-term prognosis following
myocardial infarction. BMC Cardiovasc Disord. 13:122013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Corsten MF, Dennert R, Jochems S,
Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans
S and Schroen B: Circulating MicroRNA-208b and MicroRNA-499 reflect
myocardial damage in cardiovascular disease. Circ Cardiovasc Genet.
3:499–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ishikawa M, Tanaka S, Arai M, Genda Y and
Sakamoto A: Differences in microRNA changes of healthy rat liver
between sevoflurane and propofol anesthesia. Anesthesiology.
117:1245–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tanaka S, Ishikawa M, Arai M, Genda Y and
Sakamoto A: Changes in microRNA expression in rat lungs caused by
sevoflurane anesthesia: A TaqMan® low-density array study. Biomed
Res. 33:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|