Introduction
Susceptibility-weighted imaging (SWI) has been
introduced to the clinical setting in the last decade (1–3). This
technique is extremely sensitive for the utilization of
paramagnetic material, such as deoxyhemoglobin and hemosiderin
(4,5). Combined with its excellent spatial
resolution, SWI may be important in detecting the venues system
especially in deep regions of the brain (6,7).
Deep medullary veins (DMVs) are an important part of
the deep cerebral veins system, originating from the middle part of
the cerebral medullary, converging the microvenuals around the
centrum ovale and ultimately cascading into the collecting venous
trunks (8). Under physiological
conditions, diameters of DMVs lumen were ≤0.02 mm and could not be
clearly detected. However, a previous study conducted on moyamoya
disease (MMD) suggested that in patients with severe hemodynamic
impairment, multiple DMVs were observed beside the lateral
ventricle by high-resolution magnetic resonance imaging (MRI)
(9). To the best of our knowledge,
the relationship between DMVs andhemodynamic condition or
cerebrocervical artery stenosis in patients with ischemic stroke
have yet to be invstigated.
In the current study, cerebrovascular reactivity
(CVR) as an index of hemodynamic condition was obtained by
transcranial Doppler (TCD) with CO2 stimulation.
Additionally, whether DMVs in SWI correlated with the ipsilateral
CVR in patients with ischemic stroke was examined.
Patients and methods
Patients
The clinical data of 61 consecutive patients from
the First People's Hospital of Xuzhou (Jiangsu, China) were
retrieved. The inclusion criteria for the study were: i) unilateral
(MCA) territory ischemic stroke (cerebral infarction and transient
ischemic stroke included); ii) atherosclerotic ischemic stroke with
assessable digital subtraction angiography (DSA), SWI and TCD
CO2 stimulation results prior to surgical or
interventional treatment within the first 7 days of
hospitalization. The exclusion criteria were: i) DSA-demonstrated
occlusion in the common cerebral, internal cerebral, or middle
cerebral arteries (MCAs); ii) previous arteriovenous malformation,
venous stroke, brain tumor, or demyelinating diseases, which may
influence the outcome of venous imaging; iii) previous thyroid,
respiratory, or heart disease, which may influence the mean blood
flow velocity detected by TCD; iv) multiple microbleeds located
around the lateral ventricles, which may influence the
semiquantitative assessment of DMVs; and v) poor transtemporal
penetration for ultrasound.
Study protocols were approved by the Institution
Review Board of the First People's Hospital of Xuzhou. Hemispheres
with new or recent onset of symptoms were defined as symptomatic
hemispheres (SHs), the opposite hemispheres were defined as
asymptomatic hemispheres (AHs). For each patient, risk factors of
cerebrovascular disease such as age, gender, history of
hypertension, diabetes mellitus, and current smoking were
documented.
Imaging assessment
The patients underwent SWI using a Magnetom Trio
whole body 3.0 T MRI scanner (Siemens, Erlangen, Germany). The MRI
protocol included axial T1/T2-weighted imaging, axial
diffusion-weighted imaging, axial and/or coronal T2-FLAIR imaging,
MRA and SWI. SWI images were obtained using one set of parameters
(40 mT/m gradient. TE/TR/FA: 25 msec/56 msec/20°; FOV: 230 × 115 ×
144 mm3; matrix: 512 × 254 × 72; voxel size: 0.45 × 0.45
× 2 mm3; and section thickness: 2 mm). Imaging of
maximum intensity projection (MIP) was also accumulated and
reconstituted with commercially available hardware and software.
Two experienced MRI-specialized neuroradiologists, blinded to the
patients' clinical and hemodynamic information, evaluated the
imaging of MIP individually. The number of conspicuous DMVs was
classified as: stage 1: ≤5 DMVs and stage 2: >5 DMVs. Consensus
over inter-observer discrepancies was reached through
consultation.
No serious complication occurred during the DSA
procedure. The stenosis of excranial internal carotid artery (ICA)
was analyzed according to the North American Symptomatic Carotid
Endarterectomy Trial Collaborators (10). The Warfarin-Aspirin Symptomatic
Intracranial Disease Study was employed to assess the stenosis of
intracranial vessels (11). The most
severe segments of tandem stenosis were required for the analysis.
The proportion of stenosis ≤50% was documented as mild stenosis,
50%<stenosis≤70% as moderate stenosis, and >70% was
documented as severe stenosis. Collateral compensation between
hemispheres was also classified based on DSA results. Positive
collateral compensation was defined as ipsilateral ICA territory
receiving blood flow from contralateral hemispheres or posterior
circulation, whereas isolated hemispheres or giving branches to the
contralateral sides were documented as negative collateral
compensation.
TCD CO2 stimulation
assessment
TCD recordings were made from the two MCAs via the
transtemporal route. Recordings were made with a commercially
available TCD machine (Companion II; DWL® Sipplingen,
Germany) with the probe held in position by an external fixation
device. The depth of the probe ranged between 52 and 66 mm, with an
average of 56 mm. The probes were fixed when the optimal MCA blood
flow was detected. Air was initially administered via a one-way
valve mask. Patients breathed through the mask until MCA velocity
became stable. A further 30 sec of recording was made at this
stage. The mean blood flow velocity was recorded as microvascular
flow velocity (MFV1). Air/carbon dioxide mixture (5%
carbon dioxide and 95% oxygen) was then administered via the mask.
When the MCA velocity was again stabilized, a further 30 sec of
recording was made, and the mean blood flow velocity at that time
point was recorded as MFV2. The process was repeated at
least three times, and the average value of each parameter was
recorded. Patients were required to rest >5 min between each
process. The CVR value was defined as
(MFV2-MFV1)/MFV1 × 100.
Clinical data collection
Statistical analysis
Data were analyzed using the Statistical Package for
the Social Sciences version 18.0 software for windows (SPSS, Inc.,
Chicago, IL, USA). The statistical agreement of the two observers
was analyzed using Cohen's κ value. The difference of the clinical
and hemodynamic parameters between DMV stages was analyzed using
the Student's t-test, as well as Chi-square and Fisher's exact
tests. Logistic regression was employed to determine the
independent risk factors for DMVs. For all the analyses, a
two-tailed value of P<0.05 was considered to indicate a
statistically significant difference.
Results
A total of 42 males and 19 females were included in
the present study. The mean age of the patients was 61.6±13.1, with
a range of 35–88 years. Based on the DMVs in SHs, 31 patients were
classified as grade 1, and 30 patients as grade 2. The Cohen κ
value of this binary classification was 0.806 for SHs, which showed
excellent agreement. DSA result suggested that 35 patients had mild
stenosis, 15 patients had moderate stenosis, and 14 patients had
severe stenosis in the trunk of anterior circulation. CVR was
measured to be 46.15±18.22. In AHs, 41 patients were classified as
DMVs grade 1, and 20 patients as DMVs grade 2. The Cohen κ value
was 0.821. A total of 41 patients with mild stenosis, 12 patients
with moderate stenosis, and 8 patients with severe stenosis were
detected by DSA. The value of CVR in AHs was 73.03±20.30.
The univariate analysis of AHs and SHs revealed that
five clinical and imaging indices, including age
(PAHs=0.004, PSHs=0.006), hypertension
(PAHs=0.008, PSHs= 0.020), current smoking
(PAHs=0.006, PSHs=0.021), CVR
(PAHs=0.000, PSHs=0.000), and artery stenosis
(PAHs=0.000, PSHs=0.000) exhibited
statistically significant differences between varying DMVs grades
(Table I). The subsequent
multivariate analysis indicated that CVR (ORAHs=0.925,
95% CIAHs: 0.873–0.981; ORSHs=0.945, 95%
CISHs: 0.896–0.996), and artery stenosis
(ORAH=3.147, 95% CIAH: 1.010–9.806;
ORSHs=2.882, 95% CISHs: 1.017–8.166) were
independent risk factors of DMVs (Table
II).
 | Table I.Clinical parameters between patients
of different DMVs stages in SHs and AHs. |
Table I.
Clinical parameters between patients
of different DMVs stages in SHs and AHs.
|
| DMVs stages in
SHs |
| DMV stages in
AHs |
|
|---|
|
|
|
|
|
|
|---|
| Parameters | Stage 1, n=31 | Stage 2, n=30 | P-value | Stage 1, n=41 | Stage 2, n=20 | P-value |
|---|
| Age, mean ± SD | 57.0±13.2 | 66.4±11.4 | P=0.004a | 58.5±13.1 | 68.1±10.9 | P=0.006a |
| Male (%) | 21 (67.7) | 21 (70.0) | P=0.849 | 30 (73.1) | 12 (60.0) | P=0.297 |
| Hypertension (%) | 16 (51.6) | 25 (83.3) | P=0.008a | 22 (53.7) | 19 (95.0) | P=0.020a |
| Diabetes (%) | 4
(12.9) | 7
(23.3) | P=0.335 | 5
(12.2) | 6
(30.0) | P=0.153 |
| Current smoking
(%) | 7
(22.6) | 17 (56.7) | P=0.006a | 12 (29.3) | 12 (60.0) | P=0.021a |
| Collateral
compensation (%) | 4
(12.9) | 6
(20.0) | P=0.454 | 7
(17.1) | 3
(15.0) | P=0.837 |
| CVR, mean ± SD | 55.9±16.6 | 36.1±14.0 | P=0.000a | 80.3±17.8 | 58.2±17.0 | P=0.000a |
| Stenosis mild
(%) | 25 (80.6) | 8
(26.7) |
| 34 (82.9) | 7
(35.0) |
|
|
| Moderate (%) | 4 (12.9) | 11 (36.7) |
| 6
(14.6) | 6
(30) |
|
| Severe (%) | 2
(6.5) | 11 (36.7) | P=0.000a | 1
(2.4) | 7
(35.0) | P=0.000a |
 | Table II.Multivariable analysis for risk
factors of DMVs in SHs and AHs. |
Table II.
Multivariable analysis for risk
factors of DMVs in SHs and AHs.
|
| DMVs stages in
SHs |
| DMVs stages in
AHs |
|
|---|
|
|
|
|
|
|
|---|
| Factors | Odd rates | 95% CI | P-value | Odd rates | 95% CI | P-value |
|---|
| Age | 1.038 | 0.977–1.102 | P=0.233 | 1.038 | 0.973–1.107 | P=0.263 |
| Hypertension | 1.414 | 0.275–7.269 | P=0.678 | 2.576 | 0.568–9.166 | P=0.132 |
| Current smoking | 4.214 | 0.772–23.019 | P=0.097 | 1.474 | 0.296–7.344 | P=0.636 |
| CVR | 0.925 | 0.873–0.981 | P=0.009a | 0.945 | 0.896–0.996 | P=0.036a |
| Stenosis | 2.882 | 1.017–8.166 | P=0.046a | 3.147 | 1.010–9.806 | P=0.048a |
Discussion
The present findings have demonstrated that 3.0 T
SWI was qualified in detecting the DMVs around the lateral
ventricle in patients with atherosclerotic ischemic stroke. CVR and
stenosis of the trunk of anterior cerebrocervical arteries were
independent risk factors for ipsilateral DMVs in SHs and AHs.
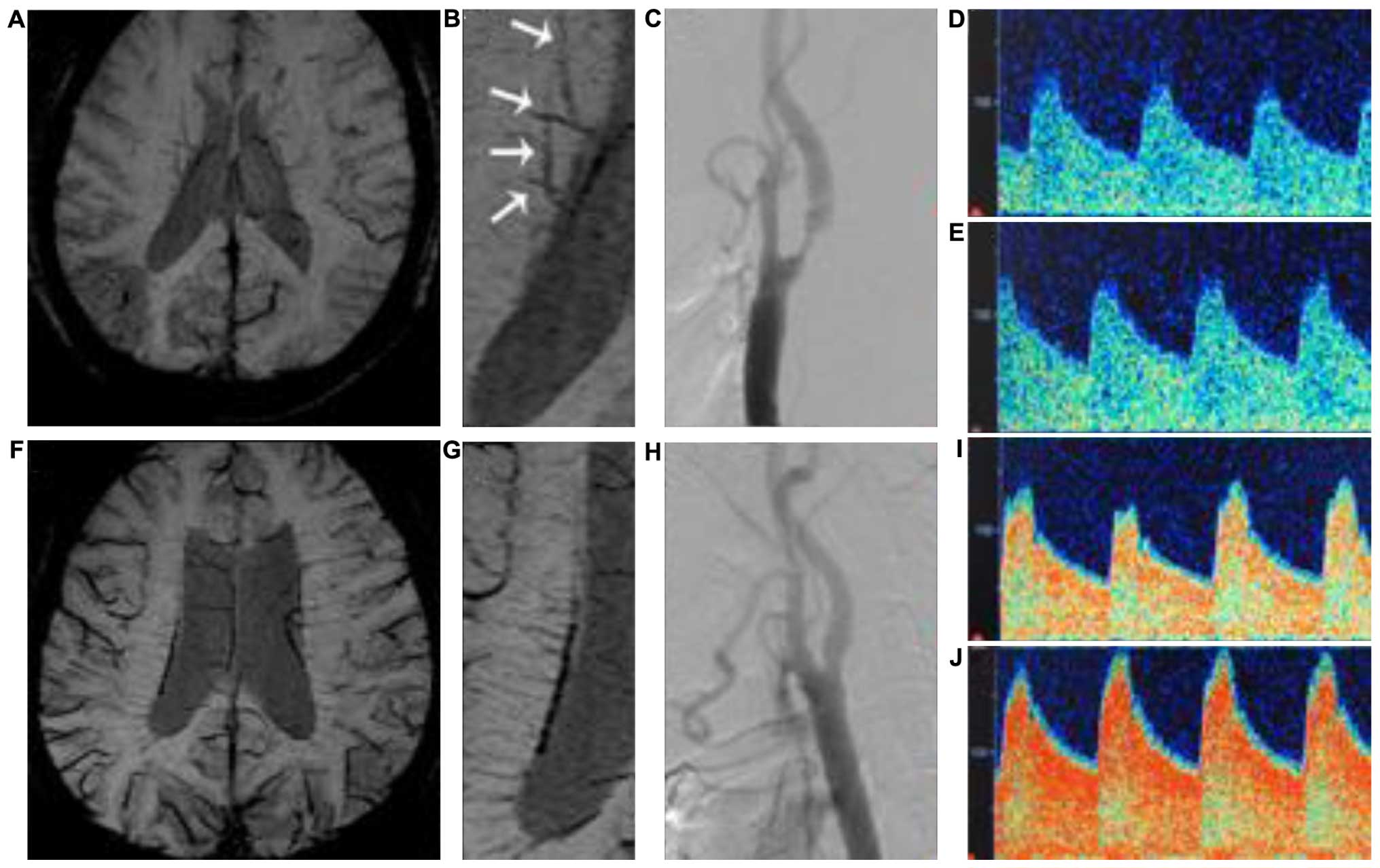
In 2010, Kesavadas et al (12), respectively, analyzed the SWI imaging
in 18 patients with occlusive/critical stenosis ischemic stroke.
Authors of that study initially suggested a significant signal loss
and increased diameter of the venous compared with contralateral
hemispheres. However, in that study, a comparison of inter-patients
was not performed due to the lack of a qualitative or quantitative
standard to define a specific segment of veins. In the present
study, a semiquantitative classification was applied based on the
numerical information of DMVs simplified from Horie's et al
(9) results of MMD. The reason for
not directly applying previous triple classification (0–5 as stage
1, 6–10 as stage 2 and >10 as stage 3) was largely due to the
unsatisfied inter-rater reliability. The Cohen κ was 0.66 in a
previous study (9) and only 0.565 in
SHs and 0.569 in AHs in the present study. The findings exhibited
unsubstantial agreements, which potentially limit the clinical
utility. This abberation may be explained by the limited resolution
of 3.0 T MRI especially for obtaining the exact number of small
signal loss from ‘Brush-like’ vessels (Fig. 1A and B). We therefore combined stages
2 and 3 together. Despite the decreased statistical efficiency, the
interrater reliability of the binary classification may be deemed
reliable.
According to the golden-standard DSA procedure, we
concluded that the degree of stenosis was an independent risk
factor of DMVs in SHs and AHs. This may be explained by the
increased ratio of deoxyhemoglobin/oxyhemoglobin in veins. Previous
findings have shown that following severe stenosis of the
cerebrocervical arteries, decreased perfusion pressure may
upregulate oxygen extraction of the brain tissue (13). A higher concentration of paramagnetic
deoxyhemoglobin in venous vessels therefore accounts for the
increased visibility of DMVs (9).
Our results suggest that in ischemic patients with multiple visible
DMVs, attention should be paid to the vascular stenosis of
ipsilateral anterior cerebrocevical arteries.
Results obtained by Horie et al (9) showed a negative correlation between
DMVs and cerebral reserve assessed by single-photon-emission
computed tomography. In the present study, we confirmed CVR as a
major part of cerebral reserve, independently contributing to the
stages of DMVs, in addition to SHs and AHs. This result supports
the specification that conspicuous DMVs indicate venous stasis in
that region (9). Decreased CVR
induced by hemodynamic impairment shows the over-dilated status of
small vessels, which reduces the velocity of the blood flow and
ultimately leads to stasis of small veins and therefore severe
signal loss in SWI (14). However,
TCD was lacking as a method for hemodynamic assessment despite a
good correlation of ‘gold-standard’ positron emission computed
tomography (15). Therefore, there
may be a discrepancy despite the TCD procedure being performed by
the same physician three times. Prospective studies should consider
other non-invasive and more accurate methods such as
perfusion-weighted imaging of high-resolution MRI.
The other limitation of the present study was that
it was performed as a preoperative study with a limited sample
size. We were not able to characterize changes of the DMVs, CVR, or
stenosis after medical/surgical treatment. Thus, the utility of
DMVs in the stratified treatment of ischemic stroke remains
unknown. In addition, since CVR and artery stenosis are independent
risk factors for subsequent ischemic stroke, whether DMVs stage
correlate with worse clinical outcome should also be prospectively
confirmed.
References
|
1
|
Abduljalil AM, Schmalbrock P, Novak V and
Chakeres DW: Enhanced gray and white matter contrast of phase
susceptibility-weighted images in ultra-high-field magnetic
resonance imaging. J Magn Reson Imaging. 18:284–290. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Ai B, Li Y, Qi H and Wu L:
Susceptibility-weighted imaging in grading brain astrocytomas. Eur
J Radiol. 75:e81–e85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haacke EM, Mittal S, Wu Z, Neelavalli J
and Cheng YC: Susceptibility-weighted imaging: Technical aspects
and clinical applications, part 1. AJNR Am J Neuroradiol. 30:19–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayaz M, Boikov AS, Haacke EM, Kido DK and
Kirsch WM: Imaging cerebral microbleeds using susceptibility
weighted imaging: One step toward detecting vascular dementia. J
Magn Reson Imaging. 31:142–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori N, Miki Y, Kikuta K, Fushimi Y, Okada
T, Urayama S, Sawamoto N, Fukuyama H, Hashimoto N and Togashi K:
Microbleeds in moyamoya disease: Susceptibility-weighted imaging
versus T2*-weighted imaging at 3 Tesla. Invest Radiol. 43:574–579.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyasaka T, Taoka T, Nakagawa H, Wada T,
Takayama K, Myochin K, Sakamoto M, Ochi T, Akashi T and Kichikawa
K: Application of susceptibility weighted imaging (SWI) for
evaluation of draining veins of arteriovenous malformation: Utility
of magnitude images. Neuroradiology. 54:1221–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang P, Chen CH, Lin WC, Lin RT, Khor GT
and Liu CK: Clinical applications of susceptibility weighted
imaging in patients with major stroke. J Neurol. 259:1426–1432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono M, Rhoton AL Jr, Peace D and Rodriguez
RJ: Microsurgical anatomy of the deep venous system of the brain.
Neurosurgery. 15:621–657. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horie N, Morikawa M, Nozaki A, Hayashi K,
Suyama K and Nagata I: ‘Brush Sign’ on susceptibility-weighted MR
imaging indicates the severity of moyamoya disease. AJNR Am J
Neuroradiol. 32:1697–1702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
North American Symptomatic Carotid
Endarterectomy Trial Collaborators: Beneficial effect of carotid
endarterectomy in symptomatic patients with high-grade carotid
stenosis. N Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schumacher HC, Meyers PM, Higashida RT,
Derdeyn CP, Lavine SD, Nesbit GM, Sacks D, Rasmussen P and Wechsler
LR: Reporting standards for angioplasty and stent-assisted
angioplasty for intracranial atherosclerosis. Stroke. 40:e348–e365.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kesavadas C, Santhosh K and Thomas B:
Susceptibility weighted imaging in cerebral hypoperfusion-can we
predict increased oxygen extraction fraction? Neuroradiology.
52:1047–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamauchi H, Fukuyama H, Fujimoto N,
Nabatame H and Kimura J: Significance of low perfusion with
increased oxygen extraction fraction in a case of internal carotid
artery stenosis. Stroke. 23:431–432. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kesavadas C, Thomas B, Pendharakar H and
Sylaja PN: Susceptibility weighted imaging: Does it give
information similar to perfusion weighted imaging in acute stroke?
J Neurol. 258:932–934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rijbroek A, Boellaard R, Vriens EM,
Lammertsma AA and Rauwerda JA: Comparison of transcranial Doppler
ultrasonography and positron emission tomography using a
three-dimensional template of the middle cerebral artery. Neurol
Res. 31:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|