Introduction
Lung cancer remains the leading cause of death from
cancer worldwide, with ~1.4 million deaths each year (1). Non-small cell lung cancer (NSCLC),
which is the predominant form of the disease, accounts for >80%
of all cases of lung cancer (2).
There are three types of NSCLC: Lung adenocarcinoma (LAC),
large-cell carcinoma (LCC) and squamous cell carcinoma (SCC)
(3). Despite great advances in
surgery, chemotherapy and radiotherapy, the long-term survival of
patients with NSCLC remains poor due to rapid growth and high rate
of recurrence and metastasis (4,5).
Therefore, it is imperative that the precise molecular mechanisms
underlying the invasion and metastasis of NSCLC are
investigated.
Metastasis suppressor 1 (MTSS1), which is also known
as MIM (missing-in-metastasis), was first identified as a potential
metastasis suppressor gene in metastatic bladder carcinoma cell
lines (6). Accumulating evidence
indicates that MTSS1 is a multifunctional molecular effector with
an important role in cancer development, carcinogenesis and
metastasis (7–9). As a scaffold protein, MTSS1 interacts
with various partners to regulate actin dynamics (8,9) and is
also involved in the transcription of effector genes of the sonic
hedgehog (Shh) signaling pathway (10). Whereas the expression of MTSS1 is
reduced in prostate and breast cancers, it is upregulated in
hepatocellular carcinoma (11).
Furthermore, although MTSS1 reportedly inhibits the invasion and
proliferation of breast, colorectal and prostate cancer cells
(12–14), it has been demonstrated to be driver
of metastasis in a subset of melanomas (15). Therefore, MTSS1 may have differential
roles in cancer malignancy, depending on tissue/cell specificity. A
previous study has demonstrated that MTSS1 is significantly
overexpressed in NSCLC, as compared with normal lung tissue; among
the subtypes of NSCLC, which include LAC, LCC and SCC, a
significant correlation was detected between decreased MTSS1
expression and poor prognosis in patients with SCC (3).
To the best of our knowledge, the present study was
the first to explore the differential roles of MTSS1 in the
invasion and proliferation of the different subtypes of NSCLC,
which may provide novel insight into the functional role of MTSS1
in cancer and may help elucidate therapeutic strategies for the
treatment of various types of cancer.
Materials and methods
Cell lines and reagents
Human NSCLC cell lines H920 (LAC) (CRL-5850), H1581
(LCC) (CRL-5878), and SW900 (SCC) (HTB-59) cell lines were
purchased from the American Tissue Culture Collection (Manassas,
VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% heat-inactivated fetal bovine serum (both
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 U/ml
penicillin-streptomycin (Sigma-Aldrich, Beijing, China) at 37°C in
an incubator with a humidified atmosphere composed of 95% air and
5% CO2. Human MTSS1 cDNA clone (SC114849) was purchased
from Origene (Beijing, China) and the full length MTSS1 cDNA
sequence was subcloned into the pcDNA 3.1 plasmid. QCM ECMatrix
24-well (8 µM) Fluorimetric Cell Invasion Assay kit (ECM554) was
purchased from Chemicon (EMD Millipore, Billerica, CA, USA).
Methylthiazoletetrazolium (MTT) Cell Proliferation Assay kit
(30–1010 K) was purchased from ATCC. Universal Tyrosine Kinase
Assay kit (MK410) was purchased from Takara Biotechnology Co.,
Ltd., (Beijing, China). Lipofectamine 2000 transfection reagent was
purchased from Thermo Fisher Scientific, Inc. Human MTSS1 shRNA
(sc-77651-V) lentiviral particles, control shRNA lentiviral
particles-A (sc-108080), and mouse anti-human monoclonal MTSS1
(SS-3; sc-101204), mouse anti-human monoclonal focal adhesion
kinase (FAK) (B-8; sc-271195), goat anti-human polyclonal
phosphorylated FAK (Tyr576/577) (sc-21831) and mouse anti-human
monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
(A-3; sc-137179) antibodies were purchased from Santa Cruz
Biotechnology, Inc., (Santa Cruz, CA, USA). Puromycin, G418 and
selective FAK inhibitor 14 were purchased from Sigma-Aldrich.
Transfection and lentiviral
transduction
MTSS1 expression vector was transfected into cells
using Lipofectamine 2000 transfection reagent, according to the
manufacturer's protocol. Pools of stable transfectants were
generated via selection with G418 (700 µg/ml), according to the
manufacturer's protocol. Lentiviral transduction of the MTSS1 shRNA
was performed and pools of stable transductants were generated via
selection with puromycin (5 µg/ml; Sigma-Aldrich), according to the
manufacturer's protocol.
Western blot analysis
Cells was lysed with a hypotonic buffer containing
2% Nonidet-P and a protease inhibitor cocktail (Sigma-Aldrich) by
sonication three times for 3 sec on ice. Following centrifugation
at 2,000 × g for 15 min at 4°C, the supernatant obtained was
obtained and used for the determination of protein concentration
via the Coomassie blue method and for subsequent steps. Equal
amount of proteins (2 mg) from each sample were separated by 10%
SDS-polyacrylamide gel electrophoresis and blotted onto a
polyvinylidene difluoride microporous membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skimmed milk
powder in Tris-buffered saline and Tween-20. Then, membranes were
incubated for 1 h with a 1:1,000 dilution of the following primary
antibodies: Mouse anti-human monoclonal MTSS1 (SS-3; cat. no.
sc-101204); mouse anti-human monoclonal focal adhesion kinase (FAK;
B-8; cat. no. sc-271195); goat anti-human polyclonal phosphorylated
FAK (Tyr576/577; cat. no sc-21831); and mouse anti-human monoclonal
anti-GAPDH (A-3; cat. no. sc-137179) purchased from Santa Cruz
Biotechnology, Inc.. Then, following washing with Tris-buffered
saline with Tween 20, membranes were incubated with horseradish
peroxidase-conjugated bovine anti-mouse (cat. no. sc-2371) or
bovine anti-goat (cat. no. sc-2350) secondary antibodies purchased
from Santa Cruz Biotechnology, Inc. (1:5,000) for 1 h. An enhanced
chemiluminescence kit (GE Healthcare, Shanghai, China) was used to
reveal the peroxidase. Three independent experiments were
performed. Western blot expression levels were quantitated using
ImageJ software version 1.42 (National Institutes of Health,
Bethesda, MD, USA).
Cell invasion assay
An QCM ECMatrix 24-well (8 µM) Fluorimetric Cell
Invasion Assay kit (Chemicon; EMD Millipore) was used to perform
In vitro cell invasion assays, according to the
manufacturer's protocol (16,17). An
insert polycarbonate membrane (pore size, 8 µM) was used. The
insert in the invasion kit was coated with a thin layer of
ECMatrix. Cells were seeded in the insert (upper chamber) at a
density of 5×104 cells/well in serum-free DMEM. A total
of 600 µl complete medium supplemented with 10% fetal bovine serum
was added to the lower chamber. Following 24-h incubation, invading
cell numbers were determined via a fluorescent cell dose curve
plotted using GraphPad Prism version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA), according to the manufacturer's protocol. Three
independent experiments were performed in duplicate.
MTT cell proliferation assay
An MTT Cell Proliferation Assay kit was used to
determine in vitro cell proliferation, according to the
manufacturer's protocol. Briefly, cells were cultured at a density
of 15×103 cells/well in 96-well tissue culture plates
and incubated at 37°C for 48 h. At the end of the culture period,
cells were washed with phosphate-buffered saline, and MTT reagents
were added according to the manufacturer's protocol. Absorbance was
measured at 570 nm using an ELISA plate reader. Three independent
experiments were performed in triplicate.
FAK activity assay
A nonradioactive isotope solid-phase ELISA kit,
which used the poly (Glu, Tyr) as a substrate (Universal Tyrosine
Kinase Assay kit; Takara Biotechnology Co., Ltd.), was used to
measure the kinase activity of FAK. FAK was purified from cells by
immunoprecipitation with a mouse anti-human monoclonal FAK antibody
(cat. no. sc-271195; Santa Cruz Biotechnology, Inc.). Briefly, 10
µg antibody was pre-adsorbed on protein A Sepharose beads (Thermo
Fisher Scientific, Inc.) in the presence of 2 mg/ml bovine serum
albumin (Thermo Fisher Scientific, Inc.), and the beads were
incubated with 1 ml lysate for 2 h at 4°C. Beads were washed 4
times with 1 ml lysis buffer (Thermo Fisher Scientific, Inc.) and
incubated for 5 min at room temperature with 40 µl high salt
radioimmunoprecipitation assay buffer (50 mM Tris, 250 mM NaCl, 1%
NP-40, 0.5% DOC, 0.1% SDS; pH 7.5) in order to elute
co-immunoprecipitated FAK. Immunoprecipitates were subjected to the
in vitro kinase assay as per the manufacturer's protocol.
Three independent experiments were performed in duplicate.
Statistical analysis
Statistical analyses were performed with SPSS 10.0
for Windows (SPSS, Inc., Chicago, IL, USA). All data values were
expressed as the mean ± standard deviation. Comparisons of means
among multiple groups were performed with one-way analysis of
variance, followed by post-hoc pairwise comparisons using
Tukey's tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
MTSS1 is stably overexpressed and
knocked down in subtypes of NSCLC
In order to determine the functional role of MTSS1
in three subtypes of NSCLC, MTSS1 was stably overexpressed and
knocked down in human H920 (LAC), H1581 (LCC) and SW900 (SCC) NSCLC
cell lines. As detected by western blot analyses, H920 (Fig. 1A) and H1581 (Fig. 1B) cells exhibited higher constitutive
expression levels of MTSS1, as compared with SW900 cells (Fig. 1C). Compared with the controls, MTSS1
expression levels were increased ~2-fold in H920 and H1581 cells
and ~3-fold in SW900 cells (Fig. 1).
Lentiviral transduction of shRNA knocked down the endogenous level
of MTSS1 by ~80% in H920 and H1581 cells and by ~85% in SW900
cells. Treatment with selective FAK inhibitor 14 induced no
significant alterations in the expression levels of MTSS1, as
compared with the MTSS1 group, in all three cell lines (Fig. 1). The results indicate that MTSS1 is
successfully overexpressed and knocked down in NSCLC cells,
respectively.
 | Figure 1.Protein levels of MTSS1 in subtypes of
NSCLC cells, including (A) H920 lung adenocarcinoma, (B) H1581
large-cell carcinoma and (C) SW900 squamous cell carcinoma human
NSCLC cells. Protein levels of MTSS1 were determined via western
blot analyses in NC cells (lane 1), VC cells stably transfected
with the empty pcDNA3.1 vector (lane 2), cells stably transduced
with SC shRNA (lane 3), cells stably transfected with MTSS1 (lane
4), cells stably transfected with MTSS1 and treated with 50 µM FAK
inhibitor 14 for 24 h (lane 5), and cells stably transduced with
MTSS1-shRNA (lane 6). GAPDH blotting was used as a loading control.
MTSS1 blot densities were normalized against GAPDH to obtain a
relative blot density. Data are expressed as the mean + standard
deviation from three independent experiments. aP<0.05
vs. the control groups (NC, VC and SC); bP<0.05 vs.
the MTSS1 group; cP<0.05 vs. the MTSS1 + FAK
inhibitor group. MTSS1, metastasis suppressor 1; NSCLC, non-small
cell lung cancer; FAK, focal adhesion kinase; NC, normal control;
VC, vector control; SC, scramble control. |
MTSS1 exerts differential effects on
invasion in subtypes of NSCLC
In order to determine the effects of MTSS1 on cell
invasion in three subtypes of NSCLC cells, in vitro cell
invasion assays were performed in H920, H1581 and SW900 cells.
Compared with the controls, overexpression of MTSS1 increased the
invading cell number of H920 and H1581 cells by ~2- and ~3-fold,
respectively (Figs. 2A and B).
Notably, this effect was abolished by treatment with selective FAK
inhibitor 14. In contrast, overexpression of MTSS1 decreased the
invading cell number by ~50% in SW900 cells, and treatment with
selective FAK inhibitor 14 enhanced this effect (Fig. 2C). Knockdown of MTSS1 decreased the
invading cell number by ~40 and 60% in H920 and H1581 cells,
respectively; whereas it increased the invading cell number of
SW900 cells ~2.5-fold (Fig. 2). The
results indicate that MTSS1 has differential effects on the
invasiveness of different subtypes of NSCLC.
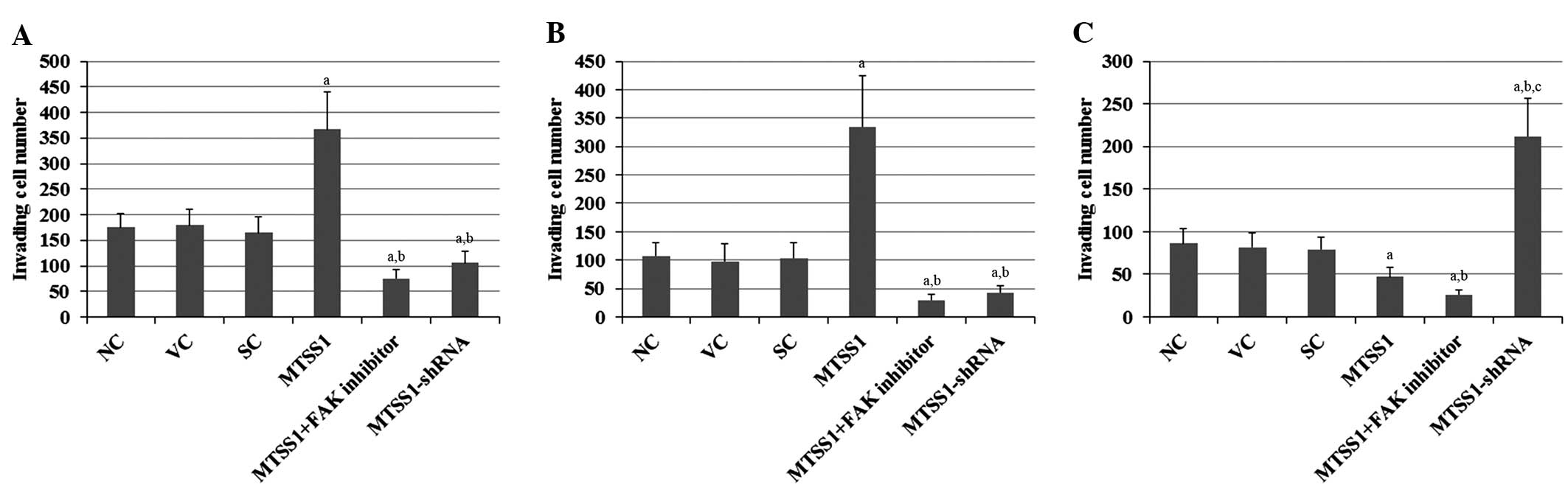 | Figure 2.Effects of MTSS1 on cell invasion in
various subtypes of NSCLC cells. In vitro cell invasion
assays were performed in (A) H920 lung adenocarcinoma, (B) H1581
large-cell carcinoma and (C) SW900 squamous cell carcinoma human
NSCLC cells. Following 24-h incubation, invading cell numbers were
determined by running a fluorescent cell dose curve in NC cells, VC
cells stably transfected with the empty pcDNA3.1 vector, SC cells
stably transduced with scramble control shRNA, cells stably
transfected with MTSS1, cells stably transfected with MTSS1 and
treated with 50 µM FAK inhibitor 14 for 24 h, and cells stably
transduced with MTSS1-shRNA. Data are expressed as the mean +
standard deviation from three independent experiments.
aP<0.05 vs. the control groups (NC, VC and SC);
bP<0.05 vs. the MTSS1 group; cP<0.05
vs. the MTSS1 + FAK inhibitor group. MTSS1, metastasis suppressor
1; NSCLC, non-small cell lung cancer; FAK, focal adhesion kinase;
NC, normal control; VC, vector control; SC, scramble control. |
MTSS1 exerts differential effects on
proliferation in subtypes of NSCLC
In order to determine the effects of MTSS1 on cell
proliferation in three subtypes of NSCLC cells, MTT in vitro
cell proliferation assays were performed in H920, H1581 and SW900
cells. Compared with the controls, overexpression of MTSS1
increased the proliferation of H920 and H1581 cells 2-fold, and
this effect was abolished by treatment with selective FAK inhibitor
14 (Figs. 3A and B). In contrast,
overexpression of MTSS1 decreased cell proliferation by ~50% in
SW900 cells, and this effect was enhanced by treatment with
selective FAK inhibitor 14 (Fig.
3C). Knockdown of MTSS1 decreased the proliferation of H920 and
H1581 cells by ~65%, whereas it increased the proliferation of
SW900 cells ~2-fold (Fig. 3). The
results indicate that MTSS1 has differential effects on the
proliferation of different subtypes of NSCLC.
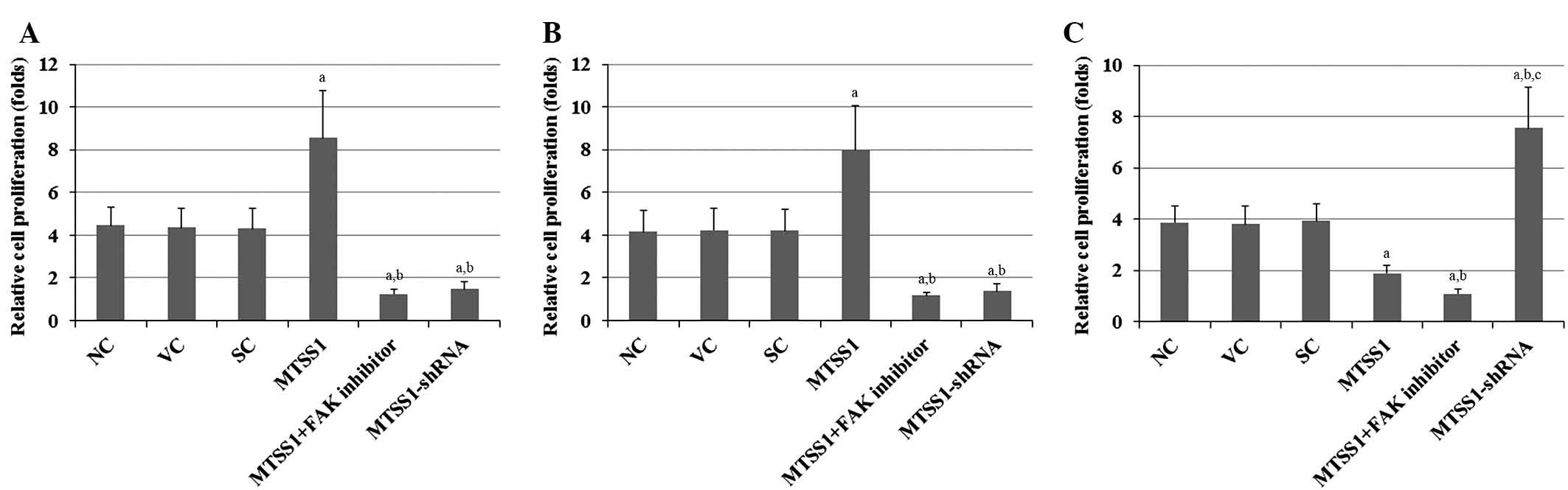 | Figure 3.Effects of MTSS1 on cell proliferation
in various subtypes of NSCLC cells, including (A) H920 lung
adenocarcinoma, (B) H1581 large-cell carcinoma and (C) SW900
squamous cell carcinoma human NSCLC cells. MTT cell proliferation
assays were performed for 48 h in NC cells, VC cells stably
transfected with the empty pcDNA3.1 vector, SC cells stably
transduced with scramble control shRNA, cells stably transfected
with MTSS1, cells stably transfected with MTSS1 and treated with 50
µM FAK inhibitor 14 for 48 h, and cells stably transduced with
MTSS1-shRNA. Cell proliferation at 48 h was expressed as fold
change relative to the NC group at 0 h (designated as 1). Data are
expressed as the mean + standard deviation from three independent
experiments. aP<0.05 vs. the control groups (NC, VC
and SC); bP<0.05 vs. the MTSS1 group;
cP<0.05 vs. the MTSS1 + FAK inhibitor group. MTSS1,
metastasis suppressor 1; NSCLC, non-small cell lung cancer; FAK,
focal adhesion kinase; NC, normal control; VC, vector control; SC,
scramble control. |
MTSS1 exerts differential effects on
the phosphorylation of FAK and FAK activity in subtypes of
NSCLC
As the findings of the present so far suggested that
MTSS1 promoted and inhibited cell invasion and proliferation in
three subtypes of human NSCLC cells through a FAK-dependent
mechanism, the effects of MTSS1 on the phosphorylation of FAK and
FAK activity in H920, H1581 and SW900 cells were investigated.
Since phosphorylation of FAK on tyrosine (Tyr) 576 and 577 within
the FAK catalytic domain is required for the full enzymatic
activity of FAK (18,19), Tyr576/577-phosphorylated FAK protein
levels were measured in the cells. Compared with the controls,
overexpression and knockdown of MTSS1 showed no significant effects
on total FAK protein levels in all three cell lines (Fig. 4). In contrast, overexpression of
MTSS1 increased FAK phosphorylation at Tyr576/577 by ~3-fold in
H920 and H1581 cells (Figs. 4A and
B), whereas MTSS1 overexpression decreased
Tyr576/577-phosphorylated FAK protein levels by ~50% in SW900 cells
(Fig. 4C). Knockdown of MTSS1
decreased Tyr576/577 phosphorylation in FAK by ~60 and 65% in H920
and H1581 cells, respectively (Figs 5A
and B), whereas MTSS1 knockdown increased Tyr576/577
phosphorylation in FAK by ~3-fold in SW900 cells (Fig. 4C). Similar trends in FAK activity
were observed in the cell lines (Fig.
5). The results indicate that MTSS1 has differential effects on
FAK activity in different subtypes of NSCLC.
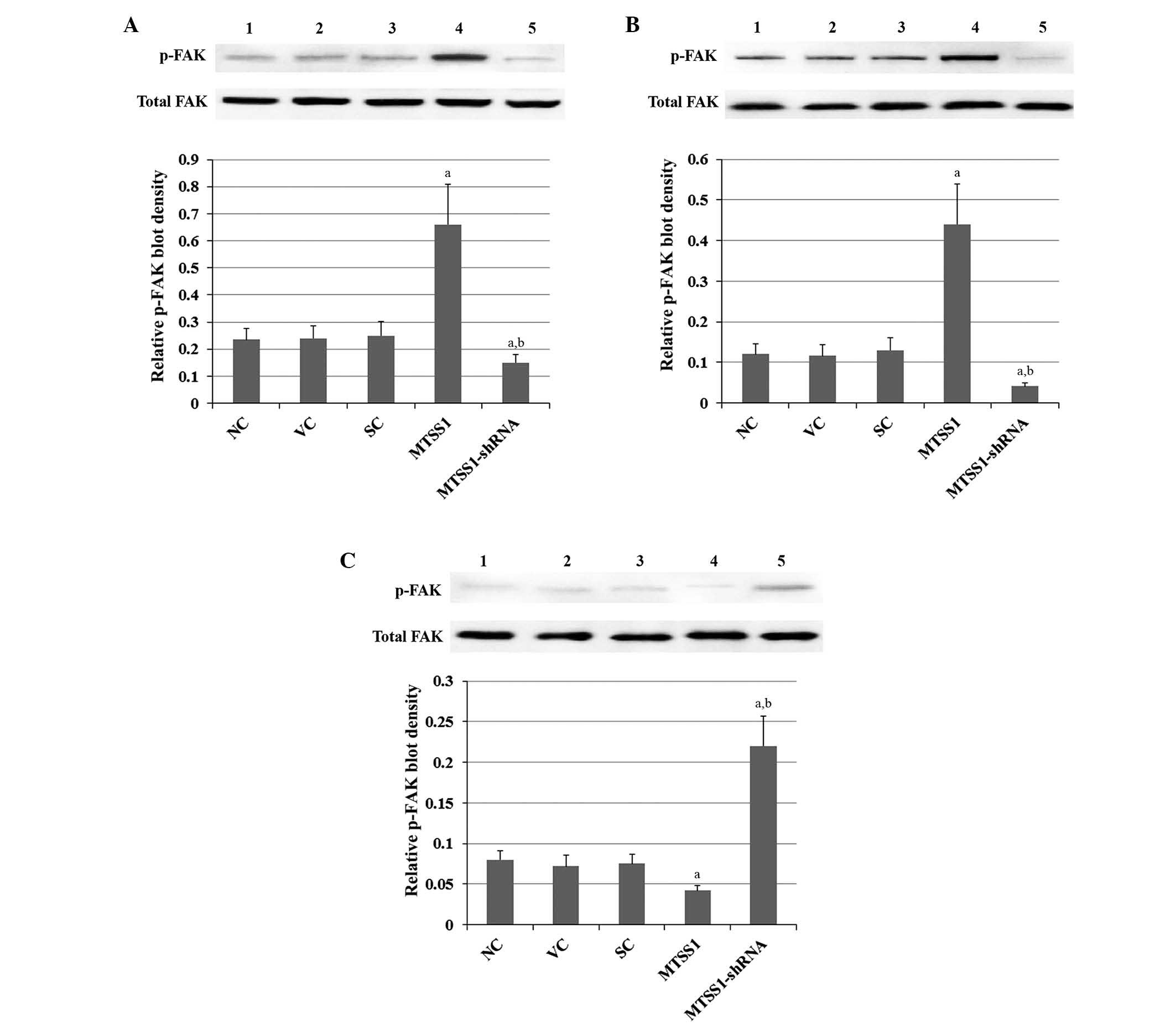 | Figure 4.Effects of MTSS1 on the tyrosine
576/577 phosphorylation of FAK in various subtypes of NSCLC cells,
including (A) H920 lung adenocarcinoma, (B) H1581 large-cell
carcinoma and (C) SW900 squamous cell carcinoma human NSCLC cells.
Levels of total FAK and p-FAK were determined by western blot
analyses in NC cells (lane 1), VC cells stably transfected
with the empty pcDNA3.1 vector (lane 2), SC cells stably
transduced with scramble control shRNA (lane 3), cells
stably transfected with MTSS1 (lane 4) and cells stably
transduced with MTSS1-shRNA (lane 5). p-FAK blot density was
normalized against total FAK to obtain a relative p-FAK blot
density. Data are expressed as the mean + standard deviation from
three independent experiments. aP<0.05 vs. the
control groups (NC, VC and SC); bP<0.05 vs. the MTSS1
group. MTSS1, metastasis suppressor 1; NSCLC, non-small cell lung
cancer; FAK, focal adhesion kinase; p-, phosphorylated; NC, normal
control; VC, vector control; SC, scramble control. |
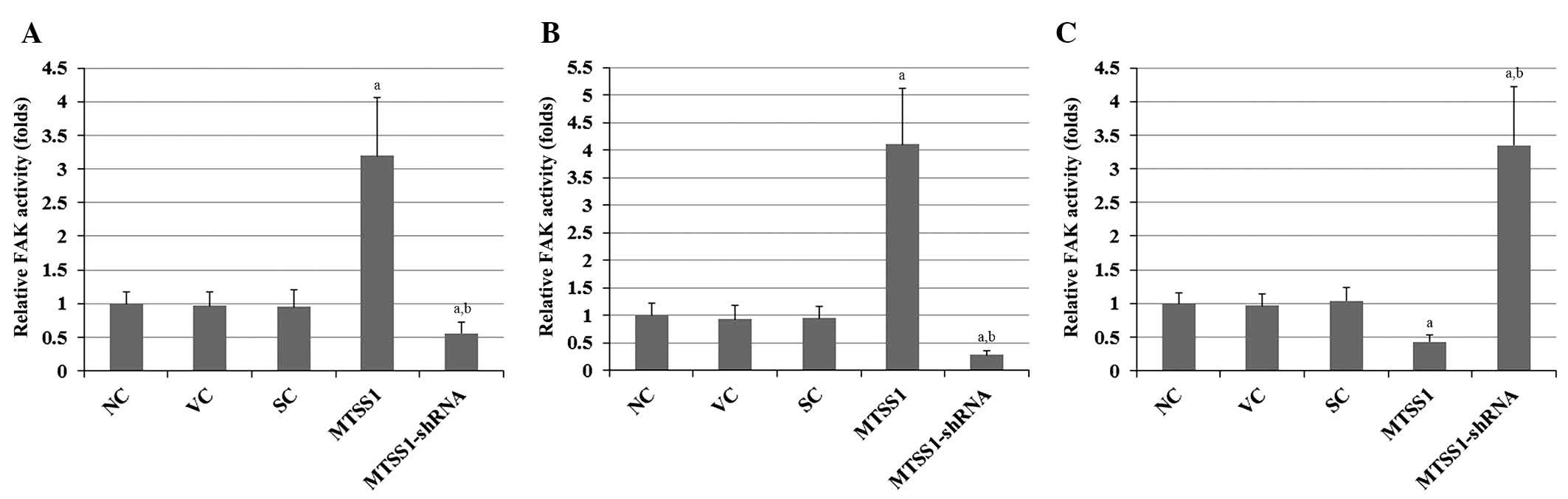 | Figure 5.Effect of MTSS1 on FAK activity in
various subtypes of NSCLC cells, including (A) H920 lung
adenocarcinoma, (B) H1581 large-cell carcinoma and (C) SW900
squamous cell carcinoma human NSCLC cells. FAK activity was
determined using a Universal Tyrosine Kinase Assay kit in NC cells,
VC cells stably transfected with the empty pcDNA3.1 vector, SC
cells stably transduced with scramble control shRNA, cells stably
transfected with MTSS1, and cells stably transduced with
MTSS1-shRNA. FAK activity is presented as fold change, relative to
the NC group (designated as 1). Data are expressed as the mean +
standard deviation from three independent experiments.
aP<0.05 vs.the control groups (NC, VC and SC);
bP<0.05 vs. the MTSS1 group. MTSS1, metastasis
suppressor 1; NSCLC, non-small cell lung cancer; FAK, focal
adhesion kinase; NC, normal control; VC, vector control; SC,
scramble control. |
Discussion
Decreased expression of MTSS1, which is a newly
discovered protein involved in tumor progression and metastasis
(3), has been associated with poor
survival rate in patients with aggressive forms of breast,
colorectal and prostate cancer (12–14).
Conversely, elevated expression of MTSS1 has been associated with
enhanced invasion and proliferation in melanoma cells and
characterizes a subgroup of primary melanomas with unfavorable
prognosis (15). Furthermore, a
recent study has demonstrated that MTSS1 is overexpressed in NSCLC
and may be an independent prognostic factor for patients with SCC
(3). To the best of our knowledge
the present study was the first to outline the differential role of
MTSS1 in invasion and proliferation in three subtypes of NSCLC.
NSCLC consists of LAC, LCC and SCC (3). In the present study, H920 (LAC), H1581
(LCC) and SW900 (SCC) cell lines were used as representative cell
models of the subtypes of NSCLC. Since all three cell lines were
derived from stage-4 NSCLC, they possess similar pathological
characteristics. Stable overexpression and knockdown of MTSS1 was
performed in the cell lines in order to explore the functional
roles of MTSS1 in NSCLC cell invasion and proliferation. Notably,
the results of the present study demonstrated that, while MTSS1
overexpression inhibited invasion and proliferation in SCC cells,
it enhanced invasion and proliferation in LAC and LCC cells. When
together with previous reports that MTSS1 respectively enhances and
inhibits tumor cell invasion and proliferation in different types
of cancer (12–15), these findings suggest that MTSS1 has
a dual functional role of oncogene and tumor suppressor in cancer,
depending on tissue/cell specificity. Previous studies have
described various similar proteins with dual roles in cancer
malignancy (19,20). For example, while Annexin A5
reportedly promotes tumorigenesis and progression in
hepatocarcinoma, breast cancer, colorectal cancer, pancreatic
cancer, bladder cancer and prostate cancer (19), it has also been demonstrated to
inhibit the malignancy of thyroid cancer (19). Moreover, high expression of TWIST,
which is a transcription factor belonging to the basic
helix-loop-helix family of proteins (20), has been associated with the initial
phase of metastatic progression in gastric and breast cancers, and
its overexpression is correlated with disease progression and a
poor clinical outcome in patients with osteosarcoma (20). As MTSS1 is associated with the
invasion and proliferation of various types of cancers and it has
been suggested that MTSS1 may serve as a prognostic indicator
and/or therapeutic target for cancer (12–15,21),
identification of the differential roles of MTSS1 in the invasion
and proliferation of various subtypes of NSCLC may provide novel
insights into the functional role of MTSS1 in cancer.
As a scaffold protein that interacts with numerous
molecules to regulate actin dynamics, MTSS1 has an important role
in carcinogenesis and metastasis (7), and is also associated with the Shh
signaling pathway (10). In the
present study, the effects of MTSS1 on the invasion and
proliferation of NSCLC cells were investigated in the presence of a
selective FAK inhibitor, which did not affect the expression of
MTSS1. The results of the present study suggested that MTSS1 may
regulate NSCLC cell invasion and proliferation through a
FAK-dependent mechanism, regardless of whether a stimulatory or
inhibitory effect is evoked. It is well-established that focal
adhesion complexes are capable of controlling cell movement by
associating with the actin cytoskeleton (22); therefore, dynamic regulation of focal
adhesion complexes and reorganization of the associated actin
cytoskeleton are crucial determinants of tumor cell invasion
(22). As a critical factor involved
in the mechanism of focal adhesion, FAK may function as a major
downstream mediator of the regulatory effects of MTSS1 on actin
dynamics. The results of the present study demonstrated that, while
MTSS1 overexpression induced FAK phosphorylation/activity in LAC
and LCC cells, it inhibited the same processes in SCC cells.
Therefore, the mechanism by which MTSS1 exerts differential effects
on the FAK phosphorylation/activity in different subtypes of NSCLC
may be dependent on specific intracellular partners that interact
with MTSS1 in specific cancer cell types. Future studies are
required in order to elucidate these underlying mechanisms.
In conclusion, the present study demonstrated that
MTSS1 has differential roles in various subtypes of NSCLC. Acting
via a FAK-dependent mechanism, MTSS1 enhances the invasion and
proliferation of LAC and LCC cells, whereas it inhibits the
invasion and proliferation of SCC cells. These findings provide
novel insight into the functional role of MTSS1 in cancer and may
help elucidate therapeutic strategies for the treatment of various
types of cancer.
References
|
1
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kayser G, Csanadi A, Kakanou S, Prasse A,
Kassem A, Stickeler E, Passlick B and Zur Hausen A: Downregulation
of MTSS1 expression is an independent prognosticator in squamous
cell carcinoma of the lung. Br J Cancer. 112:866–873. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters S, Adjei AA, Gridelli C, Reck M,
Kerr K and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23(Suppl 7): vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Zhang Y, Zhang Y, Bai X, Peng Y and
He P: TRIM31 is downregulated in non-small cell lung cancer and
serves as a potential tumor suppressor. Tumour Biol. 35:5747–5752.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee Y, Macoskay JA, Korenchukx S and
Pientay KJ: MIM, a potential metastasis suppressor gene in bladder
cancer. Neoplasia. 4:291–294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie F, Ye L, Ta M, Zhang L and Jiang WG:
MTSS1: A multifunctional protein and its role in cancer invasion
and metastasis. Front Biosci (Schol Ed). 3:621–631. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mattila PK, Salminen M, Yamashiro T and
Lappalainen P: Mouse MIM, a tissue-specific regulator of
cytoskeletal dynamics, interacts with ATP-actin monomers through
its C-terminal WH2 domain. J Biol Chem. 278:8452–8459. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattila PK, Pykäläinen A, Saarikangas J,
Paavilainen VO, Vihinen H, Jokitalo E and Lappalainen P:
Missing-in-metastasis and IRSp53 deform PI (4,5) P2-rich membranes
by an inverse BAR domain-like mechanism. J Cell Biol. 176:953–964.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Callahan CA, Ofstad T, Horng L, Wang JK,
Zhen HH, Coulombe PA and Oro AE: MIM/BEG4, a Sonic
hedgehog-responsive gene that potentiates Gli-dependent
transcription. Genes Dev. 18:2724–2729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma S, Guan X, Lee TK and Chan KW:
Clinicopathological significance of missing in metastasis B
expression in hepatocellular carcinoma. Hum Pathol. 38:1201–1206.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lei R, Tang J, Zhuang X, Deng R, Li G, Yu
J, Liang Y, Xiao J, Wang HY, Yang Q and Hu G: Suppression of MIM by
microRNA-182 activates RhoA and promotes breast cancer metastasis.
Oncogene. 33:1287–1296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Li X, Liu F, Xiao Z, He M, Shen S
and Liu S: MiR-135a promotes growth and invasion of colorectal
cancer via metastasis suppressor 1 in vitro. Acta Biochim Biophys
Sin (Shanghai). 44:838–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mustafa N, Martin TA and Jiang WG:
Metastasis tumour suppressor-1 and the aggressiveness of prostate
cancer cells. Exp Ther Med. 2:157–162. 2011.PubMed/NCBI
|
|
15
|
Mertz KD, Pathria G, Wagner C, Saarikangas
J, Sboner A, Romanov J, Gschaider M, Lenz F, Neumann F, Schreiner
W, et al: MTSS1 is a metastasis driver in a subset of human
melanomas. Nat Commun. 5:34652014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang B, Feng P, Xiao Z and Ren EC: LIM and
SH3 protein 1 (Lasp1) is a novel p53 transcriptional target
involved in hepatocellular carcinoma. J Hepatol. 50:528–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang
S, Wang W, Zhang J and Zhang Y: RNAi-mediated silencing of VEGF-C
inhibits non-small cell lung cancer progression by simultaneously
down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent
axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer.
47:2353–2363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer-a new therapeutic opportunity. Nat Rev Cancer. 5:505–515.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng B, Guo C, Guan H, Liu S and Sun MZ:
Annexin A5 as a potential marker in tumors. Clin Chim Acta.
427:42–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Huang Z, Wu S, Zang X, Liu M and
Shi J: miR-33a is up-regulated in chemoresistant osteosarcoma and
promotes osteosarcoma cell resistance to cisplatin by
down-regulating TWIST. J Exp Clin Cancer Res. 33:122014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie F, Ye L, Chen J, Wu N, Zhang Z, Yang
Y, Zhang L and Jiang WG: The impact of Metastasis Suppressor-1,
MTSS1, on oesophageal squamous cell carcinoma and its clinical
significance. J Transl Med. 9:952011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carragher NO and Frame MC: Focal adhesion
and actin dynamics: A place where kinases and proteases meet to
promote invasion. Trends Cell Biol. 14:241–249. 2004. View Article : Google Scholar : PubMed/NCBI
|



















