Introduction
Mycoplasmas belong to the class Mollicutes
which also contains Ureaplasmas, Acholeplasmas, Spiroplasmas
and the newly classified Haemoplasmas (1). Mycoplasmas are characterized by
a small size, lack of cell wall, extremely fastidious in
vitro environmental requirements and a tendency to form
centered colonies on solid media (2).
β-lactam antibiotics and vancomycin are inactive
against Mycoplasmas, as their target is the cell wall
(3). Mycoplasma and
Ureaplasma spp. are currently susceptible to agents that
interfere with protein synthesis, including tetracycline,
macrolides, aminoglycosides and chloramphenicol, and the
fluoroquinolones that inhibit topoisomerases (3,4).
However, resistance to these agents is increasing, as a consequence
of the spread of the tetM gene which confers resistance to
tetracycline (5–7). Clindamycin, fluoroquinolones or other
macrolides may be used following the failure of therapy with
tetracycline or erythromycin (8).
The extent of bacterial resistance varies
geographically, depending on the use of different antibiotics and
the history of previous antimicrobial exposure among various
populations (9). To date, with
regard to the rising infertility in China, an elevated incidence of
genital Mycoplasma infection in female outpatients was
identified by Wang et al (10); however, there is currently no data
regarding the infection prevalence of genital Mycoplasmas in
the male partners of infertile couples. For these reasons, it is
important to implement surveillance studies on the prevalence and
antimicrobial susceptibilities of these species in the Chinese male
population. The purposes of this study were as follows: i) Analyze
the prevalence of urogenital mycoplasma; ii) investigate the
susceptibilities of a large number of clinical isolates of M.
hominis and U. urealyticum to various antibiotics; and
iii) compare changes in the antibiotic susceptibilities of these
microorganisms between 2009 and 2012.
Materials and methods
Patients
Between January 1st, 2009 and December 31st, 2012, a
total of 7,374 male outpatients with suspected reproductive
disorders were enrolled from the Affiliated Hospital of Nanjing
University of Chinese Medicine (Nanjing, China), and underwent an
inspection of the reproductive system. The patients ages ranged
between 18 and 47 years. The present study was approved by the
ethical committee of the Nanjing University of Chinese
Medicine.
Specimens, culture and antimicrobial
susceptibility testing
All semen samples were obtained by masturbation and
inoculated into the Mycoplasma Susceptibility kit (cat. no.
20140317119877; Autobio Diagnostics Co., Ltd., Zhengzhou, China)
within 1 h, according to the manufacturer's guidelines. The
microbiological principle used by the Mycoplasma
identification verification and antibiotic susceptibility testing
kits was as follows: During growth, U. urealyticum and M.
hominis metabolize urea and arginine, respectively, which
changes the color of the culture medium (e.g., from yellow to red).
Susceptibility results were obtained at two concentrations for 12
antibiotics: Erythromycin, roxithromycin, josamycin, tetracycline,
doxycycline, minocycline, levofloxacin, ofloxacin, azithromycin,
clarithromycin, ciprofloxacin and sparfloxacin (Thermo Fisher
Scientific Oxoid, Ltd., Basingstoke, UK). The susceptibility of the
bacteria to each antibiotic was graded as either ‘susceptible’,
‘intermediate’ or ‘resistant’ (11).
Bacterial growth was evaluated following a two-day incubation
period at 37°C. The results were interpreted as follows: Negative
result was clear and a color change of >104 units was
considered to indicate infection. Clinical and Laboratory Standards
Institute guidelines were used to categorize the results for
bacterial susceptibility or resistance to antibiotics (12).
Statistical analysis
SPSS software, version 12.0 (SPSS, Inc., Chicago,
IL, USA) was used to conduct the data analysis. The χ2
test was used to compare the occurrence of strains susceptible or
resistant to different antibiotics. P<0.05 was considered to
indicate a statistically significant difference.
Results
Prevalence of U. urealyticum and M.
hominis
Among the 7,374 specimens tested, 3,225 (43.7%) were
positive for genital Mycoplasmas. Of these, 3,122 specimens
were positive for U. urealyticum (42.3%), 29 were positive
for M. hominis (0.4%) and 74 were positive for both (1.0%)
(Fig. 1). The distribution of M.
hominis and U. urealyticum according to age group is
shown in Table I. The prevalence
rates of U. urealyticum and of both Mycoplasmas were highest
in patients aged 25–34 years, whereas M. hominis occurred
predominantly in patients between the ages of 25 and 29 years.
 | Table I.Distribution of Ureaplasma
urealyticum and Mycoplasma hominis (single- and
co-infection) among male patients in different age groups during
the study period. |
Table I.
Distribution of Ureaplasma
urealyticum and Mycoplasma hominis (single- and
co-infection) among male patients in different age groups during
the study period.
| Age group
(years) | M.
hominis | U.
urealyticum | M. hominis +
U. urealyticum | Total |
|---|
| 18–24 | 2 (0.0) | 445
(14.1) | 8
(10.8) | 453
(100) |
| 25–29 | 17
(100.0) | 1,047 (33.3) | 23
(31.1) | 1,073 (100) |
| 30–34 | 9 (0.0) | 1,128 (35.8) | 31
(41.9) | 1,159 (100) |
| 35–39 | 1 (0.0) | 476
(15.1) | 7
(9.5) | 483
(100) |
| ≥40 | 0 (0.0) | 52
(1.7) | 5
(6.8) | 57
(100) |
| Total | 29
(100.0) | 3,148 (100) | 74 (100) | 3,225 (100) |
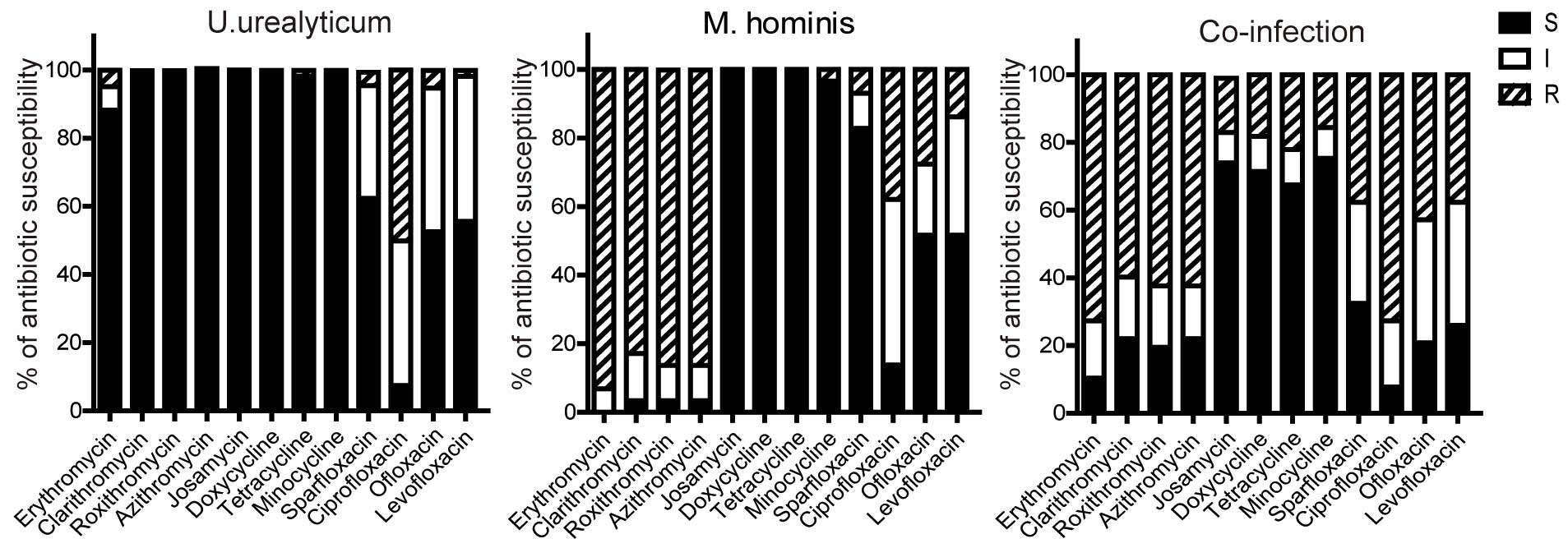
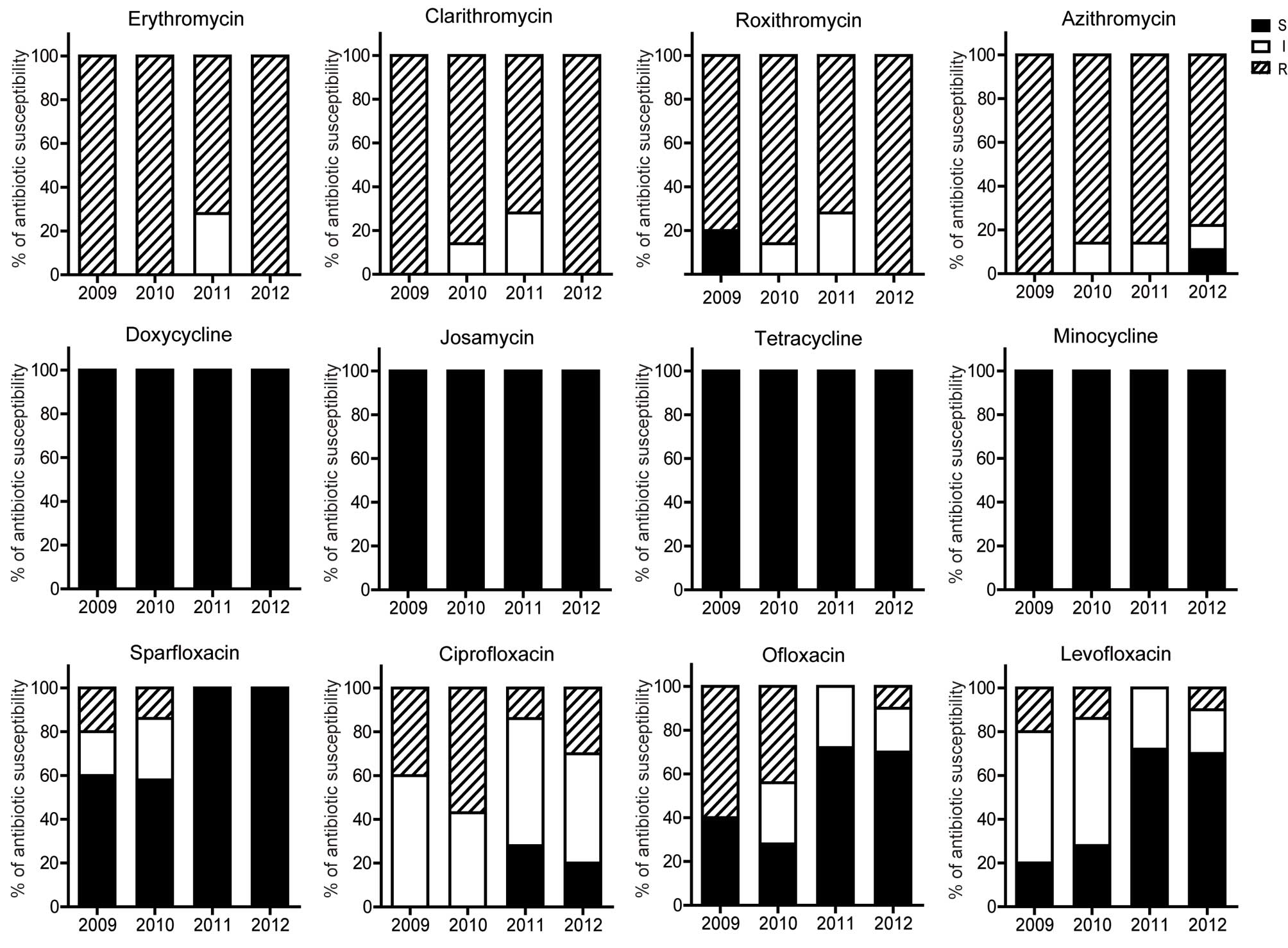
Antimicrobial susceptibility patterns
over the test period
The antimicrobial susceptibilities of M.
hominis and U. urealyticum are shown in Fig. 2. Tetracyclines (tetracycline,
minocycline, doxycycline) were the most active agents against both
genital Mycoplasmas even in the case of co-infection.
Macrolide (erythromycin, roxithromycin, azithromycin,
clarithromycin and josamycin) remained effective against the
majority of U. urealyticum clinical isolates. However,
macrolides, with the exception of josamycin, were naturally
resisted by M. hominis. Likewise, in the persons infected
with both genital Mycoplasmas, the antibacterial activity of
macrolides except for josamycin were not significant. Josamycin was
the only macrolide observed to be effective against M.
hominis and co-infection. Fluoroquinolones (ciprofloxacin) had
the lowest activity against U. urealyticum, particularly in
patients with M. hominis co-infection. The susceptibility
profiles against both Mycoplasmas over the test period did
not change significantly, despite the efficacy of ciprofloxacin to
U. urealyticum becoming increasingly diminished (Figs. 3 and 4).
Multi-drug resistant (MDR) bacteria have been
identified in numerous cases and MDR Mycoplasmas are defined
as those strains resistant to at least one agent in ≥3
antimicrobial categories (13). In
the present study, the incidence of MDR single U.
urealyticum infection was significantly reduced, as compared
with that of co-infection (1.09 vs. 33.78%; P<0.05), indicating
the presence of cross-resistance in the co-infection patients
(Fig. 5).
Discussion
Worsening environmental contamination and the
increasing incidence of sexually transmitted disease, which may
lead to infertility, have meant that an increasing number of
couples will not have their first baby without the aid of assisted
reproduction (14). Infertility is
emerging as a serious public health issue in China (15). As well as physical and chemical
factors, sexually-transmitted infections must also be taken into
consideration (16).
Mycoplasmas are among the smallest free-living
microorganisms. They are commonly isolated from the genitourinary
tract of symptomatic patients, but may be found as commensal
bacteria from asymptomatic patients. It has been reported that
infection with genital Mycoplasmas may lead to pelvic
inflammatory disease, puerperal infections, septic abortions, low
birth weight, nongonococcal urethritis, prostatitis as well as
spontaneous abortion and infertility (17–19). To
date, the effect of these microorganisms on male infertility
remains unclear, with the exception of limited studies in a few
countries (20,21). The present study described the
prevalence and antimicrobial susceptibility of U.
urealyticum and M. hominis isolated from semen samples
from Chinese patients, and may aid understanding and optimal
clinical treatment choice for these pathogens.
This study evaluated differences in the prevalence
and antibiotic resistance of U. urealyticum and M.
hominis. The overall prevalence of infection with either type
of bacteria was 43.7%. U. urealyticum was frequently
detected as a single pathogen (42.3%), and was thus significantly
associated with symptomatic patients, including loss of sperm,
which indicated its potential pathogenicity. By contrast, a single
infection of M. hominis (0.4%) was rarely found, indicative
of disproportionate incidence of these two Mycoplasmas. The
present findings are consistent with those of other studies
performed in Poland (22) and Korea
(23); however, they are distinctly
different to those of previous studies conducted in Jordan
(24) and Italy (25). This discrepancy may be a result of
variations in socioeconomic conditions and living standards. In the
present study, simultaneous colonization with M. hominis and
U. urealyticum was not common (1.0%).
In this study, the most frequent occurrence rate of
genital Mycoplasma infection was detected in patients aged
between 25 and 34 years old; the age range at which new couples
typically conceive their baby. With the exception of the age
distribution of the outpatients, no other demographic or clinical
characteristics were examined in this study. Other characteristics
should be examined in future follow-up studies.
Mycoplasmas are normally susceptible to
antibiotics that inhibit protein synthesis, but are resistant to
antibiotics that act on bacterial cell wall components because
Mycoplasmas do not possess a cell wall (26). The results of this study indicated
that there was a difference in sensitivity to the 12 antibiotics
between the isolates from single infections and co-infections.
Three tetracycline antibiotics (tetracycline, doxycycline and
minocycline) and one macrolide antibiotic (josamycin) were active
against the majority of the strains. However, four of the quinolone
antibiotics (sparfloxacin, levofloxacin, ciprofloxacin and
ofloxacin) were inactive against more than one-third of the strains
isolated in this study, particularly against the co-infection
isolates. Four macrolide antibiotics (azithromycin, erythromycin,
clarithromycin and roxithromycin) were effective against the
majority of U. urealyticum isolates, but were inactive
against the majority of bacteria isolates of the single M.
hominis and co-infections. Consistent with that of female M.
hominis isolates (10,11), semen isolated M. hominis in
this study displayed similar resistance spectrum to erythromycin,
azythromycin, roxythromycin and clarithromycin. However, a small
number of single M. hominis-positive isolates were
calculated in the study; thus, further studies including a greater
sample size of single M. hominis should be conducted.
Although, simultaneous infection with U. urealyticum and
M. hominis, was not often, but leaded an elevated MDR
compared with that of single M. hominis infection,
indicating a crucial role of cross resistance of these microbes
with distinct drug resistant spectrum. The present results support
the use of tetracycline, doxycycline, minocycline and josamycin as
first choice drugs when empirical therapy is required, whereas the
use of erythromycin and quinolones must be carefully considered.
The prevalence of the U. urealyticum and M. hominis
antibiotic resistance profiles in our study were similar to that of
female originated Mycoplasmas reported by Wang et al
(10) in China, indicating a
possible transmission of these Mycoplasmas between men and
women. Additionally, no significant difference in antibacterial
activity was observed over the study period, with the exception of
the reduced activity of ciprofloxacin, which may be attributed to
the excessive usage of the drug. Thus, differences in antimicrobial
use policies of various areas may also influence the antimicrobial
susceptibility characteristics.
In conclusion, the present study retrospectively
analyzed the prevalence and antibiotic susceptibility of U.
urealyticum and M. hominis in semen samples from the
Chinese population, in order to provide clinicians with an
evidential basis for the rational use of antibiotics, which may be
useful for avoiding treatment failure and the abuse of
antimicrobial agents.
Acknowledgements
This study was supported by Research Fund of Jiangsu
Provincial Hospital of Traditional Chinese Medicine (grant no.
Y14011).
References
|
1
|
Horner P, Blee K and Adams E: Time to
manage Mycoplasma genitalium as an STI: But not with
azithromycin 1 g! Curr Opin Infect Dis. 27:68–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Citti C and Blanchard A:
Mycoplasmas and their host: Emerging and re-emerging minimal
pathogens. Trends Microbiol. 21:196–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waites KB, Katz B and Schelonka RL:
Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol
Rev. 18:757–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCormack WM: Susceptibility of
mycoplasmas to antimicrobial agents: Clinical implications. Clin
Infect Dis. 17(Suppl 1): S200–S201. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furneri PM, Rappazzo G, Musumarra MP, Di
Pietro P, Catania LS and Roccasalva LS: Two new point mutations at
A2062 associated with resistance to 16-membered macrolide
antibiotics in mutant strains of Mycoplasma hominis.
Antimicrob Agents Chemother. 45:2958–2960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dégrange S, Renaudin H, Charron A, Bébéar
C and Bébéar CM: Tetracycline resistance in Ureaplasma spp.
and Mycoplasma hominis: Prevalence in Bordeaux, France, from
1999 to 2002 and description of two tet (M)-positive isolates of
M. hominis susceptible to tetracyclines. Antimicrob Agents
Chemother. 52:742–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereyre S, Renaudin H, Charron A, Bébéar C
and Bébéar CM: Emergence of a 23S rRNA mutation in Mycoplasma
hominis associated with a loss of the intrinsic resistance to
erythromycin and azithromycin. J Antimicrob Chemother. 57:753–756.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mardassi BB, Aissani N, Moalla I, Dhahri
D, Dridi A and Mlik B: Evidence for the predominance of a single
tet(M) gene sequence type in tetracycline-resistant Ureaplasma
parvum and Mycoplasma hominis isolates from Tunisian
patients. J Med Microbiol. 61:1254–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waites KB, Crabb DM, Bing X and Duffy LB:
In vitro susceptibilities to and bactericidal activities of
garenoxacin (BMS-284756) and other antimicrobial agents against
human Mycoplasmas and Ureaplasmas. Antimicrob Agents
Chemother. 47:161–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang QY, Li RH, Zheng LQ and Shang XH:
Prevalence and antimicrobial susceptibility of Ureaplasma
urealyticum and Mycoplasma hominis in female
outpatients, 2009–2013. J Microbiol Immunol Infect. 2014.[Epub
ahead of print].
|
|
11
|
De Francesco MA, Caracciolo S, Bonfanti C
and Manca N: Incidence and antibiotic susceptibility of
Mycoplasma hominis and Ureaplasma urealyticum
isolated in Brescia, Italy, over 7 years. J Infect Chemother.
19:621–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clinical and Laboratory Standards
Institute (CLSI): Methods for antimicrobial susceptibility testing
for human mycoplasmas; Approved guideline. CLSI Document M43-A.
CLSI. (Wayne, PA). 2–5. 2011.
|
|
13
|
Magiorakos AP, Srinivasan A, Carey RB,
Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter
G, Olsson-Liljequist B, et al: Multidrug-resistant, extensively
drug-resistant and pandrug-resistant bacteria: An international
expert proposal for interim standard definitions for acquired
resistance. Clin Microbiol Infect. 18:268–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YX, You L, Zeng Q, Sun Y, Huang YH,
Wang C, Wang P, Cao WC, Yang P, Li YF and Lu WQ: Phthalate exposure
and human semen quality: Results from an infertility clinic in
China. Environ Res. 142:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu W, Chen M, Wu W, Lu J, Zhao D, Pan F,
Lu C, Xia Y, Hu L, Chen D, et al: Gene-gene and gene-environment
interactions on risk of male infertility: Focus on the metabolites.
Environ Int. 91:188–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gimenes F, Souza RP, Bento JC, Teixeira
JJ, Maria-Engler SS, Bonini MG and Consolaro ME: Male infertility:
A public health issue caused by sexually transmitted pathogens. Nat
Rev Urol. 11:672–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crăcea E, Măicănescu-Georgescu M,
Constantinescu S and Botez D: Genital Mycoplasmas and
Chlamydiae in male infertility. Arch Roum Pathol Exp
Microbiol. 41:219–224. 1982.PubMed/NCBI
|
|
18
|
de Barbeyrac B, Bernet-Poggi C, Fébrer F,
Renaudin H, Dupon M and Bébéar C: Detection of Mycoplasma
pneumoniae and Mycoplasma genitalium in clinical samples
by polymerase chain reaction. Clin Infect Dis. 17(Suppl 1):
S83–S89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oriel JD: Role of genital mycoplasmas in
nongonococcal urethritis and prostatitis. Sex Transm Dis. 10(Suppl
4): S263–S270. 1983.
|
|
20
|
Al-Sweih NA, Al-Fadli AH, Omu AE and
Rotimi VO: Prevalence of Chlamydia trachomatis,
Mycoplasma hominis, Mycoplasma genitalium and
Ureaplasma urealyticum infections and seminal quality in
infertile and fertile men in Kuwait. J Androl. 33:1323–1329. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gdoura R, Kchaou W, Chaari C, Znazen A,
Keskes L, Rebai T and Hammami A: Ureaplasma urealyticum,
Ureaplasma parvum, Mycoplasma hominis and
Mycoplasma genitalium infections and semen quality of
infertile men. BMC Infect Dis. 7:1292007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Filipiak E, Marchlewska K, Oszukowska E,
Walczak-Jedrzejowska R, Swierczynska-Cieplucha A, Kula K and
Slowikowska-Hilczer J: Presence of aerobic micro-organisms and
their influence on basic semen parameters in infertile men.
Andrologia. 47:826–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JS, Kim KT, Lee HS, Yang KM, Seo JT
and Choe JH: Concordance of Ureaplasma urealyticum and
Mycoplasma hominis in infertile couples: Impact on semen
parameters. Urology. 81:1219–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abusarah EA, Awwad ZM, Charvalos E and
Shehabi AA: Molecular detection of potential sexually transmitted
pathogens in semen and urine specimens of infertile and fertile
males. Diagn Microbiol Infect Dis. 77:283–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salmeri M, Valenti D, La Vignera S,
Bellanca S, Morello A, Toscano MA, Mastrojeni S and Calogero AE:
Prevalence of Ureaplasma urealyticum and Mycoplasma
hominis infection in unselected infertile men. J Chemother.
24:81–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taylor-Robinson D and Bébéar C: Antibiotic
susceptibilities of Mycoplasmas and treatment of mycoplasmal
infections. J Antimicrob Chemother. 40:622–630. 1997. View Article : Google Scholar : PubMed/NCBI
|