Introduction
Hypoxic-ischemic brain damage (HIBD) in neonates
involves the cerebral injury of fetus and neonates caused by
partial or complete hypoxia, cerebral blood flow reduction or
pause, which is caused by perinatal asphyxia (1). HIBD often occurs within 24 h after
birth, and the occurrence rate of hypoxic ischemic encephalopathy
(HIE) in neonates is 3–6‰ of live births. There are different types
and different degrees of long-term sequelae in 25–30% of live
births, which is the important disease that affects the life
quality of children (2). The
diagnosis is based on consciousness, muscular tension, change of
primitive reflex, convulsion, disease course and prognosis. HIBD is
divided into mild, moderate and severe degrees, however, laboratory
tests for diagnosis are currently insufficient.
Hypoxia ischemia can activate a series of biological
chain reactions including affecting the oxidative phosphorylation
of glucose, rapid depletion of ATP in cells, accumulation of lactic
acid, depolarization of cell membrane, release of excitatory amino
acid and accumulation of Na+, Ca2+,
H2O, free radical and free fatty acid (FFA) in cells, to
cause cellular swelling and cell death (3). Hypoxia ischemia can decrease the
synthesis of protein and adipose and increase their metabolic
products such as urea nitrogen, creatinine, uric acid and FFA.
Adipocyte fatty acid-binding protein (A-FABP) is a member of the
fatty acid binding protein family. It is expressed in adipocytes
and macrophages, as well as endothelial cells and blood. As a
molecular chaperone of fatty acid, A-FABP is considered a thrifty
gene that can promote the transfer of fatty acid and energy
storage. When the body experiences stress such as starvation, the
preserved energy is released for body utilization. A-FABP is an
important regulatory factor of obesity, insulin resistance and
metabolic syndrome and the expression of A-FABP can be induced by
proliferator-activated receptor γ (PPARγ) agonist, insulin and
fatty acid (4). However, the role
and significance of A-FABP in HIBD have yet to be studied
universally. As the hypoxia ischemia lengthens, the level of FFA
increases in neonates with HIBD, and it is suggested that increased
FFA can induce the expression of A-FABP to cause an increase of
serum A-FABP.
In the present study, serum levels of A-FABP and FFA
in 42 neonates with acute phase and recovery phase HIBD were
detected, respectively, using the enzyme-linked immunosorbent assay
(ELISA) and copper colorimetric method. The relationship between
A-FABP, FFA level and severity of HIBD was analyzed, and the change
of the above indicators in the same neonate at the acute and
recovery phase was observed. The aim was to explore the
relationship between A-FABP, FFA and severity of HIBD with acute
phase and provide a diagnostic basis for diagnosis and prognosis of
HIBD.
Patients and methods
Patients
In total 42 full-term neonates with HIBD admitted
between January 2009 and January 2010 at the Department of
Pediatric, Fourth Hospital of Hebei Medical University (Hebei,
China) and Department of Pediatric, Cangzhou Central Hospital
(Cangzhou, China) were selected. There were 31 male and 11 female
cases, 17 cases of eutocia and 25 cases of cesarean delivery. The
average gestational age was 38.8±1.33 weeks, and the average weight
was 3.15±0.51 kg at birth.
Criteria of diagnosis and clinical
grading
The criteria of diagnosis and clinical stage used
were as reported earlier (5). The
neonate based on the following criteria were diagnosed as HIE: i)
There was confirmed abnormal obstetric history causing fetal
distress in the uterus, and severe fetal distress manifestation
(fetal heart rate <100/min for >5 min; and/or degree III
amniotic fluid pollution) or obvious asphyxia history during
delivery; ii) there was severe asphyxia during delivery, i.e. Apgar
score ≤3 at 1st min, ≤5 at 5th min; and/or pH ≤7.0 in blood gas of
umbilical artery; iii) there were neurological symptoms after
birth, that persisted for >24 h, such as change of consciousness
(over excitation, somnolence, coma), change of muscular tension
(increase or decrease), abnormity of primitive reflexes (sucking
reflex or moro reflex was weakened or disappeared), convulsion and
brain stem symptom when seriously ill (change of respiratory
rhythm, change of pupil, light reflex was slow or disappeared) and
increase of bregma tension; and iv) the neonates with convulsion
caused by electrolyte disturbance, intracranial hemorrhage and
birth injury were excluded. The neonates with brain injury caused
by intrauterine infection, genetic metabolic disease or other
congenital diseases were excluded.
In addition, if by computed tomography scan there
was encephaledema, there was diffuse low-density shadow complicated
with narrowed encephalocoele in brain parenchyma. If basal ganglia
and thalamus were injured there was bilateral symmetric
high-density shadow, while the cerebral infarction showed
low-density shadow in the corresponding blood supply area. The
clinical grading of HIE is shown in Table I.
 | Table I.The clinical grading of hypoxic
ischemic encephalopathy. |
Table I.
The clinical grading of hypoxic
ischemic encephalopathy.
|
|
|
| Primitive reflex |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Grade | Consciousness | Muscular tension | Embrace reflex | Sucking reflex | Convulsion | Central respiratory
failure | Change of pupil | EEG | Course of disease and
prognosis |
|---|
| Mild | Alternating of
excitement and inhibition | Normal or slightly
increased | Active | Normal | Myoclonus
sometimes | No | Normal or
enlarged | Normal | Symptoms disappear
within 72 h with good prognosis |
| Moderate | Somnolence | Decreased | Weakened | Weakened | Often | Yes | Often diminished | Low voltage,
complicated epileptiform discharge | Symptoms disappear
within 14 days with possible sequelae |
| Severe | Coma | Floppy or
intermittent muscular increased | Disappeared | Disappeared | Yes, can be
persistent | Obvious | Asymmetric or
enlarged, slow light reflex | Burst, suppression
isoelectric line | Symptoms persist for
several weeks, the mortality is high, most survivals have
sequelae |
Inclusion criteria
i) Neonates were full-term (gestational age was
between 37 and 42 weeks, birth weight between 2,500–4,000 g), Apgar
score of included neonates at 1st min was >3; ii) neonates did
not receive cortical hormone treatment. The remaining inclusion
criteria were the same as those for HIE. In total, 11 included
cases were mild, 16 included cases were moderate and 15 included
cases were severe.
Control group
Ten cases of neonates with similar gestational age
and weight to the HIBD group were selected from the full-term
neonates without hypoxia manifestation and cardiopulmonary disease
at the same period. There were 7 male cases and 3 female cases, 4
cases of eutocia and 6 cases of cesarean delivery, the average
gestational age was 38.5±1.12 weeks, the average weight was
3.29±0.52 kg at birth (Apgar score was 9′-10′-10′ or
10′-10′-10′).
Methods
Serum collection
Blood (2–3 ml) was collected from peripheral vein in
HIBD and normal neonates within 72 h after the first visit and at 7
days after birth. The blood was routinely centrifuged at 1,000 × g
for 5 min to collect serum and later preserved at −20°C. The
indicators in HIBD and normal neonates were detected at the same
time. Informed consent was obtained from the families of neonates
for serum collection.
Detection of serum A-FABP by ELISA
The reagent kit was provided by Adlitteram
Diagnostic Laboratories (San Diego, CA, USA), and plate washing and
absorbance detection were completed on a microplate reader. A-FABP
standard solution (100 µl), serum (100 µl) and 50 µl biotin-labeled
A-FABP with specific anti A-FABP antibody-coated micropores were
mixed. After incubation at 37°C for 1 h, the plates were washed for
55 min, 50 µl horse radish peroxidase-conjugated anti-biotin
antibody and 50 µl enzyme substrate solution were added for
developing coloration. After 15 min the stopping buffer was added
to terminate the reaction, and then optical density (OD) values of
different wells were read at a wavelength of 450 nm. B was the OD
value of standard and B0 was the OD value of standard at time point
0. The B/B0% value was set as a vertical coordinate and the
concentration of standard was set as a horizontal coordinate to
make a standard curve on the logarithmic coordinate paper. The
concentration of A-FABP was determined according to the OD value of
the sample on the standard curve.
Detection of FFA by copper colorimetric
method
The copper colorimetric method was used to detect
FFA according to the kit instructions (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
Detection of white blood cell, blood lipid,
glucose and C-reactive protein
White blood cell, blood lipid, glucose and
C-reactive protein were detected by Beckman Coulter LH 750, Beckman
Coulter LX20 PRO detection and Beckman Immade detection (Beckman
Coulter, Inc., Brea, CA, USA).
Statistical analysis
Data were analyzed using SPSS 11.5 (SPSS, Inc.,
Chicago, IL, USA). The data received normal distribution test
(Kolmogorov-Smirnov test), normal distributional data were
presented as mean ± standard deviation, and the difference was
analyzed by t-test and variance analysis. Non-normal distributional
data were presented as median (25 percentile, 75 percentile), the
difference was analyzed by the rank sum test and the inspection
level was a=0.05. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical data
The gender composition, the proportion of cesarean
delivery, average gestational age, and birth weight in three HIBD
neonate groups were not statistically different compared to normal
neonates (Table II).
 | Table II.Clinical parameters of HIBD and normal
neonates. |
Table II.
Clinical parameters of HIBD and normal
neonates.
| Group | Control | Case | Mild | Moderate | Severe | F | P-value |
|---|
| No. | 10 | 42 | 11 | 16 | 15 |
|
|
| FA, weeks | 38.5±1.12 | 38.8±1.33 |
39±1.35 | 38.5±1.22 | 38.7±1.29 | 0.759 | >0.05 |
| BW, kg | 3.29±0.52 | 3.15±0.51 | 2.94±0.55 | 3.10±0.35 | 3.37±0.56 | 1.914 | >0.05 |
| Male | 7 | 31 | 7 | 12 | 12 |
| >0.05 |
| Female | 3 | 11 | 4 | 4 | 3 |
| >0.05 |
| Eutocia | 4 | 17 | 6 | 4 | 7 |
| >0.05 |
| U-D | 6 | 25 | 5 | 12 | 8 |
| >0.05 |
White blood cell count and serum
C-reactive protein (CRP) in HIBD neonates at acute stage
The white blood cell count in HIBD neonates
(16.27±1.26×109/l) was significantly higher than that of
normal neonates (8.53±2.98×109/l) (P<0.01). However,
serum CRP in HIBD and normal neonates were in the normal range (CRP
<8 mg/l) (in both groups, the infectious factor was excluded,
the increase of white blood cell count in HIBD neonates was
considered to be related to the stress reaction caused by hypoxia
ischemia) (Table III).
 | Table III.Count of WBC and the content of
C-reactive protein in the acute stage of the HIBD and normal
neonates. |
Table III.
Count of WBC and the content of
C-reactive protein in the acute stage of the HIBD and normal
neonates.
| Group | Control | Case | t-test | P-value |
|---|
| No. | 10 | 42 |
|
|
| WBC
(×109/l) | 8.53±2.98 | 16.27±1.26 | −4.919 | <0.01 |
| CRP (mg/l) | <8 | <8 |
| >0.05 |
Blood lipid and glucose levels in HIBD
neonates at acute stage
High-density lipoprotein (HDL) level in HIBD
neonates (0.65±0.23 mmol/l) was significantly decreased compared
with normal neonates (0.81±0.18 mmol/l) (P<0.01). The other
blood lipid parameters and blood glucose level were not different
from normal neonates (Table
IV).
 | Table IV.Levels of blood-lipid and BG in acute
stage of HIBD and normal neonates. |
Table IV.
Levels of blood-lipid and BG in acute
stage of HIBD and normal neonates.
| Group | Control | Case | t/z | P-value |
|---|
| No. | 10 | 42 |
|
|
| TC, mmol/l | 2.72±0.97 | 2.42±0.89 | 0.941 | >0.05 |
| TG, mmol/l | 0.57
(0.29–0.80) | 0.60
(0.37–1.15) | −0.965 | >0.05 |
| HDL, mmol/l | 0.81±0.18 | 0.65±0.23 | 2.037 | <0.05 |
| LDL, mmol/l | 1.45±0.70 | 1.19±0.55 | 1.254 | >0.05 |
| ApoA, g/l | 0.80
(0.60–0.85) | 0.71
(0.70–0.80) | −0.649 | >0.05 |
| ApoB, g/l | 0.33±0.16 | 0.35±0.14 | −0.232 | >0.05 |
| BG, mmol/l | 3.90±1.12 | 3.35±1.20 | 1.207 | >0.05 |
Serum A-FABP level in HIBD neonates at
acute stage
Serum A FABP content in overall HIBD neonates at
acute stage was 21.61 (14.96, 37.67) ng/l, which was significantly
higher than 13.41 (9.64, 17.46) ng/l in the normal neonates
(P<0.05). The serum A-FABP level in severe HIBD neonates was
significantly higher than mild HIBD, moderate HIBD and normal
neonates (P<0.05). There was no difference between the mild HIBD
and moderate HIBD neonates (Fig.
1).
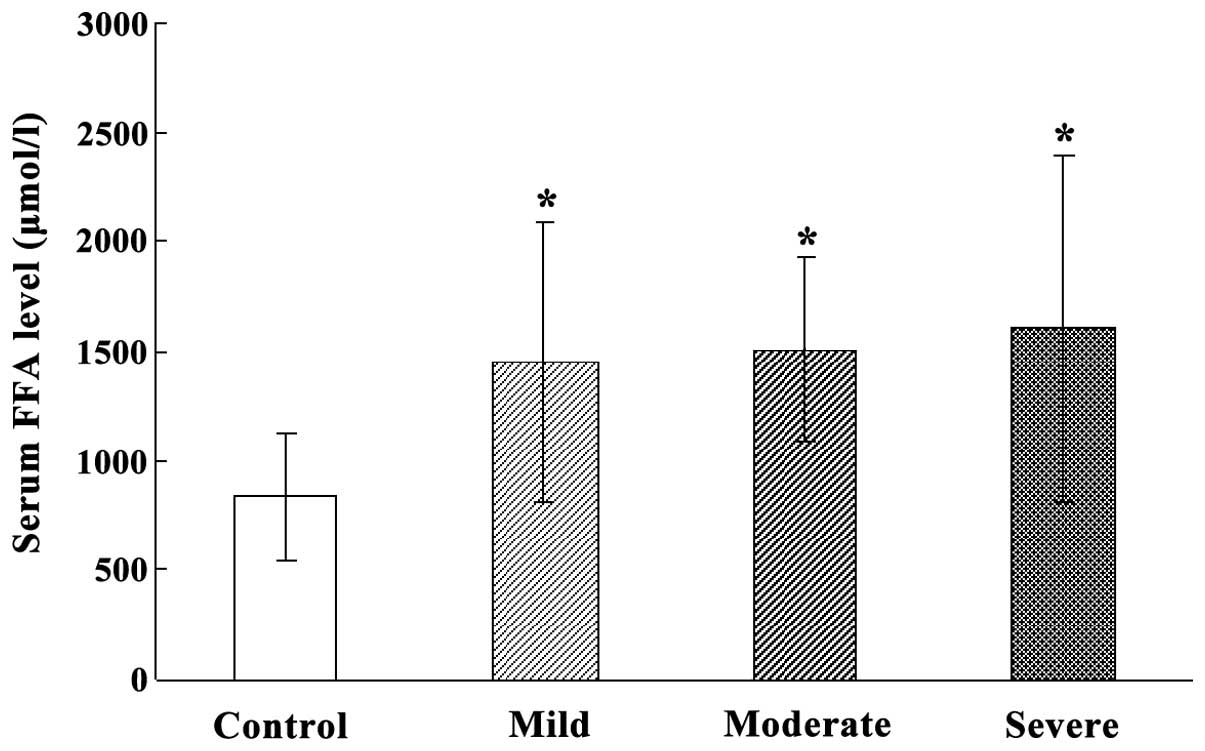
Serum FFA level in HIBD neonates at
acute stage
The overall serum FFA level in HIBD neonates at the
acute stage was significantly higher than that of normal neonates
(P<0.05). The serum FFA levels in mild, moderate and severe HIBD
neonates were significantly higher than those of normal neonates
(P<0.05). However, serum FFA level was not different among mild,
moderate and severe HIBD neonates (Fig.
2).
Changes of A-FABP and FFA levels at
recovery stage compared with acute stage
The overall A-FABP in HIBD neonates at the recovery
stage was significantly decreased compared to the acute stage
(P<0.05), which was significant in severe HIBD neonates
(P<0.05). A-FABP levels in the mild and moderate HIBD neonates
at recovery stage were decreased compared with the acute stage,
however there was no statistical difference (Fig. 3). The overall FFA content in HIBD
neonates at the recovery stage was decreased, however there was no
statistical difference compared to the acute stage.
Correlation of serum A-FABP and FFA in
HIBD neonates at the acute and recovery stages
Serum A-FABP level and FFA levels in HIBD neonates
at the acute stage were positively correlated, with the correlation
coefficient as r=0.369 (P<0.05). However, there was no
correlation at the recovery stage (Figs.
4–6).
Discussion
HIBD of neonates is important in perinatal nervous
system disease, and severe HIBD neonates leads to serious
complications of non-reversible nervous system sequelae. Hypoxia is
the key link of pathogenesis, while a series of cascade reactions
follow hypoxia. Interactions of several mechanisms cause
degeneration and necrosis, even softening of brain tissue and
further induce non-reversible brain injury (6). Statistics indicate 18–20 million live
neonates, and the occurrence rate of HIE as ~3–6‰ of live neonates,
15–20% of whom die at the neonatal period, while different types
and degrees of long-term sequelae are present in 25–30% of
survivals. Thus, this disease affects the quality of life of
children (2). At present, the
diagnosis of HIBD has been primarily based on medical history,
clinical manifestation, blood gas analysis and imageological
examination. Certain criteria (7)
are apparently extremely rigid, without flexibility, leading to an
appropriate diagnosis. The diagnostic criteria at home and abroad
lack objective and effective laboratory examination, affecting the
early diagnosis and appropriate observation of the degree of injury
and therapeutic effects in HIBD neonates (8).
When there is hypoxia ischemia, the cell membrane of
neurons is depolarized and intracellular Ca2+
concentration is rapidly increased. Hypoxia ischemia can cause the
release of a large number of oxygen radicals and the participation
of free radicals can degrade membrane phospholipid to release a
large number of FFA (9). It has been
found that phospholipase is activated and FFA is released in
moderate and severe asphyxia Wistar rat (10). FFA level in the cerebral cortex in
HIBD neonates becomes elevated with prolonging of the hypoxia
ischemia (11). In the present
study, serum FFA content level in HIBD neonates at acute stage was
significantly higher than that in normal neonates, and the serum
FFA level was also increased with aggravation of the degree of
hypoxia Overall serum FFA and FFA levels in moderate and severe
neonates was reduced, whereas serum FFA was increased in the mild
neonates. Considering that the symptoms of mild HIBD neonates were
rapidly recovered and the body weight increased satisfactorily, FFA
increase may be affected by sufficient diet, breast milk or powered
milk.
A-FABP mainly exists in adipocytes and macrophages,
the main function of which is to regulate lipid metabolism. It
regulates oxidation of fatty acid and metabolism of phospholipid
and triglyceride in several steps including assimilation, convey,
esterification and β oxidation (12). PPARγ activator, insulin, FFA and
oxidized low-density lipoprotein can upregulate A-FABP expression
(13,14). A-FABP gene knockout mice manifest as
increased fat, decreased lipolysis, increased aerobic oxidation and
reduced insulin resistance, indicating that A-FABP regulates energy
metabolism by regulating fatty acid metabolism (15). Considering that the long-chain FFA
can induce the expression of A-FABP, we hypothesized that if FFA
was increased in HIBD neonates, A-FABP expression would also be
increased in HIBD neonates. In the present study, the results
verified the hypothesis as the overall serum A-FABP in HIBD
neonates at acute stage was significantly higher than that in
normal neonates. Serum A-FABP level in severe HIBD neonates was
significantly higher than than in mild and moderate HIBD neonates.
The overall A-FABP in HIBD neonates at the recovery stage was
significantly decreased compared to the acute stage, which was
significant in severe HIBD neonates. The overall serum A-FABP and
FFA levels in HIBD neonates at the acute stage were positively
correlated.
In 2006, A-FABP was initially identified as a blood
circulation protein (16). However,
there is no specific basis that verifies that A-FABP is released by
adipocytes and macrophages or regulated by some regulatory
mechanism. Additionally, there was secretion signal sequence in the
primary and tertiary structures of A-FABP (17,18). It
was shown that the protein lacking the exocytosis positioning
signal sequence can pass plasma membrane by an unconventional
secretion mechanism (19). Rat
adipocytes lack secretion signal sequences, the release of various
proteins was completed by vesicle in rat, and A-FABP was found in
vesicle (20). There was also A-FABP
in the vesicle fragment of human cell supernatant (21). The abovementioned results have shown
that at least part of A-FABP can enter the blood circulation by
vesicle of adipocytes. Although the mechanism of how FABP4 enters
blood circulation remains to be determined, it has been
demonstrated that A-FABP was significantly increased in patients
with lipid metabolic diseases such as simple obesity, metabolic
syndrome (22,23), familial hyperlipidemia (24), non-alcoholic cirrhosis (25) and coronary heart disease (26,27).
Recent findings have shown that although there was no A-FABP in the
endothelial cells of small cerebral vessels, there was A-FABP in
endothelial cells of blood capillaries and small veins in
myocardium, skeleton muscles and tissue beside the large airway
(28). Thus we considered that
endothelial cells were one source of blood FABP4.
Different results have been identified regarding
blood lipid level in asphyxia neonates. In the detection of blood
lipid leveld in hypoxia asphyxia neonates by Yu et al
(29), serum total cholesterol and
triglyceride were significantly increased at the early stage of
asphyxia, which was more significant in severe neonates.
Time-course of the blood lipid level in asphyxia neonates,
triglyceride (TG) and apolipoprotein A1 levels were significantly
increased after birth, while HDL cholesterol (HDL-C) was decreased.
This finding was not related to asphyxia degree, and there was no
significant change of other indicators (30). In the present study, TG was increased
and HDL-C was decreased in the HIBD neonates. These results
indicate that although there was dyslipidemia in HIBD neonates, the
conventional lipid detection cannot be applied as a specific
laboratory indicator for HIBD. Serum FFA and FABP4 were
significantly increased in HIBD neonates at the acute stage, which
were increased with the increase in degree of injury, and decreased
as the recovery of disease condition.
In conclusion, the results have shown that serum FFA
and A-FABP were increased in HIBD neonates at the acute stage, and
the serum level of A-FABP and FFA at acute stage can reflect the
severity of HIBD. The detection of serum A-FABP and FFA cannot only
be applied as the indicators for the early diagnosis of HIBD, but
also provides a basis for the clinical evaluation of HIBD
treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 31271149), and the
Foundation for high-level talented person of Hebei Province (no.
A201400544) Major Projects of Education Department of Hebei (no.
ZD2016010).
References
|
1
|
Ilyukhina VA, Kataeva GV, Korotkov AD and
Chernysheva EM: [Oxygen-dependent energy deficit as related to the
problems of ontogenetic development disorders and human
sociobiological adaptation (theoretical and applied aspects)]. Zh
Evol Biokhim Fiziol. 51:77–87. 2015.PubMed/NCBI
|
|
2
|
Karadag N, Beken S, Dilli D, Zenciroglu A
and Okumus N: Successful hypothermia treatment of hypoxic-ischemic
encephalopathy in a neonate with epidermolysis bullosa. Indian J
Pediatr. 81:803–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strasser A, Stanimirovic D, Kawai N,
McCarron RM and Spatz M: Hypoxia modulates free radical formation
in brain microvascular endothelium. Acta Neurochir Suppl. 70:8–11.
1997.PubMed/NCBI
|
|
4
|
Kralisch S, Klöting N, Ebert T, Kern M,
Hoffmann A, Krause K, Jessnitzer B, Lossner U, Sommerer I, Stumvoll
M, et al: Circulating adipocyte fatty acid-binding protein induces
insulin resistance in mice in vivo. Obesity (Silver Spring).
23:1007–1013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rafay MF, Cortez MA, de Veber GA, Tan-Dy
C, Al-Futaisi A, Yoon W, Fallah S and Moore AM: Predictive value of
clinical and EEG features in the diagnosis of stroke and hypoxic
ischemic encephalopathy in neonates with seizures. Stroke.
40:2402–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Busl KM and Greer DM: Hypoxic-ischemic
brain injury: Pathophysiology, neuropathology and mechanisms.
NeuroRehabilitation. 26:5–13. 2010.PubMed/NCBI
|
|
7
|
No authors listed: Use and abuse of the
Apgar score. Committee on Fetus and Newborn, American Academy of
Pediatrics, and Committee on Obstetric Practice, American College
of Obstetricians and Gynecologists. Pediatrics. 98:141–142.
1996.PubMed/NCBI
|
|
8
|
Tataranno ML, Perrone S and Buonocore G:
Plasma biomarkers of oxidative stress in neonatal brain injury.
Clin Perinatol. 42:529–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niatsetskaya ZV, Sosunov SA, Matsiukevich
D, Utkina-Sosunova IV, Ratner VI, Starkov AA and Ten VS: The oxygen
free radicals originating from mitochondrial complex I contribute
to oxidative brain injury following hypoxia-ischemia in neonatal
mice. J Neurosci. 32:3235–3244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gardiner M, Nilsson B, Rehncrona S and
Siesjö BK: Free fatty acids in the rat brain in moderate and severe
hypoxia. J Neurochem. 36:1500–1505. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siesjö BK: Cerebral circulation and
metabolism. J Neurosurg. 60:883–908. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chmurzyńska A: The multigene family of
fatty acid-binding proteins (FABPs): Function, structure and
polymorphism. J Appl Genet. 47:39–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glatz JF and van der Vusse GJ: Cellular
fatty acid-binding proteins: Their function and physiological
significance. Prog Lipid Res. 35:243–282. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coe NR and Bernlohr DA: Physiological
properties and functions of intracellular fatty acid-binding
proteins. Biochim Biophys Acta. 1391:287–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hotamisligil GS, Johnson RS, Distel RJ,
Ellis R, Papaioannou VE and Spiegelman BM: Uncoupling of obesity
from insulin resistance through a targeted mutation in aP2, the
adipocyte fatty acid binding protein. Science. 274:1377–1379. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engl J, Ciardi C, Tatarczyk T, Kaser S,
Laimer M, Laimer E, Weiss H, Aigner F, Molnar C, Tilg H, et al:
A-FABP - a biomarker associated with the metabolic syndrome and/or
an indicator of weight change? Obesity (Silver Spring).
16:1838–1842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veerkamp JH and Maatman RG: Cytoplasmic
fatty acid-binding proteins: Their structure and genes. Prog Lipid
Res. 34:17–52. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reese-Wagoner A, Thompson J and Banaszak
L: Structural properties of the adipocyte lipid binding protein.
Biochim Biophys Acta. 1441:106–116. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radisky DC, Stallings-Mann M, Hirai Y and
Bissell MJ: Single proteins might have dual but related functions
in intracellular and extracellular microenvironments. Nat Rev Mol
Cell Biol. 10:228–234. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aoki N, Jin-no S, Nakagawa Y, Asai N,
Arakawa E, Tamura N, Tamura T and Matsuda T: Identification and
characterization of microvesicles secreted by 3T3-L1 adipocytes:
redox- and hormone-dependent induction of milk fat
globule-epidermal growth factor 8-associated microvesicles.
Endocrinology. 148:3850–3862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyake T, Ogawa E, Mikoshiba A, Kobayashi
A, Hosoe H, Kashiwabara S, Uhara H, Owada Y and Okuyama R:
Epidermal-type FABP is a predictive marker of clinical response to
systemic treatment and ultraviolet therapy in psoriatic skin
lesions. J Dermatol Sci. 68:199–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamounier-Zepter V, Look C, Alvarez J,
Christ T, Ravens U, Schunck WH, Ehrhart-Bornstein M, Bornstein SR
and Morano I: Adipocyte fatty acid-binding protein suppresses
cardiomyocyte contraction: A new link between obesity and heart
disease. Circ Res. 105:326–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stejskal D and Karpisek M: Adipocyte fatty
acid binding protein in a Caucasian population: A new marker of
metabolic syndrome? Eur J Clin Invest. 36:621–625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cabré A, Lázaro I, Cofán M, Jarauta E,
Plana N, Garcia-Otín AL, Ascaso JF, Ferré R, Civeira F, Ros E, et
al: FABP4 plasma levels are increased in familial combined
hyperlipidemia. J Lipid Res. 51:1173–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Milner KL, van der Poorten D, Xu A,
Bugianesi E, Kench JG, Lam KS, Chisholm DJ and George J: Adipocyte
fatty acid binding protein levels relate to inflammation and
fibrosis in nonalcoholic fatty liver disease. Hepatology.
49:1926–1934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karbek B, Özbek M, Bozkurt NC, Ginis Z,
Güngünes A, Ünsal IÖ, Cakal E and Delibası T: Heart-type fatty acid
binding protein (H-FABP): Relationship with arterial intima-media
thickness and role as diagnostic marker for atherosclerosis in
patients with ımpaired glucose metabolism. Cardiovasc Diabetol.
10:372011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doi M, Miyoshi T, Hirohata S, Nakamura K,
Usui S, Takeda K, Iwamoto M, Kusachi S, Kusano K and Ito H:
Association of increased plasma adipocyte fatty acid-binding
protein with coronary artery disease in non-elderly men. Cardiovasc
Diabetol. 10:442011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rueda-Valencia ML, Infante S, Campos M,
Belendez C and Saavedra LJ: Trimethoprim-sulfamethoxazole-induced
DRESS syndrome in a 4-year-old child. Ann Allergy Asthma Immunol.
116:366–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu T, Kui LQ and Ming QZ: Effect of
asphyxia on non-protein-bound iron and lipid peroxidation in
newborn infants. Dev Med Child Neurol. 45:24–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barrera-de León JC, Cervantes-Munguía R,
Vásquez C, Higareda-Almaraz MA, Bravo-Cuellar A and González-López
L: Usefulness of serum lipid peroxide as a diagnostic test for
hypoxic ischemic encephalopathy in the full-term neonate. J
Perinatol. 33:15–20. 2013. View Article : Google Scholar : PubMed/NCBI
|