Introduction
Under standard physiological conditions, vascular
smooth muscle cells (VSMCs) generally remain in a quiescent state.
However, in response to vascular damage or inflammatory
stimulations, VSMCs may undergo phenotypic changes to an
uncontrolled proliferating and migratory state (1). It has been well established that the
abnormal proliferation and migration of VSMCs in arterial walls is
crucial in the development and progression of cardiovascular
disorders, including intimal hyperplasia, arteriosclerosis and
restenosis following percutaneous coronary intervention (PCI)
(2,3).
Formononetin is an O-methylated isoflavone
phytoestrogen extracted from the root of Astragalus
membranaceus, which has been widely used in Chinese medicine
for >2,000 years. A. membranaceus has various
bioactivities, such as anti-viral, anti-oxidant, anti-tumor,
anti-diabetes, anti-inflammation, anti-atherosclerosis,
immunomodulation, hepatoprotection, hematopoiesis and
neuroprotection (4–7). As an important component of A.
membranaceus, formononetin has also been demonstrated to have
various pharmacological effects. For instance, previous studies
have indicated that formononetin is able to inhibit tumor cell
proliferation, migration and invasion (8,9), induce
tumor cell apoptosis (10),
attenuate hydrogen peroxide-induced retinal ganglion cell apoptosis
(11), as well as mediate
neuroprotection against cerebral ischemia/reperfusion (12).
Recent studies have demonstrated that formononetin
may exert protective effects against cardiovascular disorders. Huh
et al reported that formononetin promoted endothelial repair
and wound healing (13). Zhu et
al found that formononetin had neuroprotective effects against
cerebral ischemia and reperfusion injury in rats, and improved
cerebrovascular angiogenesis in human umbilical vein endothelial
cells (14). However, to the best of
our knowledge, the effect of formononetin on VSMCs has not
previously been studied.
The aim of the present study was to investigate the
effect of formononetin on PDGF-BB-stimulated VSMC proliferation and
migration, in addition to elucidating the underlying
mechanisms.
Materials and methods
Materials and agents
Formononetin was purchased from TAOTU Biotech
(Shanghai, China). Dulbecco's modified Eagle's medium (DMEM)/F12,
fetal bovine serum (FBS), and BCA Protein Assay Kit were purchased
from Life Technologies (Carlsbad, CA, USA). An enhanced
chemiluminescence kit was purchased from Pierce Biotechnology
(Thermo Fisher Scientific, Inc., Rockford, IL, USA). Recombinant
human PDGF-BB, dimethyl sulfoxide (DMSO), MTT, bovine serum albumin
(BSA) and radioimmunoprecipitation assay (RIPA) buffer were
purchased from Sigma-Aldrich (St. Louis, MO, USA). A 24-well
chamber was purchased from Corning, Inc., (Corning, NY, USA). Mouse
monoclonal antibodies against smoothelin (1:100; ab8969), α-smooth
muscle actin (α-SMA; 1:200; ab7817), desmin (1:50; ab8470), cyclin
D1 (1:50; ab6152), cyclin-dependent kinase 4 (CDK4; 1:200;
ab75511), matrix metalloproteinase 2 (MMP2; 1:100; ab86607), MMP9
(1:100; ab58803), phospho-AKT, mouse AKT (1:100; ab105731) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:50; ab8245), in
addition to goat anti-mouse secondary antibody were obtained from
Abcam (1:20,000; ab6785; Cambridge, MA, USA).
Cell culture
Human dermis VSMCs were purchased from ScienCell
Research Laboratories (Carlsbad, CA, USA). VSMCs were cultured in
DMEM/F12 medium with 10% FBS at 37°C in a humidified atmosphere of
95% air and 5% CO2.
MTT assay
An MTT assay was performed for examining the cell
proliferation. Three groups were established, as follows: Control
group, VSMCs were without any treatment; PDGF-BB group, VSMCs were
treated with PDGF-BB (30 ng/ml); and PDGF-BB + formononetin group,
VSMCs were treated with PDGF-BB (30 ng/ml) and formononetin (1 µM).
Cells (5×103) in each group were seeded into 96-well
plates, and cultured for 0, 12, 24 and 48 h, respectively. Then, 10
µl MTT (10 mg/ml) was added to the cells and incubated for 4 h
prior to termination of the reaction by removing the supernatant
and adding 100 µl DMSO to dissolve the formazan product. Following
incubation for 30 min, the optical density of each well was
measured at 570 nm using a plate reader (ELx808; BioTek
Instruments, Inc., Winooski, VT, USA).
Scratch assay
Cell migration in each group was examined using a
costar 24-well chamber (Corning Inc., Shanghai, China). The cells
were counted under a CX23 microscope (Olympus, Tokyo, Japan). In
brief, cell suspension (5×105 cells/ml) was prepared in
DMEM/F12 medium. In accordance with the manufacturer's
instructions, 500 µl DMEM/F12 with 10% FBS was added to the lower
chamber, and 300 µl cell suspension was added into the upper
chamber. In the PDGF-BB group, PDGF-BB (30 ng/ml) was also added to
the lower wells. In the PDGF-BB + formononetin group, the lower
wells contained PDGF-BB (30 ng/ml) and formononetin (1 µM). The
relative migration in the PDGF-BB group is presented as the cell
number ratio of PDGF-BB versus the control. Similarly, the relative
migration in the PDGF-BB+formononetin group is presented as the
cell number ratio of PDGF-BB+formononetin versus the control. After
24 h incubation at 37°C with 5% CO2, cells that had not
migrated through the membrane were removed, while cells that had
were stained with crystal violet dye (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min, then rinsed with water
and dried in air. The stained cells were counted and the relative
cell migration was determined.
Western blot analysis
Cells were washed with phosphate-buffered saline
once and 500 µl RIPA buffer was added to lyse the cells. Cells were
then centrifuged at 8,000 × g for 10 min at 4°C, and the
supernatant containing the protein was collected. The concentration
of protein was determined using a BCA Protein Assay kit, in
accordance with the manufacture's instructions. Next, 50 µg protein
was run on a 12% SDS-PAGE gel (Beyotime Institute of Biotechnology)
and blotted onto polyvinylidene difluoride membranes (Thermo Fisher
Scientific, Inc.), which were blocked in 5% BSA for 1.5 h at room
temperature, followed by incubation overnight at 4°C with the
indicated antibodies. The membranes were rinsed and incubated for 1
h at room temperature with the appropriate peroxidase-conjugated
secondary antibodies. Chemiluminescent detection was performed
using the enhanced chemiluminescence kit. The relative protein
expression was analyzed by Image Pro Plus software version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA) and presented as the
density ratio of FSCN1 versus GAPDH.
Statistical analysis
Data is presented as the mean ± standard deviation
of at least three independent experiments. SPSS software, version
17.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. One-way analysis of variance was used to analyze the
differences between groups. P<0.05 was considered to indicate as
statistically significant difference.
Results
Formononetin inhibited
PDGF-BB-stimulated proliferation and migration of VSMCs
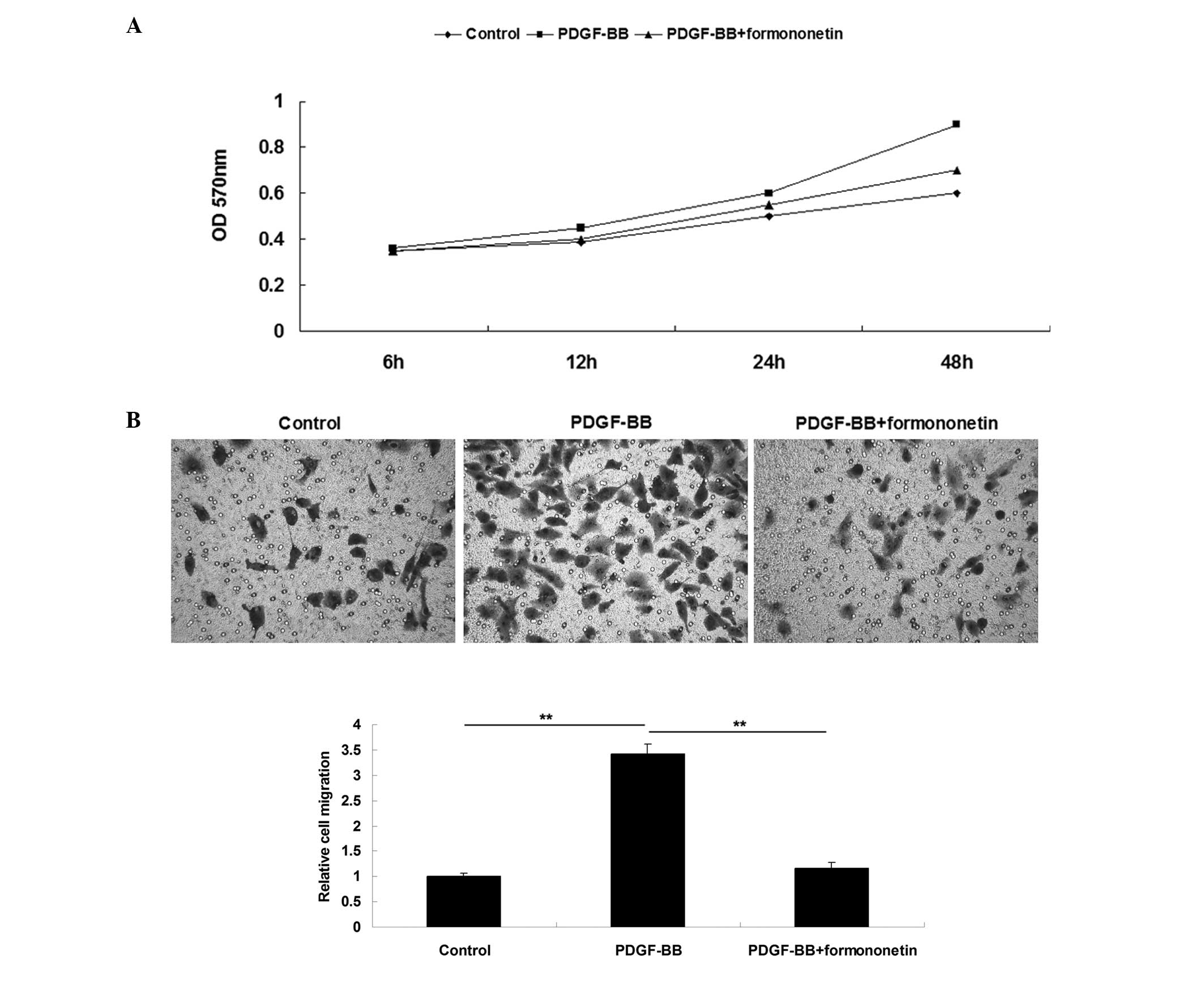
The effects of formononetin on PDGF-BB-induced VSMCs
proliferation and migration were investigated first. MTT assay data
showed that the PDGF-BB treatment enhanced the proliferation of
VSMCs compared with the control group, which was notably attenuated
by the treatment with formononetin (Fig.
1A). These data suggest that formononetin inhibits
PDGF-BB-stimulated VSMCs proliferation. Subsequently, the effect of
formononetin on the PDGF-BB-stimulated migration of VSMCs was
investigated. The results of a scratch assay showed that treatment
with PDGF-BB significantly promoted VSMCs migration compared with
the control group; however, formononetin significantly attenuated
the upregulation of PDGF-BB-induced VSMC migration (Fig. 1B), suggesting that formononetin has
an inhibitory effect on PDGF-BB-induced VSMCs migration.
Formononetin inhibited the
PDGF-BB-induced phenotype change of VSMCs
Under normal physiological conditions, vascular
smooth muscle cells (VSMCs) generally remain in a quiescent state.
However, in response to vascular damage or inflammatory
stimulation, VSMCs may undergo phenotypic changes to an
uncontrolled proliferating and migratory state (1). Smoothelin, α-SMA and desmin are markers
for the quiescent phenotype of VSMCs (15). Therefore, the expression levels of
these proteins were detected in each group. As shown in Fig. 2, administration of PDGF-BB
significantly inhibited the protein expression levels of
smoothelin, α-SMA and desmin in VSMCs, which cause VSMCs to
dedifferentiate into a proliferative phenotype. However, treatment
with formononetin significantly attenuated the PDGF-BB-induced
downregulation of α-SMA, smoothelin and desmin protein expression
in VSMCs (Fig. 2), suggesting that
formononetin has an inhibitory effect on PDGF-BB-induced phenotype
switch in VSMCs.
Formononetin inhibited the
PDGF-BB-induced expression of cell cycle-related proteins in
VSMCs
Cell cycle-related proteins, including CDK2, CDK4,
cyclin D1 and cyclin E, are crucially involved in the regulation of
cell cycle progression, as well as cell proliferation (16). It has been reported that formononetin
has effects on the expression of cell cycle-related proteins
(8). Therefore, the effect of
formononetin on VSMCs proliferation may be associated with the
expression levels of cell cycle-related proteins. Western blot
analysis was conducted to determine the protein levels of CDK2,
CDK4, cyclin D1, and cyclin E in each group. As shown in Fig. 3, administration of PDGF-BB
significantly enhanced the protein expression levels of CDK2, CDK4,
cyclin D1, and cyclin E in VSMCs; however, treatment with
formononetin significantly suppressed PDGF-BB-stimulated
upregulation of CDK2, CDK4, cyclin D1 and cyclin E, suggesting that
the suppressive effect of formononetin on PDGF-BB-induced VSMCs
proliferation may partly occur via the inhibition of the expression
of cell cycle-related proteins.
Formononetin suppressed
PDGF-BB-induced upregulation of MMP2 and MMP9 in VSMCs
It has been well established that MMP2 and MMP9 play
key roles in the regulation of cell migration (9). Therefore, the protein expression levels
of MMP2 and MMP9 were determined in the VSMCs in each group. As
shown in Fig. 4, MMP2 and MMP9 were
significantly upregulated following treatment with PDGF-BB, which
was significantly attenuated by treatment with formononetin. These
results suggest that the suppressive effect of formononetin on
PDGF-BB-induced VSMCs migration is mediated by the inhibition of
MMP2 and MMP9 protein expression.
Formononetin suppressed
PDGF-BB-induced activation of AKT signaling in VSMCs
AKT signaling pathway has been implicated in the
regulation of cell proliferation and migration, in addition the
expression of cell cycle-related proteins and MMPs (8,17).
Accordingly, the activity of AKT signaling in VSMCs was evaluated
in the present study. As shown in Fig.
5, the phospho-AKT protein expression was significantly
upregulated by treatment with PDGF-BB, when compared with the
control group, suggesting that PDGF-BB is able to activate the AKT
signaling pathway. However, treatment with formononetin effectively
suppressed PDGF-BB-stimulated upregulation of phospho-AKT protein
level in VSMCs, suggesting that formononetin is able to inhibit
PDGF-BB-induced activation of AKT signaling in VSMCs.
Discussion
The results of the present study suggest that
formononetin exerted an inhibitory effect against
PDGF-BB-stimulated VSMCs proliferation and migration. Formononetin
was able to inhibit the PDGF-BB-stimulated change of VSMCs into a
proliferative phenotype, suppressed the enhanced expression of cell
cycle-related proteins and MMPs, in addition to downregulating the
activity of AKT signaling.
VSMCs are continually stimulated by the biochemical
components in the blood compartment, which may affect their
phenotypes such as cell proliferation and migration. Thus VSMCs are
involved in the physiological and pathological processes in the
vascular wall (18,19). For example, following vascular injury
various cytokines, including PDGF-BB, are released by endothelial
cells and macrophages. These stimulate the abnormal proliferation
and migration VSMCs, a key promoter in the initiation of intimal
hyperplasia, which can further lead to arteriosclerosis and
restenosis following PCI (20–22).
Therefore, inhibition of PDGF-BB-induced VSMCs proliferation and
migration is crucial for the prevention of atherosclerosis and
restenosis. Formononetin has been shown to inhibit the
proliferation of multiple types of cancer cells. Li et al
showed that formononetin inhibited the proliferation of human
prostate cancer cells via inducing cell cycle arrest (8). Liu et al showed that
formononetin suppressed proliferation while inducing the apoptosis
of osteosarcoma cells (23).
However, the effects of formononetin on VSMCs proliferation have
been hitherto unclear. Herein, it was reported that formononetin
suppressed PDGF-BB-stimulated VSMCs proliferation. Furthermore, the
results indicate that PDGF-BB could induce VSMCs to dedifferentiate
into a proliferative phenotype, as suggested by the downregulation
of SMA, smoothelin and desmin, which is consistent with previous
studies (16,24). However, treatment with formononetin
attenuated the PDGF-BB-induced downregulation of SMA, smoothelin
and desmin, indicating that formononetin inhibited the
PDGF-BB-induced phenotype change in VSMCs.
Cell cycle-related proteins such as cyclin D1,
cyclin E, CDK2 and CDK4 are crucially involved in the regulation of
cell proliferation. Previous studies have shown that these cell
cycle-related proteins are associated with PDGF-BB-stimulated VSMCs
proliferation (25,26). Furthermore, it has been reported that
formononetin is able to mediate the expression of these proteins.
For example, formononetin promotes cell cycle arrest via the
downregulation of cyclin D1 and CDK4 expression in human prostate
cancer cells (8). The present
results suggest that treatment with formononetin significantly
attenuated the PDGF-BB-stimulated upregulation of cyclin D1, cyclin
E, CDK2 and CDK4.
MMP2 and MMP9 have been shown to play key roles in
the regulation of VSMCs migration. For instance, Ding et al
observed that resistin stimulated MMP-2 and MMP-9 expression and
VSMC migration, while neutralizing antibodies against MMP-2 and
MMP-9 effectively reversed resistin-stimulated VSMC migration
(27). In addition, MMP2 and MMP9
are involved in intimal hyperplasia. Guo et al found that
neointimal hyperplasia was reduced in MMP9−/− or
MMP2−/− mice after femoral artery injury (28). In the present study, it was shown
that treatment of PDGF-BB increased the expression levels of MMP2
and MMP9, which is consistent with the findings of Guo et al
(28). Moreover, treatment with
formononetin markedly suppressed the PDGF-BB-stimulated
upregulation of MMP2 and MMP9, suggesting that the suppressive
effect of formononetin on PDGF-BB-stimulated VSMCs migration is
partly mediated via the inhibition of MMP2 and MMP9 expression.
AKT signaling has been implicated in various
cellular biological processes, such as cell survival, apoptosis,
cell cycle progression and angiogenesis, in addition to cell
migration and invasion (29,30). In addition, it has been demonstrated
that AKT signaling is involved in regulating the expression of a
number of cell cycle-related proteins and MMPs (9,31,32).
Therefore, the activity of AKT signaling after treatment with
PDGF-BB with or without formononetin was evaluated in the present
study. Treatment with PDGF-BB appeared to enhance the
phosphorylated protein level of AKT, indicating that the activity
of AKT signaling was upregulated, which is consistent with previous
studies (15,33,34).
However, treatment with formononetin effectively suppressed
PDGF-BB-stimulated upregulation of phospho-AKT protein level in
VSMCs, suggesting that formononetin is able to inhibit
PDGF-BB-induced activation of AKT signaling in VSMCs.
In conclusion, the results of the present study
demonstrate that treatment with formononetin is able to inhibit
PDGF-BB-induced VSMC proliferation and migration via the inhibition
of phenotype switch, expression of cell cycle-related proteins and
MMPs, in addition to the activity of AKT signaling. Therefore,
formononetin may require further investigation as a potential
treatment for intimal hyperplasia, atherosclerosis and restenosis
following PCI.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central University of Central South University (grant
no. 2013zzts088).
References
|
1
|
Rodríguez AI, Csányi G, Ranayhossaini DJ,
Feck DM, Blose KJ, Assatourian L, Vorp DA and Pagano PJ: MEF2B-Nox1
signaling is critical for stretch-induced phenotypic modulation of
vascular smooth muscle cells. Arterioscler Thromb Vasc Biol.
35:430–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiong M, Cartes-Saavedra B,
Norambuena-Soto I, Mondaca-Ruff D, Morales PE, García-Miguel M and
Mellado R: Mitochondrial metabolism and the control of vascular
smooth muscle cell proliferation. Front Cell Dev Biol. 2:722014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choe N, Kwon JS, Kim JR, Eom GH, Kim Y,
Nam KI, Ahn Y, Kee HJ and Kook H: The microRNA miR-132 targets
Lrrfip1 to block vascular smooth muscle cell proliferation and
neointimal hyperplasia. Atherosclerosis. 229:348–355. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin M, Zhao K, Huang Q and Shang P:
Structural features and biological activities of the
polysaccharides from Astragalus membranaceus. Int J Biol
Macromol. 64:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agyemang K, Han L, Liu E, Zhang Y, Wang T
and Gao X: Recent Advances in Astragalus membranaceus
anti-diabetic research: Pharmacological effects of its
phytochemical constituents. Evid Based Complement Alternat Med.
2013:6546432013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Xie X, Li C and Fu P: Systematic
review of the renal protective effect of Astragalus
membranaceus (root) on diabetic nephropathy in animal models. J
Ethnopharmacol. 126:189–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu F and Chen X: A review of
pharmacological study on Astragalus membranaceus (Fisch.)
Bge. Zhong Yao Cai. 27:232–234. 2004.(In Chinese). PubMed/NCBI
|
|
8
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia WC, Liu G, Zhang CD and Zhang SP:
Formononetin attenuates hydrogen peroxide (H2O2)-induced apoptosis
and NF-κB activation in RGC-5 cells. Eur Rev Med Pharmacol Sci.
18:2191–2197. 2014.PubMed/NCBI
|
|
12
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huh JE, Nam DW, Baek YH, Kang JW, Park DS,
Choi DY and Lee JD: Formononetin accelerates wound repair by the
regulation of early growth response factor-1 transcription factor
through the phosphorylation of the ERK and p38 MAPK pathways. Int
Immunopharmacol. 11:46–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Zou L, Tian J, Lin F, He J and Hou
J: Protective effects of sulphonated formononetin in a rat model of
cerebral ischemia and reperfusion injury. Planta Med. 80:262–268.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MH, Kwon BJ, Seo HJ, Yoo KE, Kim MS,
Koo MA and Park JC: Resveratrol inhibits phenotype modulation by
platelet derived growth factor-bb in rat aortic smooth muscle
cells. Oxid Med Cell Longev. 2014:5724302014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Cai Y, Zhang W, Liu X and Liu S:
Astragaloside IV inhibits platelet-derived growth
factor-BB-stimulated proliferation and migration of vascular smooth
muscle cells via the inhibition of p38 MAPK signaling. Exp Ther
Med. 8:1253–1258. 2014.PubMed/NCBI
|
|
17
|
Guan BZ, Yan RL, Huang JW, Li FL, Zhong
YX, Chen Y, Liu FN, Hu B, Huang SB and Yin LH: Activation of G
Protein coupled estrogen receptor (GPER) promotes the migration of
renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh Migr
0. 2015. View Article : Google Scholar
|
|
18
|
Salabei JK and Hill BG: Autophagic
regulation of smooth muscle cell biology. Redox Biol. 4:97–103.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu J, Zheng Y, Hu J, Liao D, Gregersen H,
Deng X, Fan Y and Wang G: Biomechanical regulation of vascular
smooth muscle cell functions: From in vitro to in vivo
understanding. J R Soc Interface. 11:201308522013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Luo K and Hou J: Inhibitory effect
of Puerariae radix flavones on platelet-derived growth
factor-BB-induced proliferation of vascular smooth muscle cells via
PI3K and ERK pathways. Exp Ther Med. 9:257–261. 2015.PubMed/NCBI
|
|
21
|
Guan S, Tang Q, Liu W, Zhu R and Li B:
Nobiletin Inhibits PDGF-BB-induced vascular smooth muscle cell
proliferation and migration and attenuates neointimal hyperplasia
in a rat carotid artery injury model. Drug Dev Res. 75:489–496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tolva V, Mazzola S, Zerbi P, Casana R,
Albertini M, Calvillo L, Selmin F and Cilurzo F: A successful
experimental model for intimal hyperplasia prevention using a
resveratrol-delivering balloon. J Vasc Surgp. ii:S0741–S5214.
2014.
|
|
23
|
Liu Y, He J, Chen X, Li J, Shen M, Yu W,
Yang Y and Xiao Z: The proapoptotic effect of formononetin in human
osteosarcoma cells: Involvement of inactivation of ERK and Akt
pathways. Cell Physiol Biochem. 34:637–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang X, Jin Y, Zhou D, Xu G, Huang J and
Shen L: IQGAP1 promotes the phenotypic switch of vascular smooth
muscle by myocardin pathway: A potential target for varicose vein.
Int J Clin Exp Pathol. 7:6475–6485. 2014.PubMed/NCBI
|
|
25
|
Song Y, Long L, Zhang N and Liu Y:
Inhibitory effects of hydroxysafflor yellow A on PDGF-BB-induced
proliferation and migration of vascular smooth muscle cells via
mediating Akt signaling. Mol Med Rep. 10:1555–1560. 2014.PubMed/NCBI
|
|
26
|
Song MC, Park J and Kim TJ:
Diethylstilbestrol induces arrest of rat vascular smooth muscle
cell cycle progression through downregulation of cyclin D1 and
cyclin E. Mol Cell Biochem. 360:103–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Q, Chai H, Mahmood N, Tsao J,
Mochly-Rosen D and Zhou W: Matrix metalloproteinases modulated by
protein kinase Cε mediate resistin-induced migration of human
coronary artery smooth muscle cells. J Vasc Surg. 53:1044–1051.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo L, Ning W, Tan Z, Gong Z and Li X:
Mechanism of matrix metalloproteinase axis-induced neointimal
growth. J Mol Cell Cardiol. 66:116–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toker A: Achieving specificity in Akt
signaling in cancer. Adv Biol Regul. 52:78–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vasudevan KM and Garraway LA: AKT
signaling in physiology and disease. Curr Top Microbiol Immunol.
347:105–133. 2010.PubMed/NCBI
|
|
31
|
Liu D, Liu J, Lin B, Liu S, Hou R, Hao Y,
Liu Q, Zhang S and Iwamori M: Lewis y regulate cell cycle related
factors in ovarian carcinoma cell RMG-I in vitro via ERK and Akt
signaling pathways. Int J Mol Sci. 13:828–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park ES, Kang SI, Yoo KD, Lee MY, Yoo HS,
Hong JT, Shin HS, Kim B and Yun YP: Camptothecin inhibits
platelet-derived growth factor-BB-induced proliferation of rat
aortic vascular smooth muscle cells through inhibition of PI3K/Akt
signaling pathway. Exp Cell Res. 319:982–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iida M, Tanabe K, Kozawa O and Iida H:
Differential effects of intravenous anesthetics on PDGF-BB-induced
vascular smooth muscle cell migration. Cell Physiol Biochem.
33:1827–1837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Son JE, Jeong H, Kim H, Kim YA, Lee E, Lee
HJ and Lee KW: Pelargonidin attenuates PDGF-BB-induced aortic
smooth muscle cell proliferation and migration by direct inhibition
of focal adhesion kinase. Biochem Pharmacol. 89:236–245. 2014.
View Article : Google Scholar : PubMed/NCBI
|