Introduction
Doxorubicin (DOX), which belongs to the
anthracyclines family, has been used to treat cancer since the late
1960s. It is a well-established and highly effective antineopalstic
agent that is used to treat various adult and pediatric cancers,
including solid tumors, leukemia, lymphomas and breast cancer
(1). However, DOX causes various
toxic effects, the most common of which is cardiotoxicity (2). Multiple mechanisms are involved in
DOX-induced cardiomyopathy, including the increase in cardiac
oxidative stress, as evidenced by reactive oxygen species that
induce damage such as lipid peroxidation, and changes in adenylate
cyclase activity leading to apoptosis and inflammation-related
signaling pathways (3,4). It has previously been suggested that
DOX also elicits inflammatory effects by increasing the expression
levels of nuclear factor kappa-B (NF-κB), and induces the
production of various proinflammatory mediators, including tumor
necrosis factor (TNF)-α (5).
It has been demonstrated that DOX cardiotoxicity
involves cardiomyocyte apoptosis (6). Caspase activity can be influenced by
DOX, and caspase-3 activation is associated with DOX administration
(7). The forkhead box (FOX) family
of transcription factors regulate numerous cellular functions.
These transcription factors are associated with the regulation of
metabolism, cell proliferation, resistance of stress, immune system
regulation, and apoptosis (8).
FOXO3a is regulated by a number of signaling pathways, including
extracellular signal-regulated kinase (ERK), Akt, IκB kinase, and
serum glucocorticoid-related kinases (9,10).
Furthermore, FOXO3a is involved in resistance to oxidative stress
and also linked to apoptotic processes by modulating the expression
levels of proapoptotic and antiapoptotic proteins that regulate
antioxidant enzyme levels, including mitochondrial antioxidant
manganese superoxide dismutase (MnSOD) (11–13).
Hawthorn, of the rosaceae plant family, is a
traditional Chinese medicine that is used to promote digestion
(14). It has been reported that the
ketone compounds extracted from the Hawthorn leaves are able to
regulate blood lipids, blood pressure, increase coronary flow and
protect the ischemic myocardium (15–17).
Vitexin is the active ingredient extracted from hawthorn leaves,
and it has previously been demonstrated that vitexin has a
protective effect against hypoxia in the reoxygenation of
myocardial cells (18). Therefore,
the present study aimed to investigate the potential protective
effect of vitexin against DOX-induced cardiotoxicity in rats and to
elucidate the underlying molecular mechanisms in terms of oxidative
stress, inflammatory and apoptotic mediators.
Materials and methods
Materials
Vitexin with a purity of 95% (Sigma-Aldrich, St.
Louis, MO, USA) was dissolved in normal saline. The chemical
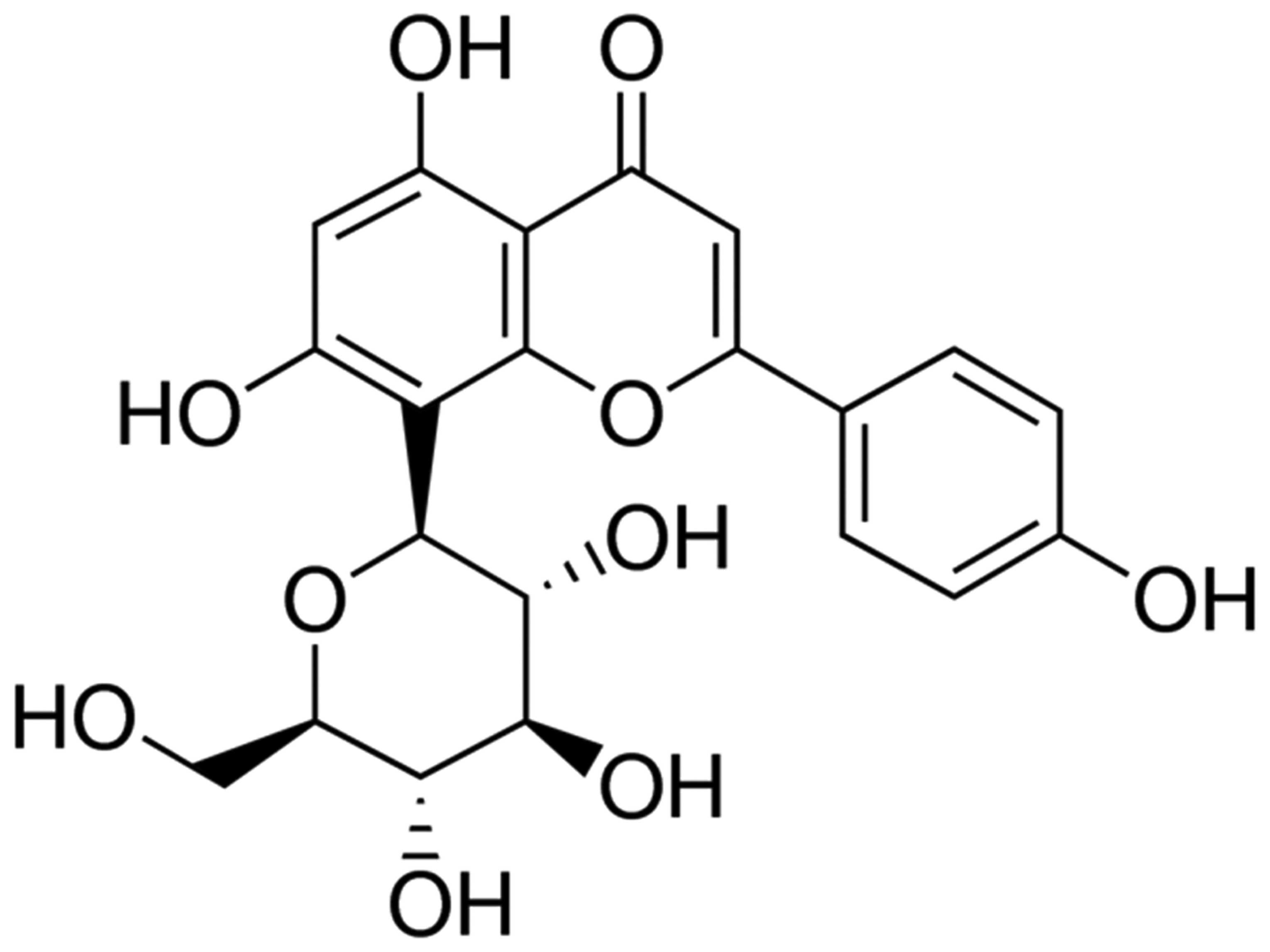
structure of vitexin is presented in Fig. 1. Casein kinase (CK), lactate
dehydrogenase (LDH), tumor necrosis factor-α (TNF-α), interleukin
(IL)-1β, IL-6, nuclear factor kappa B (NF-κB), malondialdehyde
(MDA), SOD, catalase (CAT) and myeloperoxidase (MPO) commercial
kits were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Animals and modeling
A total of 36 male Sprague-Dawley rats (age, 6–8
weeks; weight, 260±20 g) were provided by the Laboratory Animal
Center of Xinjiang Medical University (Xinjiang, China). All animal
care and experimental procedures were approved by the Animal Care
Committee of Xinjiang Medical University (Ürümqi, China). Rats were
maintained at 24±1°C (humidity, 40–80%) under pathogen-free
conditions with a 12 h light/dark cycle and ad libitum
access to food and water. Rats were randomly and equally assigned
into the control, model, and vitexin-treated groups. Control rats
received an equal volume of normal saline by intraperitoneal (i.p.)
injection at the same time points. Model group rats were induced by
i.p. injection of DOX (2 mg/kg) once a week for 4 weeks.
Vitexin-treated group rats were administered oral vitexin once
daily at doses of 30 mg/kg for 4 weeks (19). Following treatment, blood samples
were collected from the abdominal aorta and the rats were
sacrificed via an overdose of ethyl carbamate prior to the
harvesting of myocardial tissue. Blood samples were anticoagulated
with EDTA and centrifuged at 3,000 × g for 10 min at 4°C, and the
plasma was subsequently stored at −80 °C until further use.
Assessment of cardiotoxicity
indices
LDH and creatine kinase isoenzyme-MB (CK-MB) levels
were assessed in serum samples using commercially available kits
according to the manufacturer's protocol. All measurements were
performed in duplicate.
Assessment of inflammatory cytokines
in blood
TNF-α, IL-1β, IL-6 and NF-κB levels in the blood
samples were determined using ELISA kits, according to the
manufacturer's protocol. All measurements were performed in
duplicate.
Assessment of oxidative stress markers
and antioxidant enzyme activities
Lipid peroxidation was determined by estimating the
level of thiobarbituric acid reactive substances measured as MDA,
according to the manufacturer's protocol. Results were expressed as
MDA (nmol)/mg of wet tissue. Cardiac SOD activity was determined
according to the method outlined by Flohe and Otting (20). Values were expressed as U/mg protein.
Cardiac CAT activity was assessed via the determination of the
H2O2 decomposition rate at 240 nm and the
values were expressed as U/mg protein. MPO activity was assayed
using a commercially available kit, according to the manufacturer's
protocol.
Assessment of cardioprotective FOXO3a protein
expression levels by western blotting. Briefly, the cardiac left
ventricular (LV) tissue was homogenized with a lysis buffer
containing 25 mM Tris, (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1 mM
Na3VO4, 10 mM NaF, 1% (vol/vol) Triton X-100,
and 1% (vol/vol) glycerol. Equal amounts of the heart homogenate
(30 µg) were separated by 10% SDS-PAGE (wt/vol) and subsequently
transferred onto a nitrocellulose membrane (Trans-Blot Transfer
Medium; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and blocked
with 5% skimmed milk at room temperature for 60 min. Membranes were
washed three times for 5 min with Tris-buffered saline with Tween
20 (TBS-T) and incubated overnight with the appropriate primary
anti-phosphorylation-FOXO3a (p-FOXO3a; 1:2,000; 9464) and
anti-β-actin (1:500; 8457; both American Diagnostica Inc.,
Stamford, CT, USA) antibodies at 4°C. Subsequently, the membranes
were washed thrice with TBS-T and incubated with secondary
antibodies for 2 h at room temperature. Immunodetection was
performed using horseradish peroxidase-conjugated secondary
antibody (1:2,000; 5522; Cell Signaling Technology Inc., Danvers,
MA, USA) using an enhanced chemiluminescence kit (GE Healthcare
Life Sciences, Chalfont, UK). Blot quantification was performed
using ImageQuant LAS 500 software (GE Healthcare Life
Sciences).
Assessment of apoptotic markers
ELISA kits were used to analyze the levels of
caspase-3 in myocardial tissue. Briefly, cardiac LV tissue was
homogenized with a lysis buffer and equal amounts of the heart
homogenate (30 µg) were supplemented with reaction buffer with
1Asp-Glu-Val-Asp (DEVD)-p-nitroaniline and incubated at 37°C for 6
h. Caspase-3 activation was measured using a microplate reader
(Bio-Rad Laboratories, Inc.) at an absorbance of 405 nm.
Statistical analysis
Results are presented as the mean ± standard
deviation. For tests of significance between the groups, one-way
analysis of variance was performed. Comparisons between two groups
were performed using unpaired Student's t-test. SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) was used to
conduct statistical analyses. P<0.05 was considered to indicate
a statistically significant difference. All measurements were
performed at least three independent times.
Results
Biochemical cardiotoxicity
markers
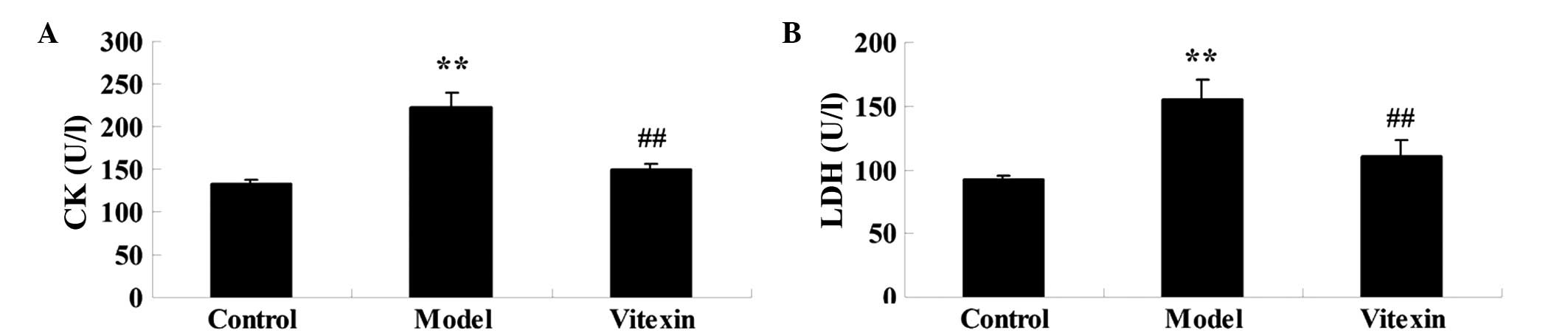
Serum markers indicating myocardial injury, LDH and
CK-MB activities were assessed. As shown in Fig. 2, the activities of LDH and CK-MB were
significantly increased in the serum of the DOX group, as compared
with the control group. Pretreatment with vitexin resulted in a
significant reduction in serum levels, as compared with the DOX
group. Rats in the vitexin group did not exhibit any significant
changes in LDH and CK-MB levels, as compared with the control
group.
Effect of vitexin on inflammatory
cytokines
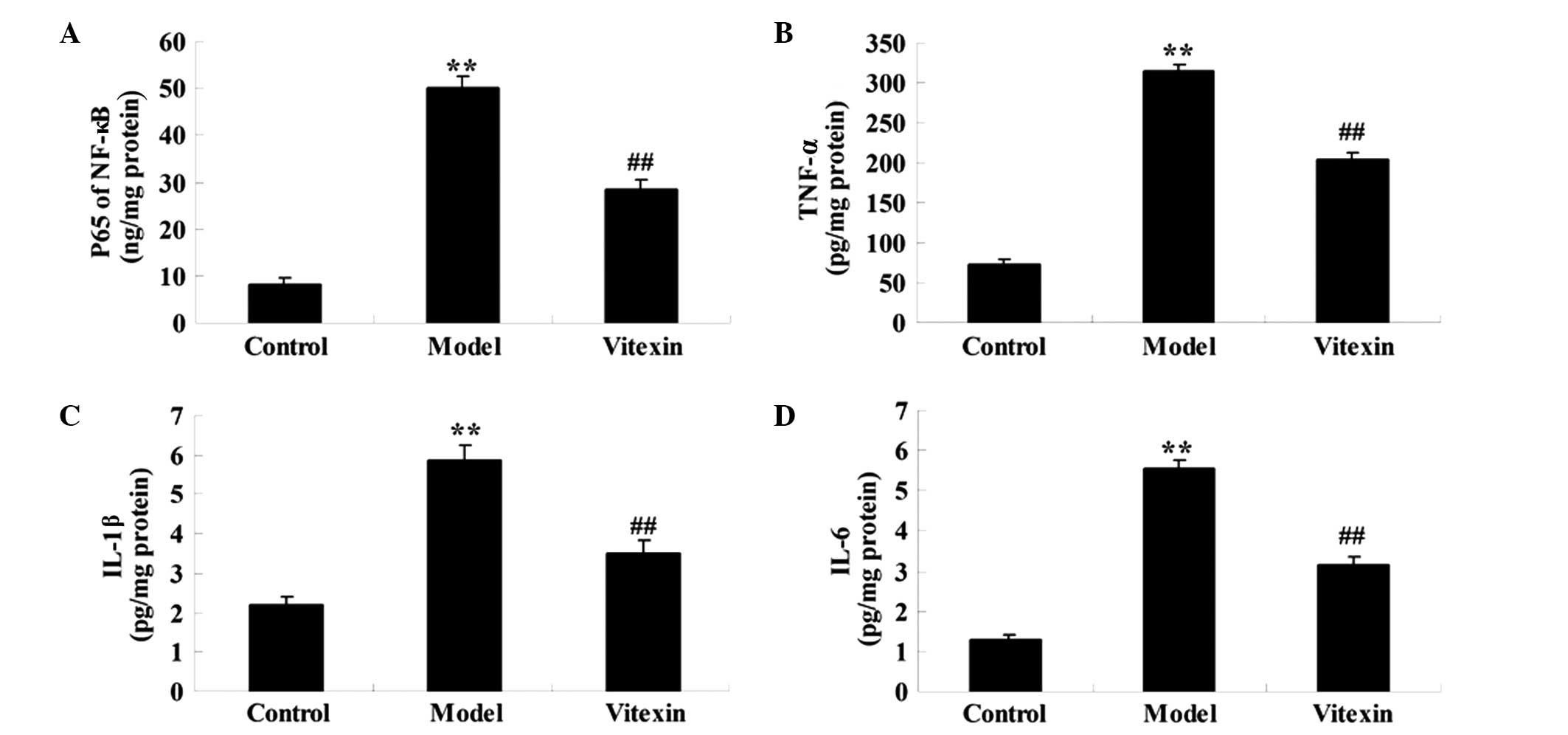
The concentration levels of TNF-α, IL-1β, IL-6 and
NF-κB in the blood represent proinflammatory mediators, which are
thought to have important roles in the development of ischemic
heart failure (21). As shown in
Fig. 3, TNF-α, IL-1β, IL-6 and NF-κB
levels markedly increased in the model group, as compared with the
control group, whereas these levels were decreased in the vitexin
group.
Oxidative stress markers and
antioxidant enzymes
As shown in Fig. 4A,
MDA levels significantly increased in the model group, as compared
with the control group (P<0.01), and vitexin significantly
inhibited MDA levels as compared with the model group (P<0.01).
As shown in Fig. 4B and C,
assessment of the myocardial antioxidant enzymatic profile of rats
in the DOX group demonstrated a significant reduction in CAT and
SOD activities, as compared with the control group (P<0.01).
Pretreatment with vitexin significantly restored CAT and SOD
activities, as compared with the DOX group (P<0.01). As shown in
Fig. 4D, MPO activity significantly
increased in the model group, as compared with the control group
(P<0.05); however, MPO activity was significantly deceased
following vitexin treatment. These results indicated that vitexin
may protect heart function by inhibiting oxidative stress.
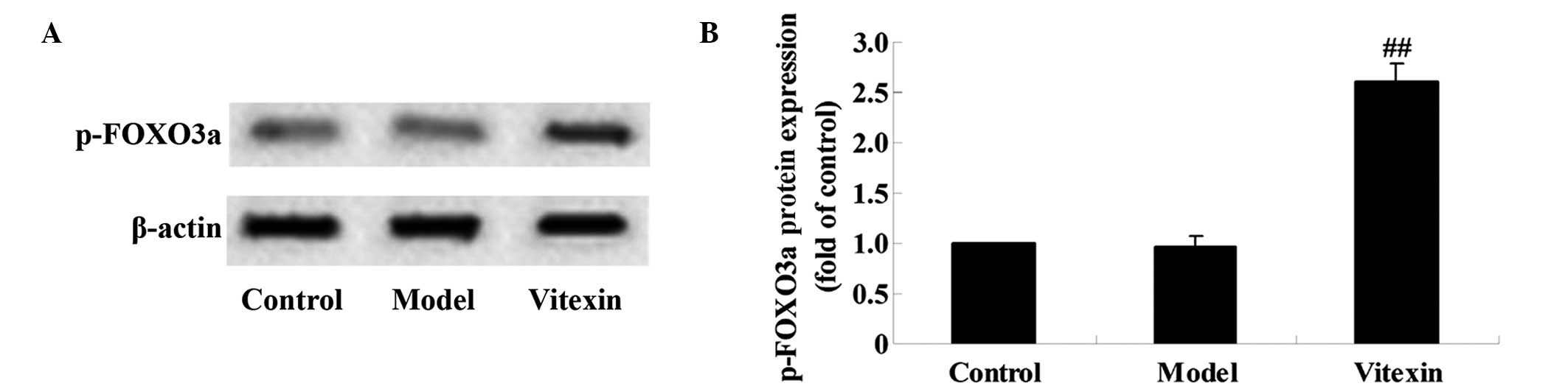
Effect of vitexin on FOXO3a
To investigate whether vitexin is able to modulate
the expression of p-FOXO3a protein, western blot analysis was
performed. The intensity measurement for proteins was determined
according to the ratio of the integrated intensity of the p-FOXO3a
band to the integrated intensity of the β-actin band in the same
sample. As shown in Fig. 5A and B,
there was no significant difference between the model and control
groups; however, the expression levels of p-FOXO3a in the vitexin
group were significantly increased compared with the control and
model groups (P<0.01). These results indicated that the
protective effect induced by vitexin against DOX-induced cardiac
failure may be associated with p-FOXO3a protein expression.
Apoptotic markers
As shown in Fig. 6,
caspase-3 activation significantly increased in the model group, as
compared with the control group (P<0.01); however, these
increased caspase-3 levels were significantly reduced by
pretreatment with vitexin, as compared with the DOX model group
(P<0.01). This result indicated that the protective effect
induced by vitexin against DOX-induced cardiac failure may be
associated with an anti-apoptotic mechanism.
Discussion
Doxorubicin (DOX) is an effective chemotherapeutic
agent that is frequently used to treat various malignancies.
However, its clinical use is hampered by the development of
cardiotoxicity. It has previously been demonstrated that
DOX-induced cardiotoxicity occurs through mechanisms other than
those that mediate its antitumor effect (22). The present study aimed to investigate
the potential cardioprotective effect of vitexin against
DOX-induced cardiotoxicity in rats and the underlying mechanisms.
The present findings indicated that pretreatment with vitexin prior
to treatment with DOX for 4 weeks improved the cardiac function of
rats, which included a decrease of LDH and CK-MB in serum. Notably,
the preservation of heart function was demonstrated to be
associated with a decrease in oxidative stress and the apoptosis in
cardiomyocytes as well as a decrease in inflammatory cytokines
levels.
DOX-induced heart failure is characterized by the
generation of free radicals in the cardiac tissue (22,23). The
present data demonstrated that the activity of SOD and CAT were
significantly decreased in the DOX group and the co-treatment of
vitexin increased SOD and CAT activity. MDA is a lipid peroxidation
marker that is used to assess lipid peroxidation due to increased
oxidative stress (24). In the
present study, blood levels of MDA were markedly increased in the
DOX group, and this was significantly reversed by pretreatment with
vitexin. MPO is an enzyme that is predominantly located in the
primary granules of neutrophils and its main function is to kill
microorganisms; however, under certain conditions, it produces
excess oxidant, which leads tissue damage (25). In the present study, MPO activity
significantly increased after DOX administration. In contrast,
pretreatment with vitexin significantly decreased MPO activity and
reduced neutrophil infiltration. These findings suggested that
vitexin is a potential antioxidant molecule that may be used to
protect the heart from DOX-induced failure.
Oxidative stress can trigger inflammatory cascades,
which are primarily mediated via NF-κB (26,27).
NF-κB is a key transcription factor that regulates inflammatory
processes (28). Various studies
have reported that NF-κB is involved in the pathogenesis of heart
failure (29,30). Activation of NF-κB induces the
activation of genetic programs that lead to the transactivation of
cytokines and chemokines. The present data demonstrated that
inflammatory cytokines levels increased in the DOX group, as
compared with the control group; whereas these levels decreased in
the vitexin group, as compared with the DOX group.
Oxidative stress evoked by DOX results in the
apoptotic death of cardiomyocytes (31,32)
through various signaling pathways, including the activation of
caspase-3 (33). The present
findings demonstrated that caspase-3 activation increased in the
model group, as compared with the control group; however, these
increased caspase-3 levels were significantly reduced by
pretreatment with vitexin, as compared with the DOX group. These
findings indicated that vitexin pretreatment may protect the heart
by decreasing the apoptotic rate of cardiomyocytes in response to
DOX.
FOXO3a has an important role in the mechanisms which
protect cells from oxidative stress-induced cell death. FOXO3a is
regulated by a number of signaling pathways, including ERK, Akt,
IκB kinase, and serum glucocorticoid-related kinases (7). FOXO3a regulates the levels of
antioxidant enzymes, including MnSOD (34). The results of the present study
demonstrated that there were no significant differences between the
model and control groups; however, the expression levels of
p-FOXO3a in the vitexin group increased, as compared with the
control and model groups. These results indicate that the
protection against DOX-induced cardiotoxicity may be associated
with p-FOXO3a protein expression.
In conclusion, these results demonstrated that
vitexin may be an effective therapeutic agent against DOX-induced
cardiotoxicity. The mechanisms investigated included the
attenuation of oxidative stress, reducing cardiac inflammatory
cytokines, increased FOXO3a, and inhibition of caspase-3
activation. We propose that vitexin may be used as an effective
therapeutic agent to prevent DOX-induced cardiomyopathy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560078), China
Postdoctoral Science Foundation (grant no. 2013M532102), and
Scientific Research Foundation for Doctors at Xinjiang Medical
University (grant no. 201006)
References
|
1
|
Poprach A, Petrakova K, Vyskoýil J, Lakomý
R, Nċmeýek R, Kocak I, Kocakova I and Vyzula R: Cardiotoxicity of
drugs used in oncology. Klinicka Onkologie: Casopis Ceske a
Slovenske Onkologicke Spolecnosti. 21:288–293. 2008.(In Czech).
PubMed/NCBI
|
|
2
|
Jain D: Cardiotoxicity of doxorubicin and
other anthracycline derivatives. J Nucl Cardiol. 7:53–62. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan MA, Singh M, Khan MS, Ahmad W, Najmi
AK and Ahmad S: Alternative approach for mitigation of
doxorubicin-induced cardiotoxicity using herbal agents. Curr Clin
Pharmacol. 9:288–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagai K, Fukuno S, Oda A and Konishi H:
Protective effects of taurine on doxorubicin-induced acute
hepatotoxicity through suppression of oxidative stress and
apoptotic responses. Anticancer Drugs. 27:17–23. 2015. View Article : Google Scholar
|
|
5
|
Wang S, Kotamraju S, Konorev E, Kalivendi
S, Joseph J and Kalyanaraman B: Activation of nuclear factor-kappaB
during doxorubicin-induced apoptosis in endothelial cells and
myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem
J. 367:729–740. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalyanaraman B, Joseph J, Kalivendi S,
Wang S, Konorev E and Kotamraju S: Doxorubicin-induced apoptosis:
Implications in cardiotoxicity. Mol Cell Biochem. 234-235:119–124.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno M, Kakinuma Y, Yuhki K, Murakoshi N,
Iemitsu M, Miyauchi T and Yamaguchi I: Doxorubicin induces
apoptosis by activation of caspase-3 in cultured cardiomyocytes in
vitro and rat cardiac ventricles in vivo. J Pharmacol Sci.
101:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Horst A and Burgering BM:
Stressing the role of FoxO proteins in lifespan and disease. Nat
Rev Mol Cell Biol. 8:440–450. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding
Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, et al: ERK promotes
tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation.
Nat Cell Biol. 10:138–148. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang W, Dolloff NG and El-Deiry WS: ERK
and MDM2 prey on FOXO3a. Nat Cell Biol. 10:125–126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kops GJ, Dansen TB, Polderman PE, Saarloos
I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH and Burgering
BM: Forkhead transcription factor FOXO3a protects quiescent cells
from oxidative stress. Nature. 419:316–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marinkovic D, Zhang X, Yalcin S, Luciano
JP, Brugnara C, Huber T and Ghaffari S: Foxo3 is required for the
regulation of oxidative stress in erythropoiesis. J Clin Invest.
117:2133–2144. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zick SM, Vautaw BM, Gillespie B and
Aaronson KD: Hawthorn Extract Randomized Blinded Chronic Heart
Failure (HERB CHF) trial. Eur J Heart Fail. 11:990–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XS, Hu XC, Chen GL, Yuan X, Yang RN,
Liang S, Ren J, Sun JC, Kong GQ, Gao SG and Feng XS: Effects of
vitexin on the pharmacokinetics and mRNA expression of CYP isozymes
in rats. Phytother Res. 29:366–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Je HG, Hong SM, Je HD, Choi YS, Seo SY,
Min YS, Chung SJ, Shin YK, Lee TJ, Park ES and Jeong JH: The
inhibitory effect of vitexin on the agonist-induced regulation of
vascular contractility. Pharmazie. 69:224–228. 2014.PubMed/NCBI
|
|
17
|
Tassell MC, Kingston R, Gilroy D, Lehane M
and Furey A: Hawthorn (Crataegus spp.) in the treatment of
cardiovascular disease. Pharmacogn Rev. 4:32–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue HF, Ying ZM, Zhang WJ, Meng YH, Ying
XX and Kang TG: Hepatic, gastric, and intestinal first-pass effects
of vitexin in rats. Pharm Biol. 52:967–971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu CC, Xu YQ, Wu JC, Hang PZ, Wang Y, Wang
C, Wu JW, Qi JC, Zhang Y and Du ZM: Vitexin protects against
cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling
pathways. Naunyn-Schmiedeberg Arch Pharmacol. 386:747–755. 2013.
View Article : Google Scholar
|
|
20
|
Flohe L and Otting F: Superoxide dismutase
assays. Methods Enzymol. 105:93–104. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma HS and Das DK: Role of cytokines in
myocardial ischemia and reperfusion. Mediators Inflamm. 6:175–183.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
El-Sayed EM, Mansour AM and Abdul-Hameed
MS: Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity
by abrogation of oxidative stress, inflammation, and apoptosis in
rats. J Biochem Mol Toxicol. 30:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Xu Y, Hua S, Zhou S and Wang K:
ALDH2 attenuates Dox-induced cardiotoxicity by inhibiting cardiac
apoptosis and oxidative stress. Int J Clin Exp Med. 8:6794–6803.
2015.PubMed/NCBI
|
|
24
|
Torun AN, Kulaksizoglu S, Kulaksizoglu M,
Pamuk BO, Isbilen E and Tutuncu NB: Serum total antioxidant status
and lipid peroxidation marker malondialdehyde levels in overt and
subclinical hypothyroidism. Clin Endocrinol (Oxf). 70:469–474.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Z, Ji W, Fu Q and Ma S: Formononetin
inhibited the inflammation of LPS-induced acute lung injury in mice
associated with induction of PPAR gamma expression. Inflammation.
36:1560–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Munoz A and Costa M: Nutritionally
mediated oxidative stress and inflammation. Oxid Med Cell Longev.
6109502013.PubMed/NCBI
|
|
27
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frantz S, Fraccarollo D, Wagner H, Behr
TM, Jung P, Angermann CE, Ertl G and Bauersachs J: Sustained
activation of nuclear factor kappa B and activator protein 1 in
chronic heart failure. Cardiovascular Res. 57:749–756. 2003.
View Article : Google Scholar
|
|
30
|
Van der Heiden K, Cuhlmann S, le Luong A,
Zakkar M and Evans PC: Role of nuclear factor kappaB in
cardiovascular health and disease. Clinical Sci. 118:593–605. 2010.
View Article : Google Scholar
|
|
31
|
Chen CT, Wang ZH, Hsu CC, Lin HH and Chen
JH: In vivo protective effects of diosgenin against
doxorubicin-induced cardiotoxicity. Nutrients. 7:4938–4954. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gulimire A, Ybadaiti T, Rena K and Ting
FW: Protective effect of total flavonoids of H. rhamnoides
L. sunsp. Turkestanica rousi against adriamycin-induced
cardiotoxicity in rats. Xin Jiang Yi Ke Da Xue Xue Bao. 33:383–385.
2010.
|
|
33
|
Dash SK, Chattopadhyay S, Ghosh T, Dash
SS, Tripathy S, Das B, Bag BG, Das D and Roy S: Self-assembled
betulinic acid protects doxorubicin induced apoptosis followed by
reduction of ROS-TNF-alpha-caspase-3 activity. Biomed Pharmacother.
72:144–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim AD, Kang KA, Piao MJ, Kim KC, Zheng J,
Yao CW, Cha JW, Hyun CL, Kang HK, Lee NH and Hyun JW:
Cytoprotective effect of eckol against oxidative stress-induced
mitochondrial dysfunction: involvement of the FoxO3a/AMPK pathway.
J Cell Biochem. 115:1403–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|