Introduction
Hypertension causes renal injury and is a major
factor inducing progressive organ damage in end-stage renal disease
(1). Sympathetic nerve activity
(SNA) is increased in patients with chronic kidney disease and has
an important and distinct role in renal disease-associated
hypertension (2,3). A long-standing hypothesis proposes that
neurogenic hypertension results from alterations to renal function
via the actions of renal nerves on renal vascular resistance,
tubular sodium reabsorption and renin release (4,5).
Catheter-based renal denervation (RD) has been introduced into
clinical practice to selectively denervate efferent and afferent
renal sympathetic fibers (6–8). Other than resulting in marked and
sustained blood pressure reductions, RD has also been demonstrated
to reduce renal resistive index, and the incidence of albuminuria
without adversely affecting glomerular filtration rate or renal
artery structure (9).
The kidneys are critical to the regulation of blood
pressure via modulation of sodium and water excretion (10). One mechanism by which the kidneys are
posited to maintain fluid homeostasis is using the renal
sympathetic nerves (11). RD is
widely reported to cause an increase in sodium, potassium and water
excretion in several mammalian species (12–15).
However, controversy remains regarding the immediate changes to
renal excretory functions following RD (15,16). No
appreciable differences were observed by Salman et al in the
mean arterial pressure (MAP) and plasma sodium (PNa) between
denervated and innervated SD rats (15,16).
In the present study, the changes to renal function
and renal sodium/potassium handling were investigated in
spontaneously hypertensive rats (SHR) subjected to RD, with the aim
of functionally characterizing the sympathetic nerve control of the
kidney. The current study addressed this aim using a correlation of
renal sodium/potassium excretions and renal function by combining
surgical and chemical procedures to achieve RD.
Materials and methods
Animals
All experimental procedures conformed to the Animal
Ethics Committee guidelines of Guangxi Medical University (Nanning,
China), and received committee approval. Twelve-week-old male SHR
(n=16 animals) and age-matched Wistar Kyoto (WKY) rats (n=8
animals) were obtained from Vital River Laboratories Co., Ltd.
(Beijing, China). The animals were housed in controlled
environmental conditions consisting of a 12-h light/dark cycle and
22 ± 2°C. All animals were fed ad libitum throughout the
study with standard rat chow (Vital River Laboratories Co., Ltd.)
for at least seven days before the study. Following an acclimation
period of two weeks, SHR were randomly assigned to the renal
denervated (RDNX; n=8) or sham (n=8) groups.
Surgical preparation of animal and
experimental protocol
Rats were fasted overnight prior to the surgery.
Briefly, anesthesia was induced using pentobarbital sodium at a
dose of 50 mg/kg (intraperitoneally; Sigma-Aldrich, St. Louis, MO,
USA), and the kidneys were exposed through midline abdominal
incisions and surgically denervated with the aid of a
stereomicroscope (Shanghai Third Optical Instrument Factory,
Shanghai, China). Denervation was accomplished by incising all
visible nerves along the renal artery, and the renal vessels were
surrounded with cotton swabs previously soaked in 10% (v/v) phenol
solution (Shanghai Biological Engineering Co., Ltd., Shanghai,
China) for 2 min. Sham RD (sham group) treatment entailed identical
anesthetic and surgical procedures, but the renal nerves were left
intact. After two weeks, tail arterial pressure was estimated by
the tail-cuff method (17), and
urine was collected over 24 h. Under anesthesia with 200 mg/kg
ketamine (intramuscular injection; Jiang Su Heng Rui Medicine Co.,
Ltd., Suzhou, China), blood was drawn via abdominal aortic
puncture, and the kidneys were removed.
Serum and urine lithium concentrations were measured
with an AA-7000 atomic absorption spectrometer (Shimadzu, Kyoto,
Japan), while serum and urine sodium, potassium, protein and
creatinine concentrations were determined by spectrophotometer
(Roche Diagnostics, Basel, Switzerland). To confirm that the
chemical RD was achieved in the rats, renal tissue and serum
noradrenaline (NE) content was analyzed in each experimental group
using high-performance liquid chromatography (HPLC) with
electrochemical detection (ECD) (LC-20A; Shimadzu).
Calculations
Urine flow rate (UFR) was calculated by the
following formula: UFR (µl/min/kg) = UV/T × BW, in which UV (µl) is
the urine volume, T (min) is the time and BW (kg) is the body
weight of the rat (16).
The clearances were calculated using the usual
formula: Cx = Ux × UFR/Sx, in which Cx is the clearance of
substance ‘x’, Ux is the urine concentration of ‘x’ and Sx is the
serum concentration of ‘x’. Glomerular filtration rate (Ccr) was
considered to indicate the clearance of creatinine. The fractional
excretion of ‘x’ (FEx) was calculated as: Cx/Ccr. The fractional
distal reabsorption rate of sodium was calculated by the following
formula: FDRNa =
[(FELi-FENa)/FELi]x100.
FELi and FDRNa were calculated as markers of
proximal and distal sodium handling, respectively (18). FELi = ULi ×
Scr/SLi x Ucr. Serum and urine sodium,
potassium, protein and creatinine concentrations were determined
using a spectrophotometer (Roche Diagnostics) and lithium
concentration by inductively coupled plasma mass spectrometry
(7500CE; Agilent Technologies, Inc., Santa Clara, CA, USA).
Histological examination
Kidney tissues (coronal slices) were fixed with 10%
paraformaldehyde and embedded in paraffin (Shanghai Biological
Engineering Co., Ltd.). Coronal sections of the kidney (4-µm-thick)
were stained with periodic acid-Schiff and Masson-trichrome stains
(Fuzhou Maixin Biotech, Co., Ltd., Fuzhou, China), and examined
blind using a DP72 brightfield microscope (Olympus Corporation,
Tokyo, Japan) to assess glomerular and arterial morphology.
Histological scores were assessed using Image-Pro Plus version 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA). Glomerular
injury score was calculated as previously described (19,20). At
least 50 glomeruli were randomly selected in each rat and the mean
glomerular injury score was calculated. The severity of
tubulointerstitial injury was evaluated by the interstitial
fibrosis (IF) score, as described previously (19,20). The
percentage of interstitial fibrotic areas per cortical field
(magnification, ×100) was calculated, and the mean percentage from
10 randomly selected fields of view was determined as the IF score
for each rat. The medial thickness-to-lumen ratio was calculated as
described previously (21). For
this, 5 regions of the interlobular artery from each rat were
evaluated, and the average ratio was calculated.
Statistical analysis
Values are presented as mean ± standard error.
Statistical analysis was performed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). Comparisons between multiple groups were
evaluated using one-way analysis of variance followed by the
Dunnett test. P<0.05 was considered to represent a statistically
significant difference.
Results
General observations
The body weights of the rats were similar in all
three groups (RDNX group, 231±15 g; sham group, 238±13 g; and WKY
group, 229±16 g). RD was confirmed by assessment of renal tissue
and serum NE content (Table I). As
Table I shows that serum NE content
was significantly lower in RDNX compared with the sham group
(P<0.05), and did not differ between the RDNX and WKY groups.
The kidney NE content was significantly lower in the RDNX group
compared with the sham group (P<0.05), and did not differ
between the RDNX and WKY groups. No significant differences in
serum sodium, potassium or creatinine, and serum or urine protein
concentrations were observed among the three groups. There was no
observably significant difference in Ccr among the three groups.
The kidney weight/body weight ratio of the rats was not affected by
bilateral renal denervation (RDNX group, 3.515±0.14 g/kg; sham
group, 3.64±0.29 g/kg; and WKY group, 3.5ww6±0.23 g/kg).
 | Table I.Parameters of renal function and
sodium handling in rats. |
Table I.
Parameters of renal function and
sodium handling in rats.
| Parameter | RDNX | Sham | WKY | P-value |
|---|
| Kw/Bw, g/kg |
3.515±0.14 |
3.64±0.29 |
3.56±0.23 | 0.31 |
| MAP, mmHg |
96±7a |
131±10 |
89±8a | <0.05 |
| S-NE, ng/ml |
14.02±2.37a |
23.04±8.77 |
13.41±3.95a | <0.05 |
| K-NE, ng/mg |
0.95±0.21a |
1.35±0.18 |
1.01±0.24a | <0.05 |
| S-Na, mmol/l |
142.78±1.09 |
142.12±1.36 |
141.43±1.22 | 0.67 |
| S-K, mmol/l |
4.56±0.29 |
4.88±0.32 |
4.51±0.24 | 0.17 |
| S-Cr, mmol/l |
30.78±3.96 |
32.88±4.09 |
34.00±3.21 | 0.26 |
| S-Pro, g/l |
55.16±7.68 |
57.19±5.31 |
60.57±8.60 | 0.23 |
| U-Pro, mg/24 h |
2.35±0.42 |
2.35±0.74 |
2.07±0.47 | 0.54 |
| FEK,
% |
47.04±4.80 |
52.77±4.78 |
45.75±6.41 | 0.19 |
| FELi,
% |
18.13±2.21a |
15.24±1.78 |
17.35±2.17a | 0.03 |
| CNa,
µl/min |
5.39±1.83a |
3.63±1.27 |
5.59±1.97a | 0.04 |
| FDRNa,
% |
94.55±9.33 |
94.21±7.01 |
93.97±10.65 | 0.08 |
| Ccr, ml/min·kg |
0.48±0.13 |
0.45±0.15 |
0.52±0.14 | 0.60 |
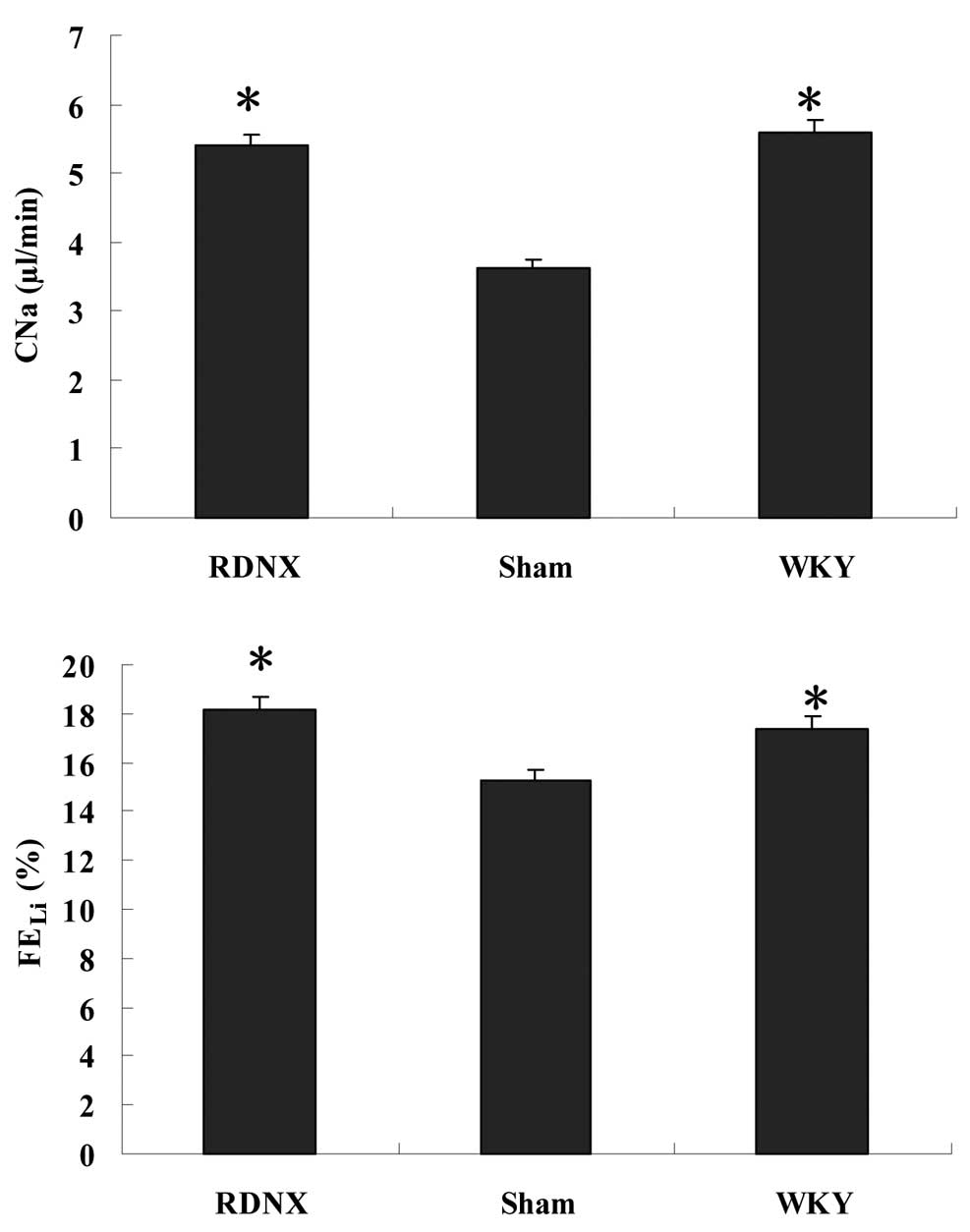
Effects of RD on renal
sodium/potassium excretions
FEK was lower in the RDNX group compared
with the sham group, but no significant difference in
FEK was found among the three groups (Table I). RD generated a significantly
(P<0.05) higher sodium clearance (CNa) compared with
the sham group (Fig. 1). Sham and
WKY groups showed significant differences for FEK and
CNa. Furthermore, the FELi was significantly (P<0.05)
higher in the RDNX group compared with the sham group.
FEK, CNa and FELi exhibited no
significant differences between the RDNX and WKY groups. In
contrast with these observations, RD did not significantly alter
FDRNa (P>0.05) in SHRs compared with the sham
group.
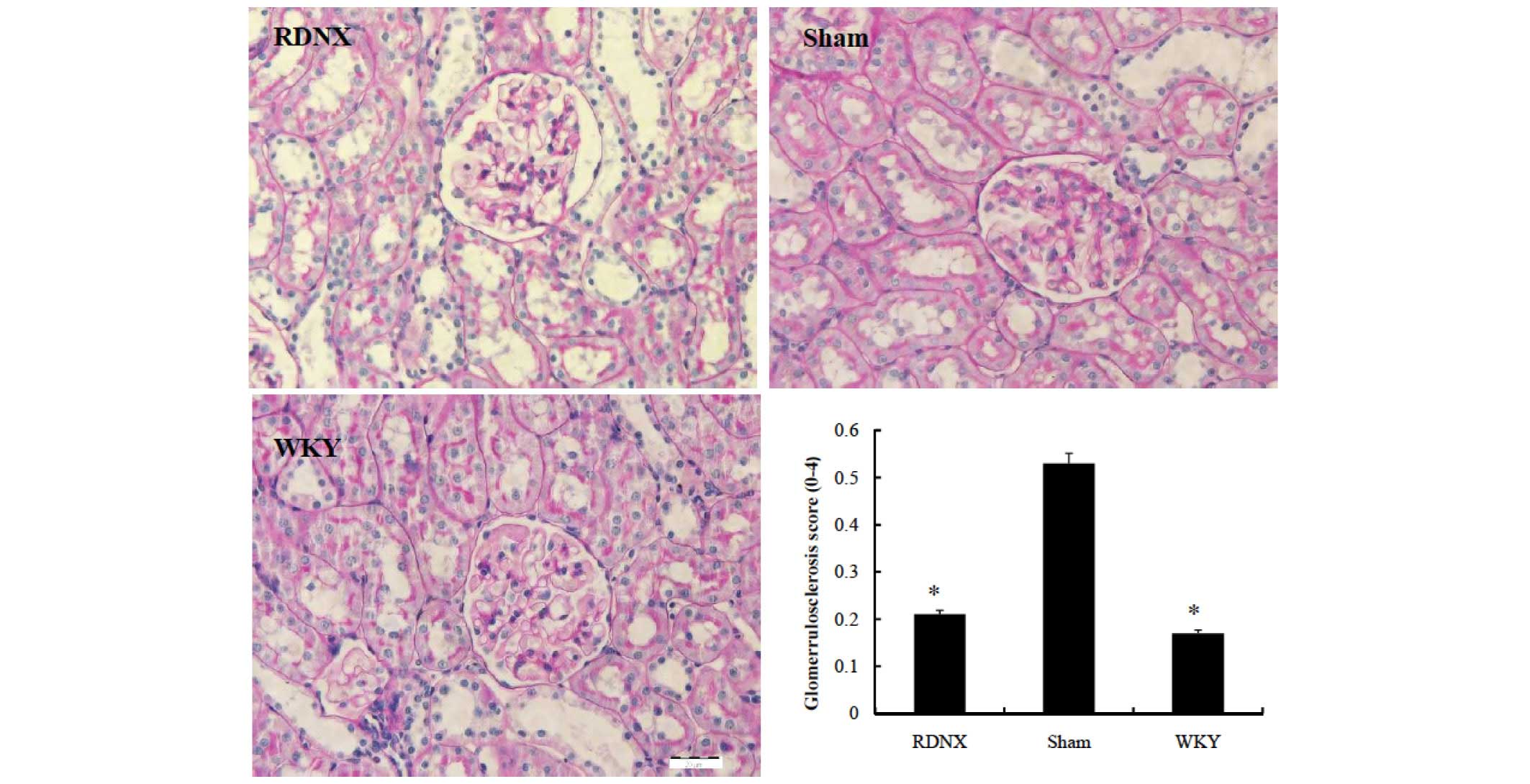
Effects of RD on renal
histopathology
Less glomerular morphological change, assessed by
the glomerular injury score, was noted in the RDNX group compared
with the sham group (Fig. 2). The
glomerular injury score was lower in the RDNX group than in the
sham group (2.5±0.1 vs. 0.7±0.2, respectively; P<0.05). To
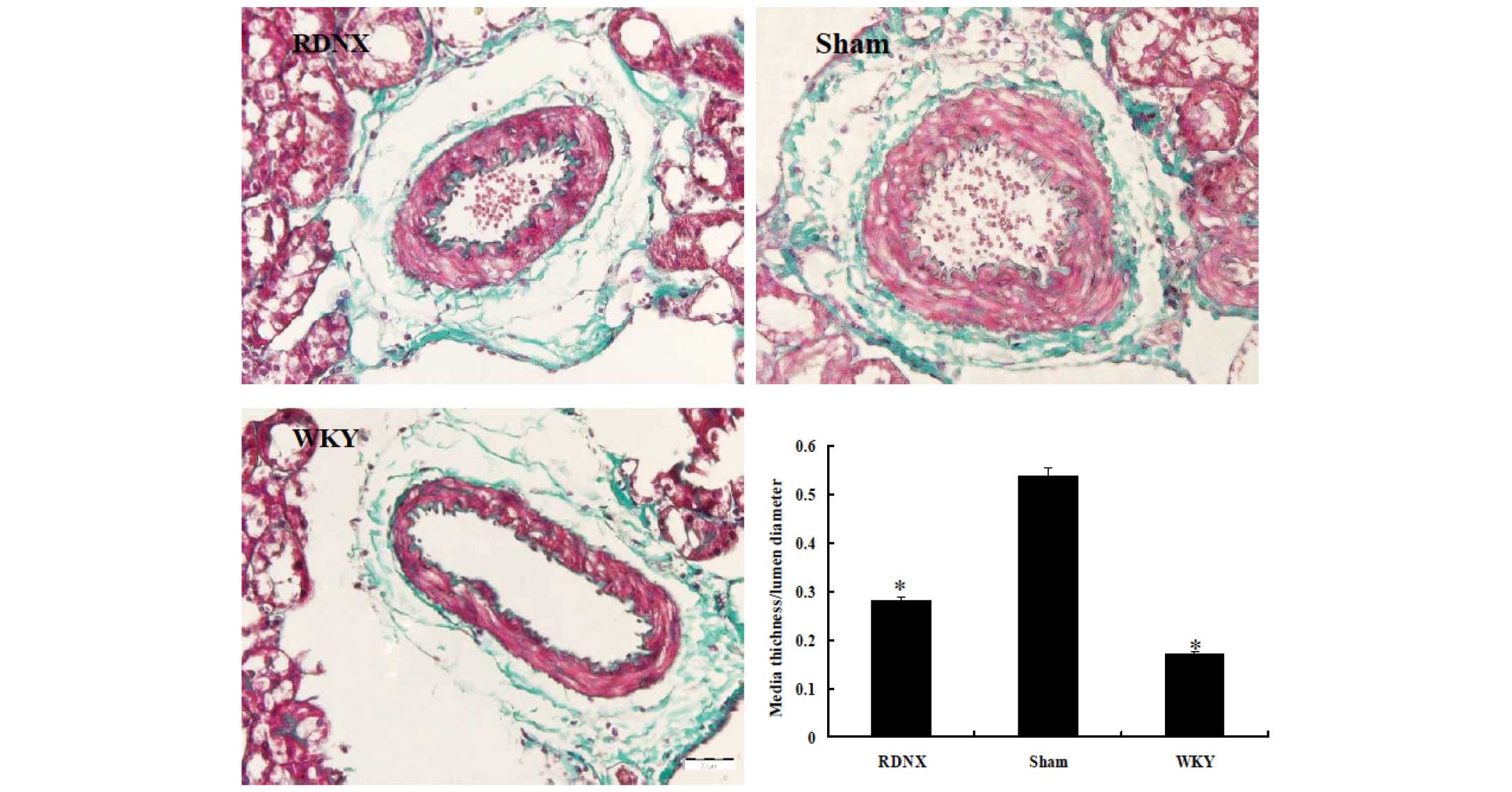
assess changes to the interlobular artery, a parameter of
hypertension-related vascular damage, the wall-to-lumen ratio was
examined. The medial thickness-to-lumen diameter ratio was higher
in the sham group than in the WKY group, indicating that this had
been caused by sham treatment (Fig.
3). RD significantly decreased the ratio to a similar level as
that of the WKY group. The severity of tubulointerstitial injury
was evaluated by IF score, revealing no significant difference in
IF score among the three groups (Fig.
4).
Discussion
Renal sympathetic nerves utilize NE as a
neurotransmitter, which affects the renal arterioles,
juxtaglomerular granular cells and tubules of the kidney (22). Using a rat model of genetic
hypertension, several manifestations of renal sodium excretion,
glomerular and arterial morphological structure was reported in the
present study to be substantially alleviated when RD is performed
by chemical sympathectomy.
In the current study, SHRs were selected due to
previous evidence of increased renal sympathetic discharge in this
hypertension model (23). The kidney
has an important role in the regulation of blood pressure via
modulation of sodium and water excretion (10). A previous study of SHRs suggested
that an impaired pressure-diuresis relationship exists in these
animals, such that greater perfusion pressures are required to
achieve the same level of diuresis when compared with WKY rats
(24). Furthermore, a previous study
using isolated perfused kidneys from SHRs revealed an intrinsic
renal abnormality in sodium excretion that may contribute to the
maintenance of hypertension in SHR (25). Renal sympathetic nerves and
circulating catecholamine are involved in the regulation of sodium
and water excretion in the kidney (26). In the present study, a significant
change to sodium excretion was observed following RD. Higher
CNa was observed in the denervated SHRs compared with
rats with intact renal nerves. The observed natriuresis following
RD has been reported in several studies on acute and chronic RD of
different experimental hypertensive rats (21,27,28).
Together, these and previous results implicated renal sympathetic
activity in sodium regulation (16).
It has previously been revealed that NE released
from renal sympathetic nerve endings acts on the basolateral
membranes of epithelial cells to stimulate tubular sodium and water
reabsorption at the proximal tubule, thick ascending limb of the
loop of Henle and the distal nephron (29). Electric stimulation of the renal
nerves in acute experiments has been demonstrated to enhance sodium
reabsorption, particularly in the proximal convoluted tubule.
Low-frequency renal nerve stimulation directly affects proximal
tubular sodium reabsorption and rennin release in the absence of
changes to renal hemodynamics (30).
Furthermore, several previous studies indicated that RD results in
an increased urine flow rate that is attributed to a decreased
absolute and fractional reabsorption of sodium in the proximal
convoluted tubule (31,32). Conversely, several previous studies
have reported that bilateral RD in 3- to 8-week-old SHRs delays the
development of hypertension, associated with reduced sodium
reabsorption by the proximal tubule, the loop of Henle and the
distal convolution (33–35). In the present study, following
bilateral RD, FELi was elevated when compared with the
sham-operated group, consistent with the withdrawal of sympathetic
stimulation. While the results of the present study indicated no
significant effect of RD on FDRNa, it may be that RD
results in decreased sodium reabsorption by the proximal convoluted
tubule, but not the distal convoluted tubule.
The renal mechanisms of potassium excretion have
varied upon the application of a diversity of techniques including
renal clearance, micropuncture, microperfusion and
electrophysiological studies (15).
However, a paucity of information exists on the role of the
adrenergic mechanisms in the regulation of renal potassium
reabsorption and secretion. It is established that potassium freely
diffuses in the renal corpuscular membrane, and the majority of its
filtered load is reabsorbed by proximal tubular epithelial cells.
However, potassium excretion in the urine depends on controlled
secretion in the distal nephron (36). Salman et al (15) demonstrated that RD in Sprague Dawley
rats caused a significantly higher renal potassium excretion in
absolute terms and as a fraction of the filtered load. However, the
present study did not report significant changes to plasma
potassium levels and urinary potassium excretion, suggesting that
decreased renal sympathetic nerve activity of SHR has a more direct
tubule natriuretic effect in the proximal segment than at the
sodium-potassium exchange site.
Hypertension is an established consequence of
chronic renal disease, and is often observed in patients with focal
segmental glomerulosclerosis and with membranoproliferative
glomerulonephritis (37).
Furthermore, several lines of evidence suggest that sympathetic
overactivity, through functional and morphological alterations to
renal physiology and structure, may contribute to kidney injury and
chronic kidney disease progression (38). In the present study, renal
sympathetic denervation aided amelioration of glomerular sclerosis
in SHR. To assess changes to the small arteries, a parameter of
hypertension-related vascular damage, the wall-to-lumen ratio was
examined. The current data indicated that RD not only reverses
glomerular sclerosis, but also greatly enlarges the lumen size of
the interlobular artery in these genetically hypertensive rats.
There is much experimental evidence and clinical data to indicate
that drugs that reduce SNA have a renoprotective effect.
Moxonidine, which decreases sympathetic nerve activity, was
revealed to have renoprotective effects in patients, in addition to
in experimental rats with renal failure (39–41).
Furthermore, catheter-based RD selectively targets efferent and
afferent renal nerves and functionally denervates the kidney,
reducing blood pressure in clinical trials (38), and provide renoprotection in diabetic
and Dahl salt-sensitive rats by ameliorating the effects of
excessive renal sympathetic signals (3,42).
Together, these observations confirm that RD considerably improves
glomerular sclerosis and hypertension-associated renal vascular
damage, which indicates a role of the overactive sympathetic
nervous system in this pathophysiological state.
In conclusion, the present data demonstrate that
renal nerves are significantly involved in the regulation of renal
tubular sodium reabsorption in SHR. Furthermore, if the kidney is
prevented from sympathetic nerve stimulation, structural changes
due to early stage hypertensive nephropathy, namely glomerular
sclerosis and vascular damage, are abolished.
Acknowledgements
The present study was supported by research funding
from Guangxi Provincial Education Office (grant no. 201202ZD025),
the Opening Project of the Science Experiment Center, Guangxi
(grant no. KFJJ2011-35) and the National Natural Science Foundation
of Guangxi (grant no. 2012GXNSFAA239004).
References
|
1
|
Bakris GL, Williams M, Dworkin L, Elliott
WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W and Sowers J:
Preserving renal function in adults with hypertension and diabetes:
A consensus approach. National kidney foundation hypertension and
diabetes executive committees working group. Am J Kidney Dis.
36:646–661. 2000. View Article : Google Scholar
|
|
2
|
Neumann J, Ligtenberg G, Klein II, Koomans
HA and Blankestijn PJ: Sympathetic hyperactivity in chronic kidney
disease: Pathogenesis, clinical relevance and treatment. Kidney
Int. 65:1568–1576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagasu H, Satoh M, Kuwabara A, Yorimitsu
D, Sakuta T, Tomita N and Kashihara N: Renal denervation reduces
glomerular injury by suppressing NAD(P)H oxidase activity in dahl
salt-sensitive rats. Nephrol Dial Transplant. 25:2889–2898. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiBona GF and Kopp UC: Neural control of
renal function. Physiol Rev. 77:77–197. 1997.
|
|
5
|
Guyton AC, Coleman TG, Cowley AW, Scheel
KW, Manning RD and Norman RA: Arterial pressure regulation:
Overriding dominance of the kidneys in long-term control and in
hypertension. Am J Med. 52:584–594. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krum H, Schlaich M, Whitbourn R, Sobotka
PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar
S, et al: Catheter-based renal sympathetic denervation for
resistant hypertension: A multicentre safety and proof-of-principle
cohort study. Lancet. 373:1275–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esler MD, Krum H, Sobotka PA, Schlaich MP,
Schmieder RE and Böhm M: Renal sympathetic denervation in patients
with treatment-resistant hypertension (The Symplicity HTN-2 Trial):
A randomised controlled trial. Lancet. 376:1903–1909.
2010.Symplicity HTN-2 Investigators. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krum H, Sobotka P, Mahfoud F, Böhm M,
Esler M and Schlaich M: Device-based antihypertensive therapy:
Therapeutic modulation of the autonomic nervous system.
Circulation. 123:209–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahfoud F, Cremers B, Janker J, Link B,
Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, et
al: Renal hemodynamics and renal function after catheter-based
renal sympathetic denervation in patients with resistant
hypertension. Hypertension. 60:419–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guyton AC: Blood pressure control-special
role of the kidneys and body fluids. Science. 252:1813–1816. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacob F, Ariza P and Osborn JW: Renal
denervation chronically lowers arterial pressure independent of
dietary sodium intake in normal rats. Am J Physiol Heart Circ
Physiol. 284:H2302–H2310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonjour JP, Churchill PC and Malvin RL:
Change of tubular reabsorption of sodium and water after renal
denervation in the dog. J Physiol. 204:571–582. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blake WD and Jurf AN: Renal sodium
reabsorption after acute renal denervation in the rabbit. J
Physiol. 196:65–73. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boer PA, Morelli JM, Figueiredo JF and
Gontijo JA: Early altered renal sodium handling determined by
lithium clearance in spontaneously hypertensive rats (SHR): Role of
renal nerves. Life Sci. 76:1805–1815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salman IM, Sattar MA, Abdullah NA, Ameer
OZ, Basri F, Hussain NM, Yam MF, Swarup KR, Rathore HA, Kazi RN, et
al: Role of renal sympathetic nervous system in the control of
renal potassium handling. J Nephrol. 23:291–296. 2010.PubMed/NCBI
|
|
16
|
Salman IM, Sattar MA, Abdullah NA, Ameer
OZ, Hussain FB, Hye Khan MA, Yam MF, Rathore KR, Kazi RN, Salman HM
and Johns EJ: Renal functional & haemodynamic changes following
acute unilateral renal denervation in sprague dawley rats. Indian J
Med Res. 131:76–82. 2010.PubMed/NCBI
|
|
17
|
Kubota Y, Umegaki K, Kagota S, Tanaka N,
Nakamura K, Kunitomo M and Shinozuka K: Evaluation of blood
pressure measured by tail-cuff methods (without heating) in
spontaneously hypertensive rats. Biol Pharm Bull. 29:1756–17581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou J, Li Y, Yan CH, Wei FF, Zhang L and
Wang JG: Blood pressure in relation to interactions between sodium
dietary intake and renal handling. Hypertension. 62:719–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Namikoshi T, Tomita N, Fujimoto S, Haruna
Y, Ohzeki M, Komai N, Sasaki T, Yoshida A and Kashihara N:
Isohumulones derived from hops ameliorate renal injury via an
anti-oxidative effect in dahl salt-sensitive rats. Hypertens Res.
30:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Namikoshi T, Tomita N, Satoh M, Haruna Y,
Kobayashi S, Komai N, Sasaki T and Kashihara N: Pioglitazone
enhances the antihypertensive and renoprotective effects of
candesartan in zucker obese rats fed a high-protein diet. Hypertens
Res. 31:745–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddi AS and Bollineni JS:
Selenium-deficient diet induces renal oxidative stress and injury
via TGF-beta1 in normal and diabetic rats. Kidney Int.
59:1342–1353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kowalski R, Kreft E, Kasztan M, Jankowski
M and Szczepanska-Konkel M: Chronic renal denervation increases
renal tubular response to P2X receptor agonists in rats:
Implication for renal sympathetic nerve ablation. Nephrol Dial
Transplant. 27:3443–3448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beevers G, Lip GY and O'Brien E: ABC of
hypertension: The pathophysiology of hypertension. BMJ.
322:912–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roman RJ and Cowley AW Jr: Abnormal
pressure-diuresis-natriuresis response in spontaneously
hypertensive rats. Am J Physiol. 248:F199–F205. 1985.PubMed/NCBI
|
|
25
|
Heckmann U, Zidek W and Schurek HJ: Sodium
reabsorption in the isolated perfused kidney of normotensive and
spontaneously hypertensive rats. J Hypertens Suppl. 7:S172–S173.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vieira-Coelho MA and Moura E: Effect of
clonidine on renal sodium handling in spontaneously hypertensive
rats. J Pharmacol Sci. 119:122–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katholi RE, Naftilan AJ, Bishop SP and
Oparil S: Role of the renal nerves in the maintenance of DOCA-salt
hypertension in the rat. Influence on the renal vasculature and
sodium excretion. Hypertension. 5:427–435. 1983.
|
|
28
|
Katayama T, Sueta D, Kataoka K, Hasegawa
Y, Koibuchi N, Toyama K, Uekawa K, Mingjie M, Nakagawa T, Maeda M,
et al: Long-term renal denervation normalizes disrupted blood
pressure circadian rhythm and ameliorates cardiovascular injury in
a rat model of metabolic syndrome. J Am Heart Assoc. 2:e0001972013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Healy V, Thompson C and Johns EJ: The
adrenergic regulation of proximal tubular Na +/H+ exchanger 3 in
the rat. Acta Physiol (Oxf). 210:678–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DiBona GF: Neural control of the kidney:
Functionally specific renal sympathetic nerve fibers. Am J Physiol
Regul Integr Comp Physiol. 279:R1517–R1524. 2000.PubMed/NCBI
|
|
31
|
Greenberg SG, Enders C and Osborn JL:
Renal nerves affect rate of achieving sodium balance in
spontaneously hypertensive rats. Hypertension. 22:1–8. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rogenes PR and Gottschalk CW: Renal
function in conscious rats with chronic unilateral renal
denervation. Am J Physiol. 242:F140–F148. 1982.PubMed/NCBI
|
|
33
|
Rudd MA, Grippo RS and Arendshorst WJ:
Acute renal denervation produces a diuresis and natriuresis in
young SHR but not WKY rats. Am J Physiol. 251:F655–F661.
1986.PubMed/NCBI
|
|
34
|
Oparil S, Sripairojthikoon W and Wyss JM:
The renal afferent nerves in the pathogenesis of hypertension. Can
J Physiol Pharmacol. 65:1548–1558. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kline RL: Renal nerves and experimental
hypertension: Evidence and controversy. Can J Physiol Pharmacol.
65:1540–1547. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giebisch G: Renal potassium transport:
Mechanisms and regulation. Am J Physiol. 274:F817–F833.
1998.PubMed/NCBI
|
|
37
|
Ljutić D and Kes P: The role of arterial
hypertension in the progression of non-diabetic glomerular
diseases. Nephrol Dial Transplant. 18 Suppl 5:v28–v30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petras D, Koutroutsos K, Kordalis A,
Tsioufis C and Stefanadis C: The role of sympathetic nervous system
in the progression of chronic kidney disease in the era of catheter
based sympathetic renal denervation. Curr Clin Pharmacol.
8:197–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amann K, Nichols C, Tornig J, Schwarz U,
Zeier M, Mall G and Ritz E: Effect of ramipril, nifedipine and
moxonidine on glomerular morphology and podocyte structure in
experimental renal failure. Nephrol Dial Transplant. 11:1003–1011.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fenton C, Keating GM and Lyseng-Williamson
KA: Moxonidine: A review of its use in essential hypertension.
Drugs. 66:477–496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krespi PG, Makris TK, Hatzizacharias AN,
Triposkiadis P, Tsoukala C, Kyriaki D, Votteas V and Kyriakidis M:
Moxonidine effect on microalbuminuria, thrombomodulin and
plasminogen activator inhibitor-1 levels in patients with essential
hypertension. Cardiovasc Drugs Ther. 12:463–467. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luippold G, Beilharz M and Mühlbauer B:
Chronic renal denervation prevents glomerular hyperfiltration in
diabetic rats. Nephrol Dial Transplant. 19:342–347. 2004.
View Article : Google Scholar : PubMed/NCBI
|