Introduction
It is generally acknowledged that, whenever
indicated, wounds should be carefully sutured (1). The simple interrupted suture (SIS) and
horizontal mattress suture (HMS) are two common wound closure
methods. SIS is used as the wound is kept clean and smooth, and it
aligns tissues well. HMS is chiefly used in big tension wounds, in
repair of blood vessel rupture or in closing the peritoneum, to
create a smooth inner wall (2,3).
However, the differences in the resulting fibrotic processes of the
two types of suture remain to be clarified. Thus, a self-comparison
model was formed to examine the different fibrotic processes of the
two sutures in corneal wound healing.
Since the cornea is transparent, avascular and
composed of simple substances, it is an ideal site to investigate
the process of wound healing (2).
When the cornea is injured, the quiescent keratocytes in the wound
stroma become apoptotic, and the keratocytes nearby are activated.
The activated keratocytes proliferate and differentiate into
spindle-shaped fibroblasts that migrate towards the wound area
(4). After arriving at the wound
edge, some of these cells enlarge and differentiate into
myofibroblasts within the stroma (5). Myofibroblasts fill the wound in an
interwoven and interconnected network pattern, and express
filaments of α-smooth muscle actin (α-SMA) to promote wound
contraction (6). Myofibroblasts are
less translucent than static keratocytes and they further decrease
stromal transparency by altering the organization and composition
of the extracellular matrix (ECM) (7). Vimentin is one of the many intermediate
filaments of mesenchymal cells such as fibroblasts and endothelial
cells (8). Vimentin is needed during
tissue repair and its overexpression plays an unappreciated
pathogenic role in corneal fibrotic conditions (9). Fibroblasts and myofibroblasts
synthesize the ECM components, including collagen types I and III,
and fibronectin (10). These
proteins are involved in the fibrosis of wound healing.
In the present study, we utilized a rabbit model to
test the hypothesis that the two types of sutures differ in the
fibrotic processes they stimulate.
Materials and methods
Animals
In total, 45 healthy male New Zealand white rabbits
(Peking University Health Science Center, Beijing, China), weighing
2.0–2.5 kg, were used in the present study. Five rabbits were
sacrificed as normal controls. The remaining 40 rabbits were
divided into 4 groups (n=10) and were sacrificed at 14 days, 21
days, 3 months and 6 months after the initial incision. The present
study was conducted in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The Institutional Animal Care and
Use Committee of Peking University approved the animal protocol
(permit no. LA2012–54).
Surgeries were performed under general and topical
anesthesia. General anesthesia was induced with a muscle injection
of ketamine HCl (0.1 ml/kg) and Su-Mian-Xin (0.1 ml/kg). Topical
anesthesia was applied with Benoxil. Efforts were made to minimize
suffering.
Animal surgery
The surgeries were randomized to the left or right
eye of the rabbits, but surgery was performed to only one eye for
each rabbit. The surgical procedure was as follows: After
sterilization of the palpebrae and the conjunctival sac, the
palpebrae were opened by use of a spatula, and the superior and
inferior recti muscles were stabilized with 5–0 sutures. Two
‘I’-shaped incisions on each side of the cornea, parallel to the
corneal median vertical line were made. The lengths of the top and
bottom ends of the vertical wounds were 3 mm, the vertical length
of the wound was 6 mm, and the incision depth was 1/2 of the
corneal thickness. The vertical flaps to the left and right were
separated 1.5 mm through the lamellar stroma. After washing the
wounds with 0.9% normal saline, 0.2 ml of aqueous was aspirated
from the anterior chamber with a 1.0 ml syringe at the vertical
limbus position to reduce the corneal tension. The SIS wound was
closed with five interrupted 8–0 silk sutures (Snake, Germany),
while the HMS wound was closed with three interrupted horizontal
mattress 8–0 silk sutures (Fig. 1E).
After finishing the sutures, 0.5 ml tobramycin was injected into
the subconjuctival space, and erythromycin ointment was applied to
the conjunctival sac. Ofkoxacin eye drops were administered into
the wounded eye 3 times per day for 1 month. Ten days after the
surgery, the sutures were removed under anesthesia. The animals
were sacrificed in a randomized manner on days 14 and 21, and
months 3 and 6 after surgery.
Regular images
Photos were obtained at 14 and 21 days, and at 3 and
6 months after surgery under general and topical anesthesia. The
regular images captured were obtained using a Topcon OMS 800
Surgery Microscope with an NC Video 1.0 system (Newcom Technology
Co., Ltd., Shenzhen, China).
Tissue processing
In total, 50 corneas were harvested, including 40
corneas from the four treated groups (n=10), and 10 corneas from
the five blank control rabbits once they were sacrificed. Half of
the corneas in each group were used for detection of
immunofluorescence, while the remaining 50% of the corneas were
used for quantitative polymerase chain reaction (qPCR) analysis.
The corneas used for immunofluorescence were fixed in 4%
paraformaldehyde (PFA) immediately after death. After 1 h, the
corneas were harvested from the eyeballs and were placed in 4% PFA
solution for 24 h at 4°C. After fixation, the corneas were washed
twice in 1X phosphate-buffered saline (PBS) for 30 min. The corneas
were then dehydrated in a 30% sucrose solution, embedded into OCT
(Sakura Finetek, Torrance, CA, USA), frozen in liquid nitrogen for
3 min and preserved in a refrigerator at −80°C. The prepared
samples were cut into 7 µm sections using a Leica Freezing
Microtome (Nussloch, Germany) 1 day prior to the subsequent
procedures. The frozen sections were immersed in ice-cold acetone
for 10 min prior to drying in air for 15 min, and were kept in a
−80°C refrigerator for immunofluorescence detection on the
following day.
The central corneas of the eyeballs used for qPCR
were marked with a 12-mm diameter trephine (Meisinger, Centennial,
CO, USA) and excised with scissors to remove the rims. The obtained
grafts were cut vertically in half and were preserved in labeled
freezing tubes (Corning Inc., Acton, MA, USA) and kept at −80°C
until mRNA assessment. The primers used to amplify the five genomic
sequences are shown in Table I.
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Gene | Forward primer | Temperature | Reverse primer | Temperature | bp | Source |
|---|
| α-SMA |
CTGACCGTATGCAGAAGGAAA | 58.0 |
AGAAACAGAGCAGGGAAGTGAC | 57.7 | 222 | NM_001101682 |
| Vimentin |
CAGGCAAAGCAGGAGTCAA | 56.8 |
TCTTCCATTTCACGCATCTG | 56.7 | 116 | XM_002717420 |
| Collagen type I |
CTGGACGGATTGAAAGGACA | 58.1 |
GGACCAACACTGCCATCACT | 57.6 | 173 | NM_001195668 |
| Collagen type
III |
TGTTCCTTTTGTTCTAATCTTGTCA | 58.3 |
TATAGCACCATTGAGACATTTTGA | 57.4 | 199 | S83371 |
| GAPDH |
GAGCACCAGAGGAGGACGA | 57.8 |
TGGGATGGAAACTGTGAAGAG | 57.3 | 103 | NM_001082253.1 |
Immunofluorescence
Immunofluorescent staining was performed to detect
α-SMA, vimentin, and collagen types I and III, which are markers of
myofibroblasts, fibroblasts, and new ECM protein deposition,
respectively. Frozen sections were air dried and washed three times
in 1X PBS for 5 min. The sections were then blocked with goat serum
for 1 h and repaired with 1X Frozen Sections Antigen Repair Agent
(Beyotime Biotech, Jiangsu, China) for 5 min at room temperature.
Subsequently, 1:100 primary antibodies were applied overnight in a
moist chamber set at 4°C. The following day, 1:300 diluted
anti-mouse IgG secondary antibodies, conjugated with fluorescein,
were applied at room temperature for 1 h. After washing 3 times in
PBS for 5 min, DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL,
USA) was mounted for staining of the nuclei and the samples were
covered with coverslips. The primary antibodies used were:
monoclonal α-SMA antibody (mouse anti-rabbit; dilution of 1:200;
Sigma, St. Louis, MO, USA; cat. no. A5228), monoclonal vimentin
antibody (mouse anti-rabbit, dilution of 1:500; Abcam, Cambridge,
UK; cat. no. ab30436), collagen type I (monoclonal mouse
anti-rabbit; dilution of 1:500; Abcam; cat. no. ab292), and
collagen type III (monoclonal mouse anti-rabbit; Abcam; dilution of
1:500; cat. no. ab7778). The tissues were observed and photographed
under a Leica fluorescence microscope camera (Leica DM3000). The
negative controls were prepared using PBS, rather than primary
antibodies.
Semi-quantitative PCR
The mRNA levels of a-SMA, collagen type I and III
were analyzed by qPCR. Five corneal grafts of the rabbits that did
not undergo surgery were used as negative controls to minimize the
potentially confounding effects of natural variability in gene
expression. Total RNA was extracted using an RNA purification kit
(Invitrogen Life Technologies, Austin, TX, USA), according to the
manufacturer's instructions. First-strand cDNA was synthesized
using a synthesis kit (Avian Myeloblastosis Virus First-Strand cDNA
Synthesis kit, Life Technologies, Carlsbad, CA, USA). The cDNA
samples were then divided into tubes containing a PCR reaction
mixture (Applied Biosystems Life Technologies, Foster City, CA,
USA) with specific primers (Table
I). For each treatment, a PCR reaction mixture was prepared,
containing 1 µl of cDNA, 10 µl of PCR master mix (SYBR-Green;
Applied Biosystems Life Technologies), 1 µl of each primer and 7 µl
of ddH20 in a total volume of 20 µl. Semi-quantitative
RT-PCR was performed with an StepOnePlus Real-Time PCR instrument
(Applied Biosystems Life Technologies). The samples were assayed in
triplicate. The cycling conditions were initiated at 95°C for 2
min, followed by 40 cycles consisting of a 10-sec melting interval
at 95°C and a 40-sec interval for annealing at 60°C. Immediately
after the amplification, melt curve protocols were performed to
ensure that primer-dimers and other non-specific products were
minimized or eliminated. For data analyses, the cycle threshold
(CT) of each gene for the surgery corneal grafts was normalized to
the corresponding value for the control cornea and this value was
used to calculate the fold-change with the 2−ΔΔCT
method. The results are presented as significant increases or
decreases in mRNA detected in the experimental groups compared with
their respective controls.
Statistical analysis
The corneal samples were analyzed individually (not
pooled) ≥3 times. The individual data values from each corneal
sample were statistically analyzed using SPSS statistical software
package, version 17.0 (SPSS, Inc., Chicago, IL, USA). The paired
t-test was used to compare mRNA values between the two
differentially sutured groups. One-way ANOVA was used to compare
mRNA values of different time points in each group. The statistical
results with P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphology results
Clinical observations of wound healing following
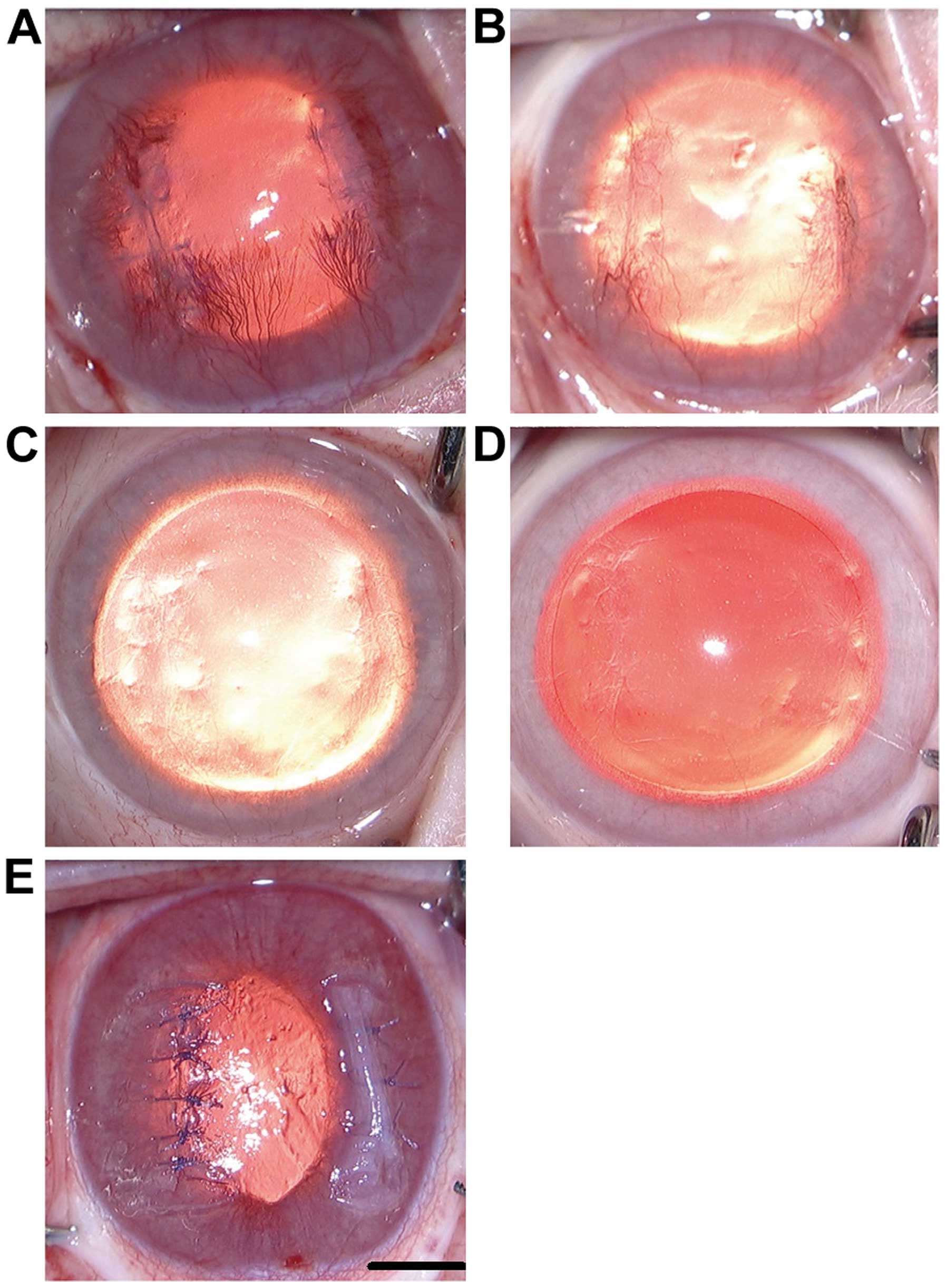
corneal incisions closed with SIS or HMS. Images of clinical signs
of the wound-healing process were captured. Fig. 1E shows an image directly after the
procedures. SIS closure is shown on the left wound, while HMS
closure is shown on the right wound. The neovascularization of
wounds reached a peak on day 14 (Fig.
1A) and began to fade away. At 3 months, neovascular vessels
and scarring tissue had virtually disappeared (Fig. 1C). A comparison revealed that the
scars of HMS wounds were denser than those of SIS wounds at month 3
(Fig. 1C). Fibrosis of the two sides
were apparent on day 21 (Fig. 1B).
The scars appeared to show no apparent disparities between the two
sides at month 6 (Fig. 1D).
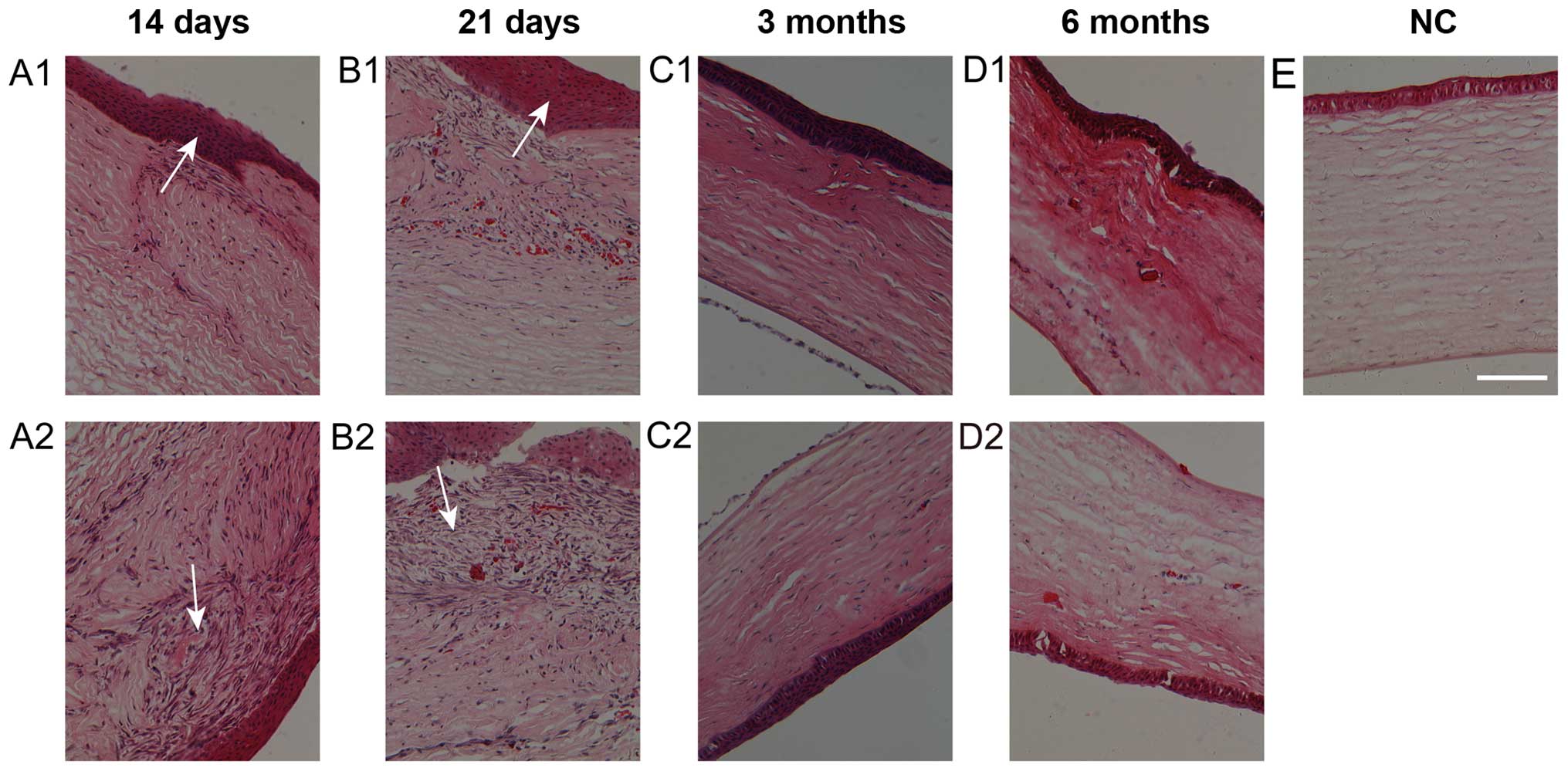
Hematoxylin and eosin staining of the
HMS and SIS wounds following surgery
The two types of sutured wounds appeared more
cellular than normal corneas. The epithelial cells of the wounds
showed proliferation and multilayered morphology at days 14 and 21
(Fig. 2A1, A2, B1 and B2). At 3 and
6 months after surgery, the fibrotic cells in the wound area had
almost disappeared (Fig. 2C1, D1 and C2,
D2). Three months after surgery, the fibrotic cells in the
wound area had almost disappeared (Fig.
2C1 and C2). However, the hypercellular interwoven tissues
under the epithelium in HMS wounds were more abundant than in the
SIS wounds on days 14 and 21 (Fig. 2A1,
A2, B1 and B2). The wounds on the two sides showed signs of
normal recovery to almost normal corneal tissues (Fig. 2C-E).
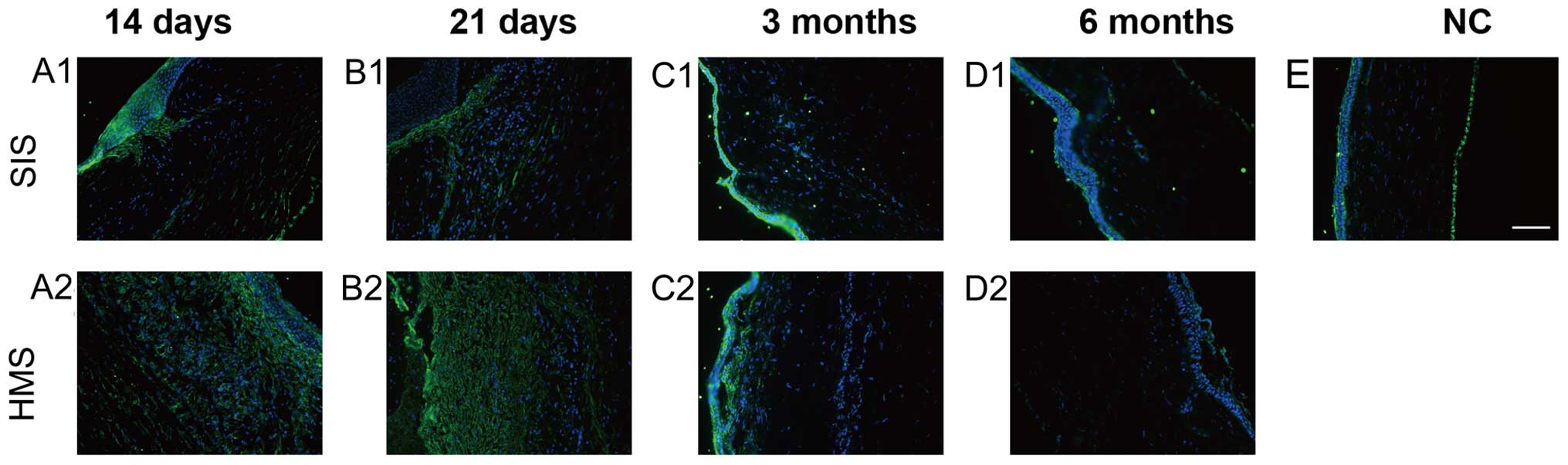
Immunofluorescence
The α-SMA-positive cells emerged in the wound areas
on the two sutures and reached a peak at day 21 (Fig. 3B1 and B2). At 3 and 6 months, the
α-SMA staining on HMS wounds was much more intense than that on the
SIS wounds on 21 day (Fig. 3B1 and
B2). At 3 months, the α-SMA-stained cells had nearly
disappeared, except for the region immediately under the epithelium
(Fig. 3C1, D1 and C2, D2). The
analysis of control samples demonstrated this antibody reacts with
stromal and endothelial cells in the normal cornea (Fig. 3E).
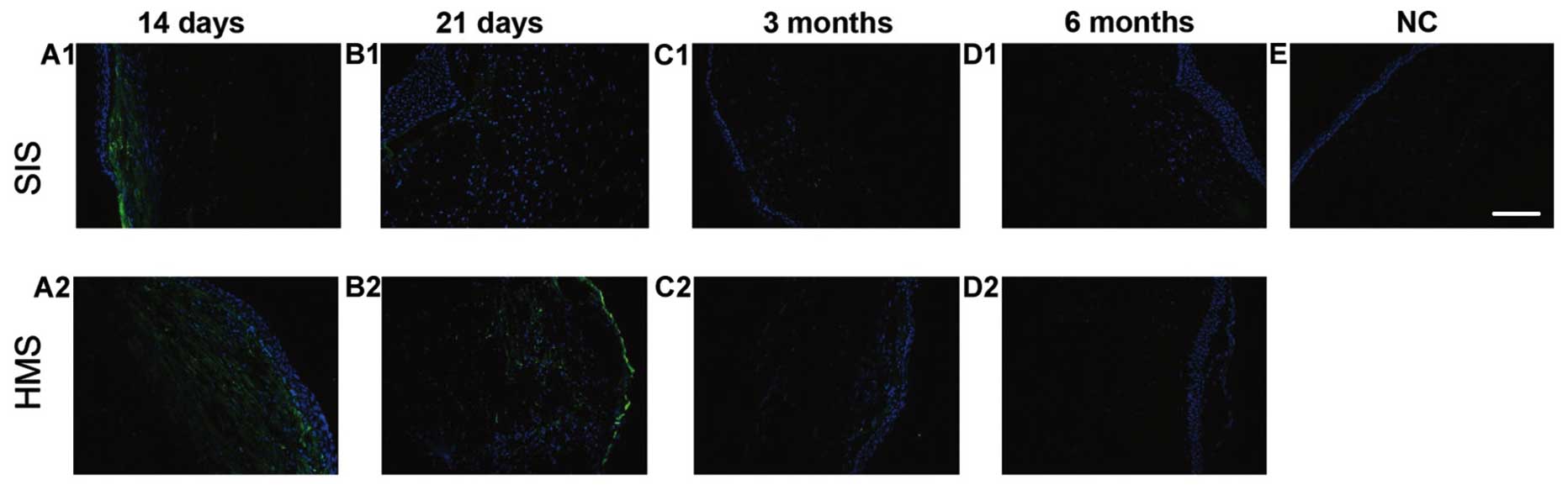
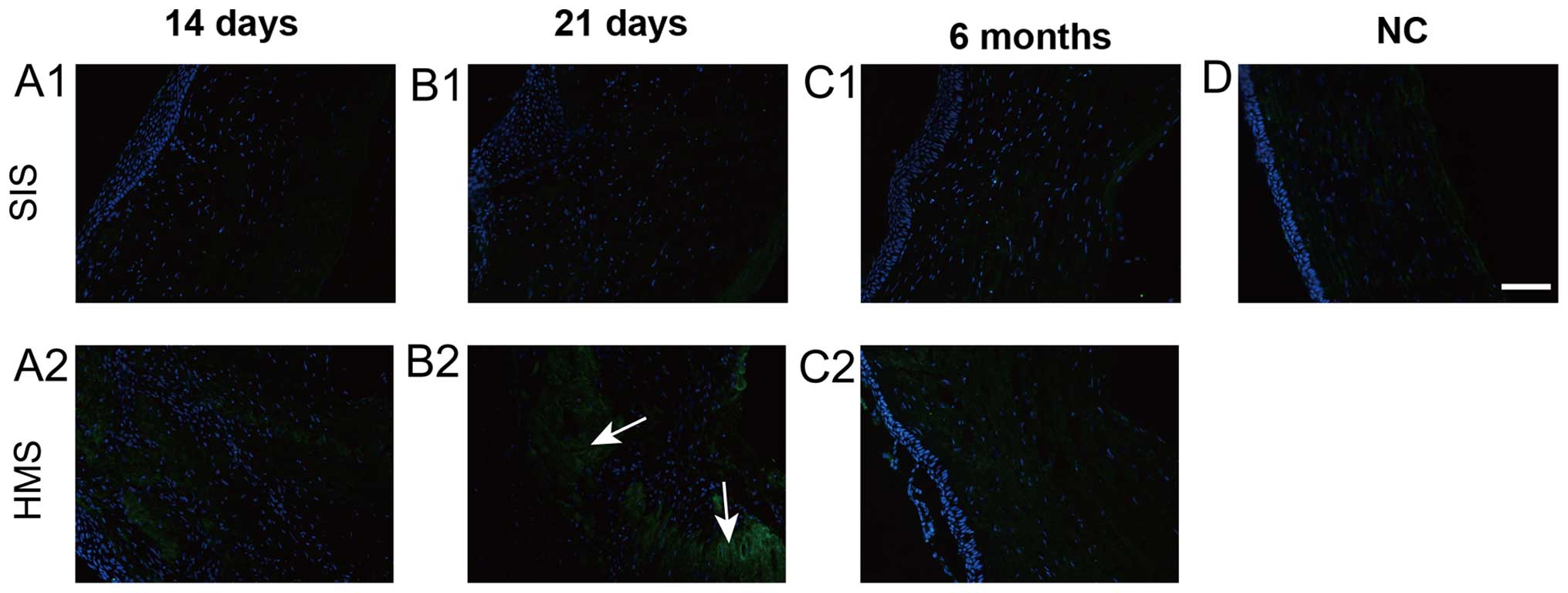
The number of vimentin-positive cells on both wounds
was noted on days 14 and 21 (Fig. 4A1,
A2, B1 and B2). At 3 and 6 months after surgery, the
vimentin-positive cells were barely visible (Fig. 4C1, D1 and C2, D2). There was no
positive staining in the normal cornea, indicating vimentin did not
stain keratocytes (Fig. 4E). During
the study, the peak of detection of the myofibroblast's marker
α-SMA was on day 21 (Figs. 3B1, B2 and
5A), which was later than that of the fibroblast marker
vimentin. Vimentin expression reached its peak on day 14 (Figs. 4A1, A2 and 5B).
Collagen type I was found in the entire cornea
except in the fibrotic region, especially on day 21 (Fig. 6B1 and B2). At that point the
expression pattern of collagen type I resembled that of a ring with
the center region full of fibrotic tissue containing collagen type
III and α-SMA. The normal cornea staining demonstrated collagen
type I expression in the stroma (Fig.
6D).
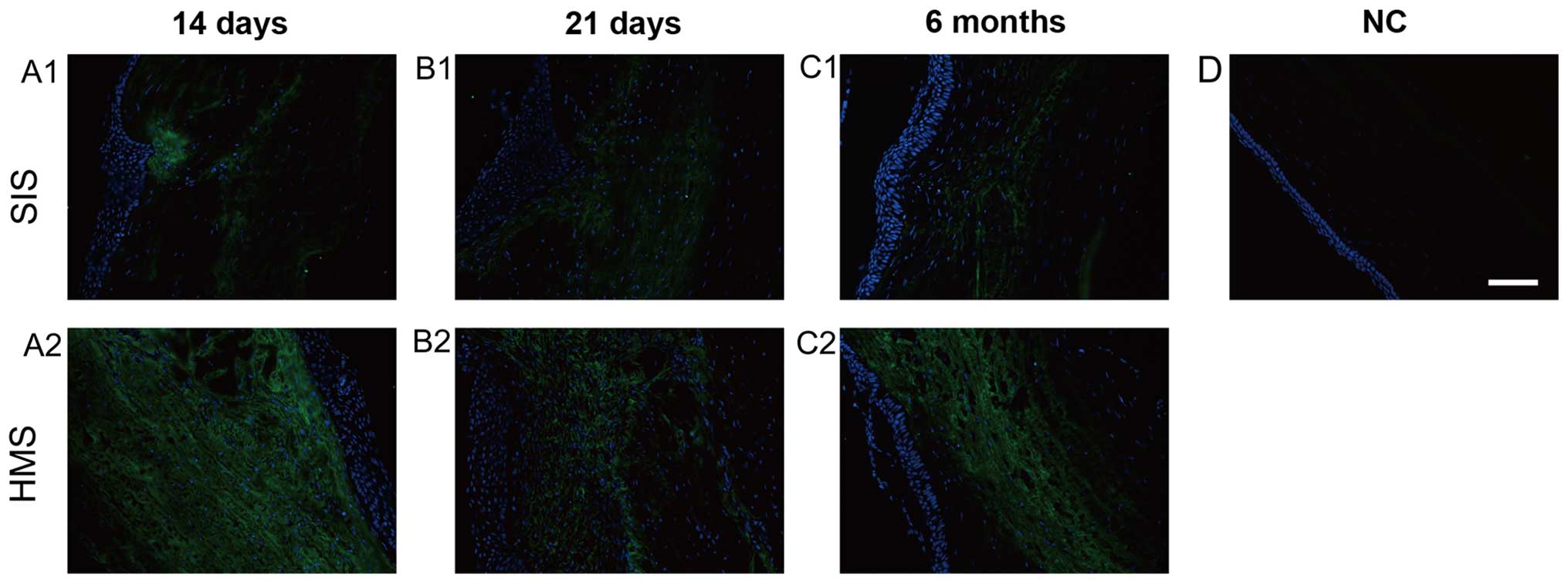
The staining of collagen type III in HMS wounds was
most intense on days 14 and 21 (Fig.
7A1, A2, B1 and B2). The expression of collagen type III was
gradually decreased after 6 months (Fig.
7C1 and C2). Notably, the immunofluorescence of collagen type
III in HMS wounds was much stronger than that in SIS wounds on day
21, and month 6 (Fig. 7A1-C1 and
A2-C2). There was no positive staining of collagen type III in
normal corneas in the present study (Fig. 7D).
In comparing the proteins, the positive expression
of collagen type I in SIS wounds was gradually reduced to normal
levels, as shown by immunofluorescence (Fig. 6C1 and C2), while the α-SMA gradually
receded to the level of the subepithelial region (Fig. 3C1 and C2). Collagen type III was
gradually replaced by collagen type I in the two types of sutured
wounds after day 21 (Figs. 6 and
7).
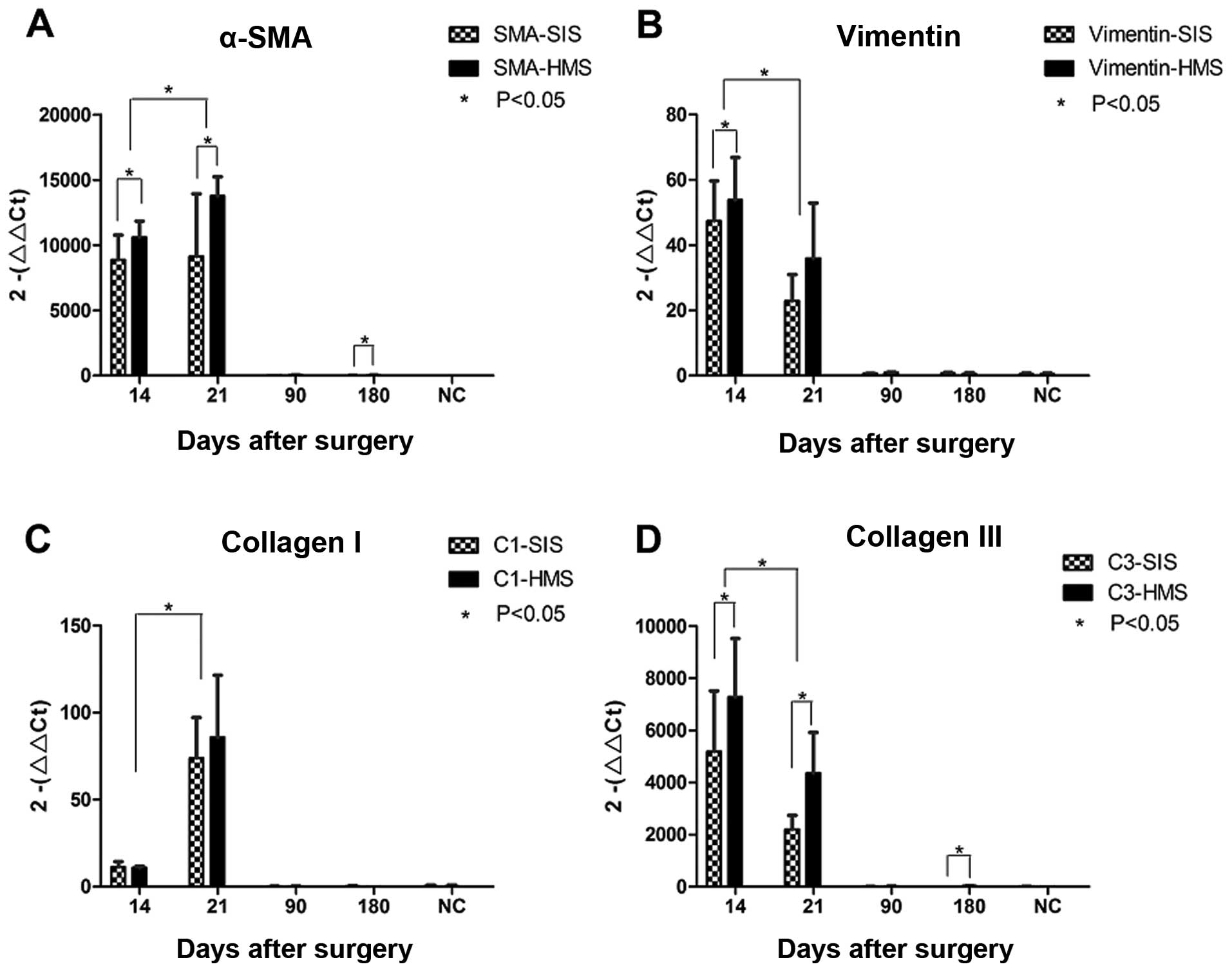
qPCR results
Changes in the expression of four mRNAs from SIS and
HMS wounds during the course of corneal wound healing were compared
using semi-quantitative PCR. Each of the four mRNA expressions was
high on days 14 and 21, but low on months 3 and 6 (Fig. 5). On day 21, the mRNA expression of
α-SMA and collagen type I reached a peak (Fig. 5A and C). However, the highest
expression for vimentin and collagen type III mRNAs was evident on
day 14 (Fig. 5B and D). According to
statistics, the difference in the mRNA expression of α-SMA,
vimentin and collagen type III in SIS and HMS wounds was
statistically significant (P<0.05). On day 14 (P=0.031) and 21
(P=0.05) as well as at 6 months (P=0.035), the HMS wounds produced
increaesd α-SMA mRNA than the SIS wounds. As for vimentin, the HMS
wounds generated increased mRNA than the SIS wounds on day 14
(P=0.023). For collagen type III, the HMS wounds produced more mRNA
than the SIS wounds on days 14 (P=0.001) and 21 (P=0.039), and
month 6 (P=0.006). However, for collagen type I, the mRNA
expression between SIS and HMS wounds showed no distinct
differences (Fig. 5C).
Discussion
The rabbit model of cornea
An opportunity to study the wound healing response
in SIS and HMS. While corneal trauma is extremely common, human
tissue appropriate for histologic analysis is difficult to obtain.
Although patient specimens of penetrating keratoplasty are
available, they are often combined with other corneal lesions and
cannot be used to investigate normal wound-healing processes.
In the present study, a rabbit model of corneal
wound healing was used to overcome these problems. This model
correlates general imaging findings with histological and
expression analyses at different time-points following a
standardized surgical procedure and follow-up. The rabbits were
used as the animal models, partly because their eyes are
sufficiently large for practical surgical operations. However, the
regenerative capacity of the rabbit endothelium is much better than
that of humans. Therefore, 1/2 corneal thickness wounds were made
in order to compensate for the differences between human and rabbit
eyeballs.
The major potential problem of this rabbit model was
the use of the same cornea to compare two wounds, as results were
potentially confounding between the two wounds. To verify the
spacing between two corneal wounds that would preclude interference
from one healing process to the other, we initially created
numerous corneal wounds and optimized these parameters in the
model. It was verified by histological and immunofluorescence
assays that no positive cells or proteins were stimulated in the
intermediate region between the two selected wounds Additionally,
our model design comparing two wounds in the same cornea avoids the
discrepancies that are irremediably found using different
corneas.
HMS wounds exhibit more fibrosis than
SIS wounds in the early healing stages
We used immunofluorescence to mark fibrotic related
proteins in the two wounds. The results show that the fluorescence
intensities of HMS wounds are stronger than those of the SIS wounds
during the early stages. Furthermore, results of qPCR show the
mRNAs of these fibrotic proteins are abundantly expressed in HMS
wounds during the early stage, supporting these findings. The
findings of the images captured (Fig.
1) and the histological sections (Fig. 2) are in agreement with that HMS
wounds cause more fibrosis than SIS wounds during the early steps
of the wound-healing process.
It has previously been noted that some cells in the
anterior stroma can simultaneously express vimentin and α-SMA
(8). However, our results suggest
that, the time of peak for vimentin expression is ~10–14 days after
surgery while that of α-SMA is at around day 21. Thus, it is
possible that the transformation of fibroblasts into myofibroblasts
lasts ~5–7 days in this in vivo model, and accounts for this
finding.
Scarring of the cornea is mainly due to improper
deposition of ECM components (10).
Collagen type III, a main component of early granulation tissue, is
gradually decomposed and replaced by normal collagen tissues
(6). However, its role in the
wound-healing process is incompletely understood (11). Boote suggested that the upregulation
of mRNA for collagen type III increased at 2 weeks and decreased at
4 weeks (12). Our results showed
that the collagen type III protein and its related mRNA expression
reached a peak on day 14 and decreased on day 21. Additionally, the
positive expression of collagen type III gradually leveled to a
pre-suture point, as indicated by the immunofluorescence.
Furthermore, collagen type I, the normally existing collagen in the
cornea, may fluctuate in the wound-healing process (6). We have found that the collagen type III
may be gradually replaced by collagen type I in the two types of
sutured wounds after day 21. In the remodeling phase, which can
last for years, the ECM is remodeled into a structure approaching
normal tissue (13).
It is generally accepted that the interaction
between epithelial and mesenchymal cells is crucial in abnormal
wound repair (7). In our model, HMS
evidently breaks the normal interaction of epithelium and matrix.
The present results have shown how HMS artificially turned the
cornea lamellar stroma out and thus generated more fibrosis than
SIS at the beginning of the healing process. Nevertheless, although
mattress sutures can produce surface scarring, early removal of
these sutures can limit this damage (14). We hypothesize the reason for
differences between SIS and HMS not becoming evident after 3 months
may be the removal of the stitches at 10 days post-suturing. In our
pilot study, the optimal time of stitch removal was on day 10.
Stitches removed at a time earlier than day 9, resulted in a wound
being unable to close adequately, while stitches removed after 10
days were starting to show corneal tissue degradation.
Methodological considerations
Our study was limited by a lack of protein
quantitative analyses. This is due to the fact that these fibrotic
proteins are not very abundant in a linear scar, and our attempts
to identify them by western blot analysis repeatedly failed.
However, based on the general imaging findings, histological
analyses, immunofluorescence and qPCR results, enough evidence was
provided to show a difference in the fibrotic tissues between HMS
and SIS during the early stages of wound healing.
In conclusion, wounds in the cornea create scars
that induce blurred vision. Good stitching can reduce scar
formation. In our rabbit model of cornea wound healing, HMS
produced increased fibrosis compared to SIS in the early stages of
wound healing, albeit similar outcomes are yielded after 3 months.
This observation suggests that it may be beneficial to select SIS
over HMS, if only for the more benign initial healing process. A
better understanding of wound closure methods may impact corneal
wound healing and provide important insights for enhancing the
vision of patients undergoing corneal injury suturing.
Acknowledgements
We are grateful for the technical support of Li
Ying, who works for the Vision Repair and Rehabilitation lab of
Peking University Third Hospital.
References
|
1
|
Thomas JR and Somenek M: Scar revision
review. Arch Facial Plast Surg. 14:162–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henderson J, Sutcliffe M and Gillespie P:
Epitendinous suture techniques in extensor tendon repairs - an
experimental evaluation. J Hand Surg Am. 36:1968–1973. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kan CD and Yang YJ: Double telescopic
anastomosis with interrupted suture technique in acute aortic
dissection. Ann Thorac Surg. 91:1630–1631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fini ME and Stramer BM: How the cornea
heals: Cornea-specific repair mechanisms affecting surgical
outcomes. Cornea. 24(Suppl 8): S2–S11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myrna KE, Pot SA and Murphy CJ: Meet the
corneal myofibroblast: The role of myofibroblast transformation in
corneal wound healing and pathology. Vet Ophthalmol. 12(Suppl 1):
25–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klingberg F, Hinz B and White ES: The
myofibroblast matrix: Implications for tissue repair and fibrosis.
J Pathol. 229:298–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weis AJ, Huxlin KR, Callan CL, DeMagistris
MA and Hindman HB: Keratocyte apoptosis and not myofibroblast
differentiation mark the graft/host interface at early time-points
post-DSAEK in a cat model. PLoS One. 8:e756232013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaurasia SS, Kaur H, de Medeiros FW,
Smith SD and Wilson SE: Dynamics of the expression of intermediate
filaments vimentin and desmin during myofibroblast differentiation
after corneal injury. Exp Eye Res. 89:133–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bargagna-Mohan P, Paranthan RR, Hamza A,
Zhan CG, Lee DM, Kim KB, Lau DL, Srinivasan C, Nakayama K, Nakayama
KI, et al: Corneal antifibrotic switch identified in genetic and
pharmacological deficiency of vimentin. J Biol Chem. 287:989–1006.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karamichos D, Guo XQ, Hutcheon AE and
Zieske JD: Human corneal fibrosis: An in vitro model. Invest
Ophthalmol Vis Sci. 51:1382–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volk SW, Wang Y, Mauldin EA, Liechty KW
and Adams SL: Diminished type III collagen promotes myofibroblast
differentiation and increases scar deposition in cutaneous wound
healing. Cells Tissues Organs. 194:25–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boote C, Du Y, Morgan S, Harris J,
Kamma-Lorger CS, Hayes S, Lathrop KL, Roh DS, Burrow MK, Hiller J,
et al: Quantitative assessment of ultrastructure and light scatter
in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci.
53:2786–2795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo S and Dipietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuber TJ: The mattress sutures: Vertical,
horizontal, and corner stitch. Am Fam Physician. 66:2231–2236.
2002.PubMed/NCBI
|