Introduction
Deep burns cause severe damage and tissue
regeneration and wound healing is difficult (1). The survival of a transplanted free skin
flap is closely related to local micro-vessel density,
hemoperfusion and nearby cell migration (2). The stress reaction after a deep burn
causes generalized resistance to insulin and a negative nitrogen
balance (3). A local injection of
insulin has been shown to be safe and effective in promoting wound
healing while exerting little influence on the plasma glucose
levels in a diabetic rat model (4).
However, as studies in humans have not been conducted, there are
many questions that remain unanswered and it is not even clear that
a local application of low-dose insulin is beneficial for wound
healing.
The aim of the present study was to examine the
effects of local low-dose insulin treatment after operation for
deep burns, providing valuable information and a reference for
clinical use.
Subjects and methods
Subjects information
A total of 165 patients with deep burns were
enrolled in the study in The Second Affiliated Hospital of Kunming
Medical University (Yunnan, China) from January, 2013 to January,
2016. Exclusion criteria for the study were the presence of
co-existing diseases including acute lung injury, severe
infections, malnutrition, diabetes, scarring tissues, other severe
underlying diseases and failed free flap transplantation. After
obtaining the approval of the ethics committee of The Second
Affiliated Hospital of Kunming Medical University and informed
consent of patients or their relatives, the cases were divided into
5 equal size groups, by random assignment: A blank control group, a
saline control group, a low-dose insulin group, median dose insulin
group and a high dose insulin group. The baseline data in each
group were compared to the data to the other groups and differences
were considered statistically significant (P<0.05; Table I).
 | Table I.Comparison of inter-group baseline
data. |
Table I.
Comparison of inter-group baseline
data.
| Group | Case | Male/female | Age | Course of disease,
h | Deep II° | III° | Extent of burn (%
TBSA) | Limbs | Chest | Abdomen | Back |
|---|
| Blank control | 33 | 19/14 | 52.4±10.3 | 1.2±0.4 | 17 | 16 | 35.6±7.8 | 16 | 3 | 4 | 10 |
| Saline control | 33 | 20/13 | 51.3±11.4 | 1.3±0.5 | 18 | 15 | 36.4±7.2 | 18 | 2 | 2 | 11 |
| Low dose | 33 | 21/12 | 53.2±12.2 | 1.4±0.7 | 19 | 14 | 35.8±7.5 | 17 | 3 | 3 | 10 |
| Median dose | 33 | 20/13 | 54.5±13.5 | 1.1±0.6 | 16 | 17 | 36.3±7.6 | 15 | 4 | 2 | 12 |
| High dose | 33 | 21/12 | 52.7±12.6 | 1.3±0.5 | 18 | 15 | 35.9±7.7 | 16 | 5 | 3 | 9 |
| F
(χ2) |
| 0.357 | 0.625 | 0.514 | 0.633 |
| 0.249 |
|
| 3.335 |
|
| P-value |
| 0.986 | 0.349 | 0.636 | 0.959 |
| 0.302 |
|
| 0.993 |
Treatment method
All the patients had similar free skin flap
transplantation operations performed by one nursing team, and
prophylaxis for infections and strengthening of nourishments
according to the standard medical procedures for burns were
followed. The individuals in the blank control group had no local
subcutaneous drug injections. The saline control group patients
were subcutaneously injected with 2 ml of saline once daily for 14
days. The individuals in the insulin groups received local insulin
injections at a total volume of 2 ml once a day for 14 days: The
low insulin group received 0.5 units regular insulin injections,
the median dose group had 1.0 units regular insulin injections and
the high dose group had 2.0 units regular insulin injections.
Observation indexes
Wound healing and flap survival conditions were
assessed with a variety of methods and compared amongst groups.
The healing rate of wounds was determined by using a
transparent film to manually trace the wound outline and then its
area calculated with the imageJ software using the formula: Healing
rate = area of wound healed/total area × 100%.
The flap survival in each case was studied taking
digital images of the flap with a camera (Canon, Inc., Tokyo,
Japan), and then calculating the survival rate using the image
analysis software image-Pro Plus.v 6.0 (Microsoft Corporation,
Redmond, WA, USA). The survival rate was calculated as: survival
areas/design area × 100%.
The wound blood flow volumes were measured by laser
doppler flowmetry according to a previous study (5).
Skin flap biopsies of each healed-unhealed interface
tissue (0.5 × 0.5 × 0.5 mm) were processed for hematoxylin and
eosin staining and histological observation by conventional
fixation, embedding and sectioning.
CD31 immunohistochemical detection in biopsies was
used to measure microvessel quantity. Briefly, sample tissues were
incubated overnight at 4°C, with rabbit polyclonal anti-CD31
antibody (Abcam, Cambridge, MA, USA; catalog no.: ab28364;
dilution: 1:50). After washing 3 times with phosphate-buffered
saline, the samples were incubated for 1 h goat anti-rabbit
monoclonal tetramethylrhodamine isothiocyanate-labelled secondary
antibody (Abcam; catalog no.: ab6718; dilution: 1:2000). Cell
nuclei were dyed using DAPI. A laser scanning confocal microscope
(Lucid Inc., Rochester, NY, USA) was used to observe the samples
and estimate the blood vessel density by counting the number of
vessels in five sections and working out an average number.
The expression levels of heat shock protein
(HSP)-90, vascular endothelial growth factor (VEGF), transforming
growth factor (TGF)-β and interleukin (IL)-1 were evaluated using
an ELISA kit strictly adhering to the manufacturer's instructions
(R&D Systems, Inc., Minneapolis, MN, USA).
Conventional biochemical methods were used to detect
the levels of glucose in plasma.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Numerical data were expressed as mean ±
standard deviation. For comparison between groups, single-factor
ANOVA analysis was selected, while pairwise comparisons were made
by the least significant difference test. Categorical data were
analyzed with the Chi-square test and expressed as number of cases
and percentage. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparisons of wound healing and flap
survival conditions
The wound healing time of the low-dose insulin group
was significantly shorter while the high-dose insulin group took
the longest time to heal, and differences were statistically
significant (P<0.05). The healing rate at 14 days
post-intervention was higher than that after 7 days for all groups,
however, the healing rate for the high dose insulin group was the
lowest of them all, indicating that a high dose of insulin actually
impaired normal healing (Table
II).
 | Table II.Comparison between wound healing and
flap survival conditions. |
Table II.
Comparison between wound healing and
flap survival conditions.
| Group | Wound healing time,
days | 7 days healing rate,
% | 14 days healing rate,
% | Total flap survival
rate, % | 7 days survival rate,
% | 14 days survival
rate, % |
|---|
| Blank control | 27.5±4.2 | 47.2±10.3 | 53.9±11.2 | 70.5±6.9 | 61.5±7.2 | 68.5±7.4 |
| Saline control | 26.6±4.5 | 48.8±11.2 | 55.7±11.6 | 72.3±6.8 | 63.2±7.3 | 69.7±7.5 |
| Low dose | 18.2±3.3 | 72.8±8.6 | 86.5±10.4 | 92.5±5.4 | 83.4±6.3 | 90.6±6.6 |
| Medium dose | 22.4±5.2 | 56.9±12.3 | 70.3±12.6 | 78.8±6.6 | 72.1±7.8 | 76.5±7.3 |
| High dose | 31.3±5.6 | 33.4±14.2 | 48.4±15.5 | 67.9±6.3 | 64.4±7.1 | 65.3±7.2 |
| F |
8.245 |
9.654 | 10.772 |
7.648 |
8.524 |
8.639 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
The total flap survival rate in the low-dose insulin
group was the highest while the high-dose insulin group had the
lowest survival rate of all the groups, and differences were
statistically significant (P<0.05). There were no significant
differences between the survival rates for the blank control,
saline control, and high-dose insulin groups. The survival rate for
the low-dose and medium-dose insulin groups was higher at 14 days
than at 7 days.
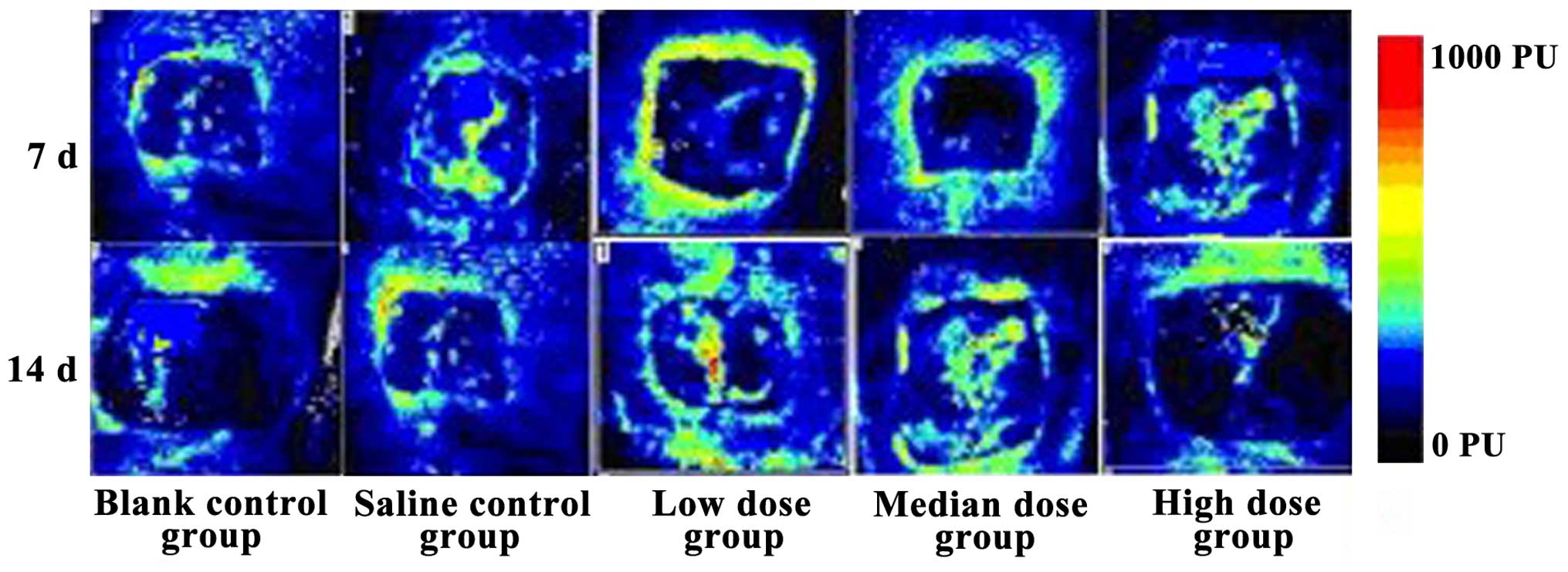
Comparison of wound blood flow
volume
The blood flow volume in the low-dose insulin group
was the best of them all after 14 days, while that of the high-dose
insulin level was the worst. The flows were superior after 14 days
than after 7 days for all the groups, and the differences were
statistically significant (Fig.
1).
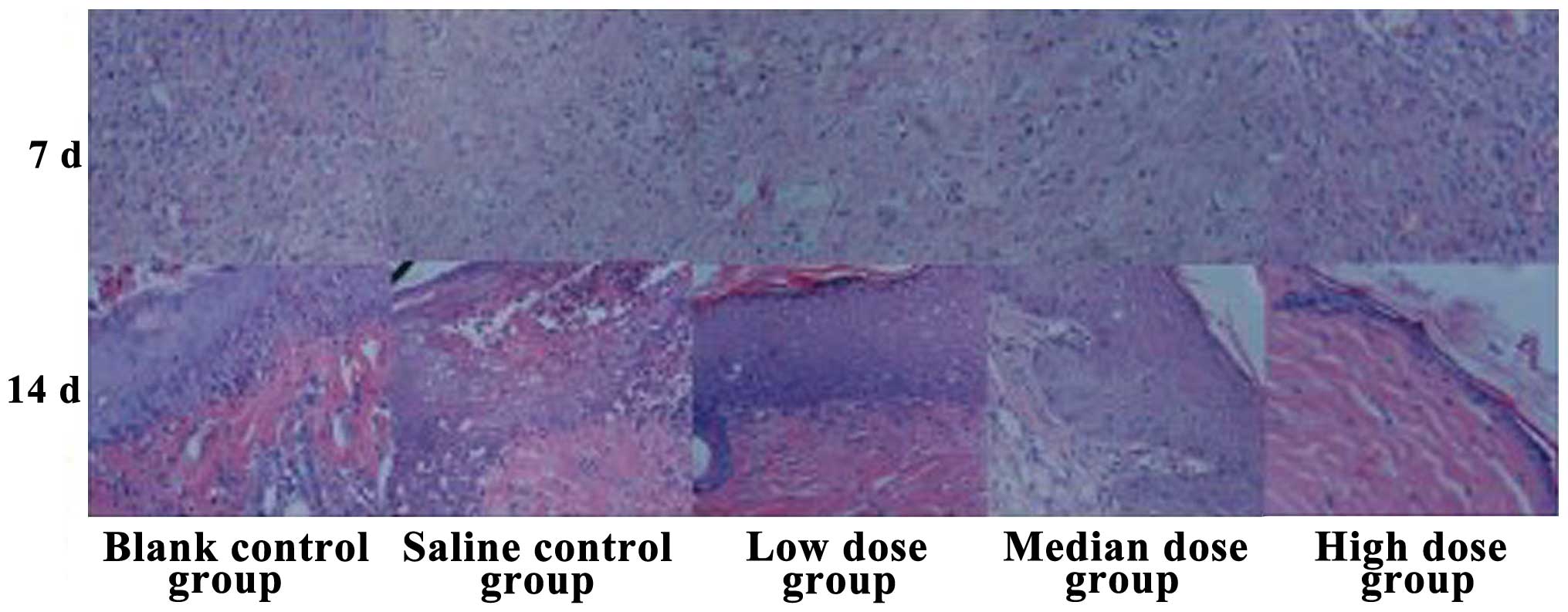
Histological observation of wound
healing
After 7 days of treatment the low-dose insulin group
cases had a reduced number of inflammatory cells in the wound, the
fibroblast counts had increased, the capillary components had
increased, and partial regenerated epithelial tissue was observed.
By contrast, the high-dose insulin group had the highest number of
inflammatory cells present in the wound and the least counts of
fibroblasts. Furthermore, after 14 days, the low-dose insulin group
showed a regenerated epidermal structure, rete pegs were clearly
visible at the epidermal and dermal attachment sites, new vessels
increased, and fibroblast cells were arranged in neat rows. By
contrast, the high-dose insulin group had fewer regenerated
epidermal structures that were too fragile to cover all the
epidermal layers, albeit with abundant inflammatory cell
infiltration (Fig. 2).
Comparison of expression levels of
HSP-90, VEGF, TGF-β and IL-1
Expression levels of HSP-90, VEGF, TGF-β and IL-1 at
7 days were higher than those at 14 days for all groups, however,
they were highest in the low-dose insulin group. The differences in
the blank control group, saline control group and high-dose group
were not obvious (Table III).
 | Table III.Expression levels of HSP-90, VEGF,
TGF-β and IL-1. |
Table III.
Expression levels of HSP-90, VEGF,
TGF-β and IL-1.
|
| HSP-90, ng/ml | VEGF, µg/ml | TGF-β, pg/ml | IL-1, pg/ml |
|---|
|
|
|
|
|
|
|---|
| Group | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days |
|---|
| Blank control | 25.2±6.4 | 16.8±6.5 | 14.3±8.2 | 12.9±8.4 | 45.9±15.2 | 24.8±11.2 | 73.2±32.6 | 39.7±14.2 |
| Saline control | 26.3±6.9 | 17.2±6.8 | 15.2±7.6 | 13.5±7.8 | 44.8±14.7 | 26.5±11.4 | 75.7±36.7 | 36.5±13.9 |
| Low dose | 63.4±12.3 | 42.5±12.6 | 44.8±10.3 | 36.5±10.5 | 156.3±43.2 | 85.7±36.5 | 247.3±62.4 | 112.3±48.5 |
| Medium dose | 52.6±13.7 | 33.7±13.9 | 32.8±11.2 | 27.6±11.3 | 120.5±45.7 | 64.5±32.4 | 215.8±70.2 | 86.4±36.5 |
| High dose | 24.8±6.6 | 15.5±6.3 | 14.4±7.2 | 12.7±7.3 | 43.2±15.8 | 23.9±10.3 | 69.8±35.5 | 37.2±12.5 |
| F | 10.523 |
8.659 |
8.645 |
9.203 | 15.426 | 13.258 | 14.527 | 13.625 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
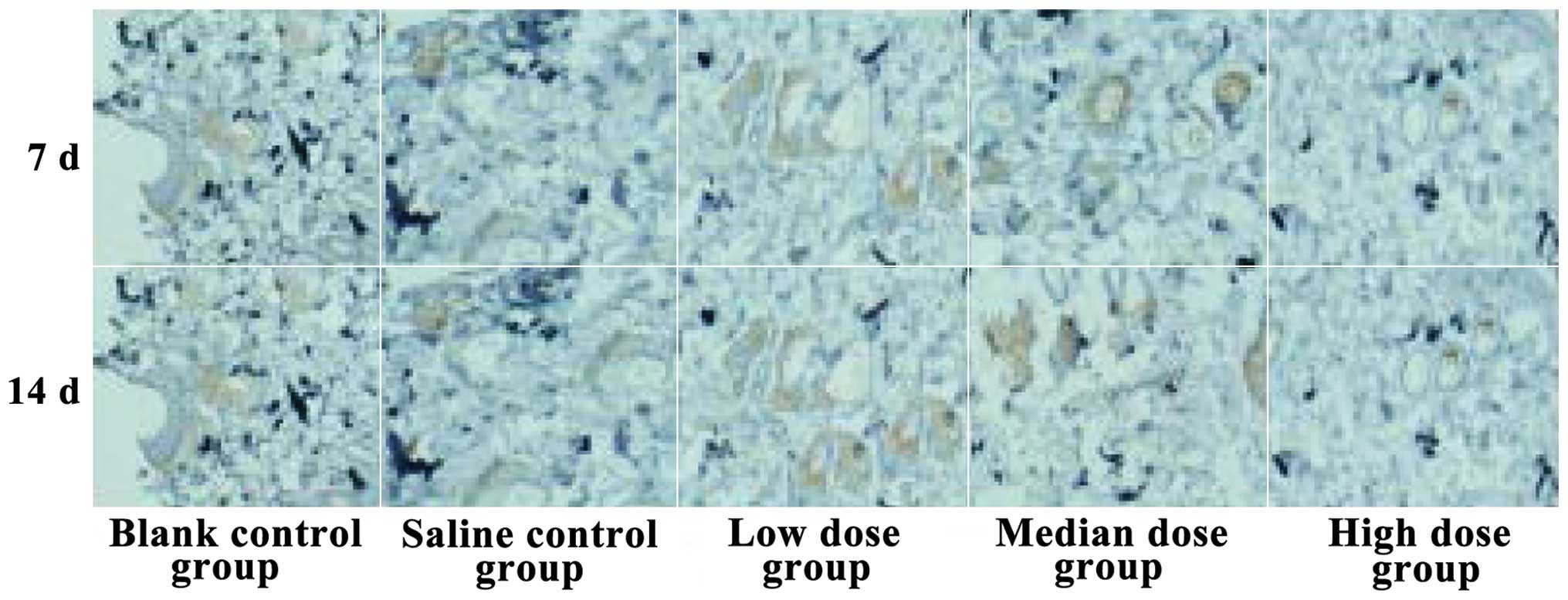
Comparison of microvessel
quantity
The microvessel quantities of the low-dose insulin
group were the highest of them all at 14 days, while the quantities
in the high-dose insulin group were the lowest. The differences
found were statistically significant (P<0.05, Fig. 3).
Comparison of blood sugar level
The plasma glucose levels in patients gradually
declined, and comparative differences over time were statistically
significant (P<0.05; Table
IV).
 | Table IV.Comparison of blood sugar level
(mmol/l). |
Table IV.
Comparison of blood sugar level
(mmol/l).
| Group | Before
treatment | 7 days | 14 days |
|---|
| Blank control | 7.6±1.5 | 7.1±1.3 | 6.3±1.1 |
| Saline control | 7.7±1.4 | 6.9±1.6 | 6.4±1.3 |
| Low dose | 7.5±1.5 | 7.0±1.4 | 6.5±1.2 |
| Medium dose | 7.5±1.3 | 6.8±1.2 | 6.3±0.9 |
| High dose | 7.6±1.4 | 6.5±1.1 | 6.1±1.6 |
| F | 0.625 | 4.625 | 4.967 |
| P-value | 0.754 | 0.063 | 0.052 |
Discussion
It is possible that the local insulin injections
promote wound healing by stimulating the expression of VEGF, which
in turn increases the number of blood capillaries. Additionally,
glucose and amino acid entry into cells and the acceleration of
protein synthesis, with increased fat synthesis and steatolysis
inhibition promote wound granulation (6). Furthermore, the increase in the
expression of epidermal growth factor and basic fibroblast growth
factor in wound tissues promotes fibroblast proliferation,
increasing the hydroxyproline content in wound tissue, advancing
the epidermal cell proliferation cycle and increasing synthesis of
collagen fibers in the wounded tissue (7). The initial stage of repair and the
proliferation of apoptosis after deep burns has the following
adverse effects: Growth factor expression is weakened, granulation
of the wounded tissues is not normal and consequently the survival
rate of free flaps is not high due to the lack of a nurturing
environment. Previous experiments conducted on animals have
revealed that local application of low-dose insulin can promote
wound healing in a diabetic rat model with scalds (8). High glucose conditions may lead to
suppression of vascular endothelial cell proliferation, weakened
cell contacts and increased apoptosis of the cells. At the same
time, the decreased expression of VEGF delays the formation of new
vessels, and the accumulation of advanced glycosylation end
products leads to an extended inflammatory response and restrained
collagen synthesis. The resulting microcirculation disturbance
results in problems, such as insufficiency of local blood supply
and local neurotrophic disturbances.
Whether the insulin is injected via subcutaneous,
infiltration or intravenous injection, its actions depend on its
ability to regulate the blood sugar metabolism. Insulin is a
polypeptide with a short half-life in vivo. It may be
decomposed by proteases in tissue when directly applied to wounds,
and therefore reapplications are needed for treatment. The problem
of how to continually release insulin in an effective manner to
promote the growth of granulation tissue and wound healing is a
known concern (9). Previous findings
have shown that local infiltration injections increase the
absorption efficiency of insulin while exerting little influence on
blood sugar contents (10). The
possibility that insulin may promote wound healing following simple
deep burn skin flap transplantation in humans and its possible
mechanism were analyzed in this clinical case-control study.
Regular insulin was chosen because of its higher purity, easy
absorbability, fast actions, and infimal probability of causing
anaphylactic reactions (11). From
the research findings it may be concluded that the wound healing
rate of the low-dose insulin group significantly improved, the
wound healing time shortened, the flap survival rate increased and
the wound blood flow volume increased. Re-epithelization in the
low-dose insulin group was faster, the infiltration of inflammatory
cells was reduced, and fibroblast and new blood vessel counts were
increased. The high-dose insulin group had the lowest wound healing
rate, longest wound healing time and lowest total survival rate of
flap of all of the groups in the study. By contrast, the
medium-dose insulin group values were also better than the control
group values. This suggests that the insulin effect on wound
healing is related to its concentration. When the concentration is
increased above a certain threshold, the effect is weakened,
probably restraining normal wound healing speed. From this, it
seems clear that a low-dose insulin can promote the regeneration of
wound tissue (12,13).
Expression levels of HSP-90, VEGF, TGF-β and IL-1
were highest after 7 days in the low-dose insulin group, and the
medium-dose insulin group took the second place. The comparative
differences of the other 3 groups were not significant.
After all the biological cells including prokaryotic
and eukaryotic cells were stimulated, the HSP-90 is a protein
released in response to factors such as increased temperature,
pathogen invasion, ischemia, hypoxia and contact to
physical-chemical elements in different tissues, it is thought to
protect cells from harmful stimuli. HSP-90 can promote wound
healing, decrease ischemia reperfusion injury in flaps and increase
survival rate of transplanted flaps (14). VEGF is an important regulatory factor
for new vessels and the strongest mitogen, in the growth factor
family, that promotes endothelial cell proliferation. It also
increases the permeability of capillaries, thus promoting the
formation of new blood vessels (15). TGF-β mainly comes from blood
platelets, it can promote the chemotaxis, migration, proliferation,
and differentiation of cells and the formation of granulation
tissue by extracellular accretion and secretion, essential factors
of wound healing (16,17). IL-1 makes the generation of cells,
neutrophils and lymphocytes possible by chemotaxis. It stimulates
the fibroblasts to produce collagen and controls the formation of
scarring tissue, playing a crucial role in wound healing. Local
low-dose insulin acts independently of blood sugar metabolism in
promoting wound healing, and it plays an important regulatory role
in the course of regeneration of cell factors, growth factors and
inflammatory reactions of multiple epithelia. Accordingly, this
study showed that while the microvessel quantity of low-dose
insulin group increased, the blood sugar levels did only slightly
fluctuate.
In conclusion, the local application of low-dose
insulin can markedly promote wound healing and the survival of
transplanted flaps after operation for deep burns, and this is
probably associated with the stimulation of higher expression
levels of HSP-90, VEGF, TGF-β and IL-1, which nevertheless, exert
little influence on systemic blood glucose. Our approach has
clinical practical value, and should be tested using similar
clinical control studies with a larger population of patients.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (no. 81071552).
References
|
1
|
Yu G, Ye L, Tan W, Zhu X, Li Y and Jiang
D: A novel dermal matrix generated from burned skin as a promising
substitute for deep degree burns therapy. Mol Med Rep. 4:23–25.
2016.
|
|
2
|
Lei J, Hou C, Duan P, Hao Z, Zhai Y and
Meng Y: Clinical application of modified skin soft tissue expansion
in early repair of devastating wound on the head due to electrical
burn. Zhonghua Shao Shang Za Zhi. 31:406–409. 2015.(In Chinese).
PubMed/NCBI
|
|
3
|
Chi Y, Chai J, Xu C, Luo H and Zhang Q:
Apelin inhibits the activation of the nucleotide-binding domain and
the leucine-rich, repeat-containing family, pyrin-containing 3
(NLRP3) inflammasome and ameliorates insulin resistance in severely
burned rats. Surgery. 157:1142–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang M, Xue X, Xie P and Zhang J: Effects
of nerve growth factor-insulin composite gel on deep second degree
scald wound healing in diabetic rats. Zhongguo Xiu Fu Chong Jian
Wai Ke Za Zhi. 27:182–188. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Bircher A, de Boer EM, Agner T, Wahlberg
JE and Serup J: Guidelines for measurement of cutaneous blood flow
by laser Doppler flowmetry. A report from the Standardization Group
of the European Society of Contact Dermatitis. Contact Dermatitis.
30:65–72. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong J, Tang Q, Li W and Tian F: Our
preliminary experiences in topical administration of insulin in
addition to vacuum assisted closure for wound healing in diabetic
patients. Minerva Chir. 70:389–391. 2015.PubMed/NCBI
|
|
7
|
Kim SM, Kim YH, Jun YJ, Yoo G and Rhie JW:
The effect of diabetes on the wound healing potential of
adipose-tissue derived stem cells. Int Wound J. 13(Suppl 1): 33–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salazar JJ, Ennis WJ and Koh TJ: Diabetes
medications: Impact on inflammation and wound healing. J Diabetes
Complications. 30:746–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhall S, Silva JP, Liu Y, Hrynyk M, Garcia
M, Chan A, Lyubovitsky J, Neufeld RJ and Martins-Green M: Release
of insulin from PLGA-alginate dressing stimulates regenerative
healing of burn wounds in rats. Clin Sci (Lond). 129:1115–1129.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azevedo F, Pessoa A, Moreira G, Santos MD,
Liberti E, Araujo E, Carvalho C, Saad M and Lima MH: Effect of
topical insulin on second-degree burns in diabetic rats. Biol Res
Nurs. 18:181–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Attia EA, Belal DM, El Samahy MH and El
Hamamsy MH: A pilot trial using topical regular crystalline insulin
vs. aqueous zinc solution for uncomplicated cutaneous wound
healing: Impact on quality of life. Wound Repair Regen. 22:52–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XJ, Meng C, Chinkes DL and Herndon
DN: Beneficial effects of insulin on cell proliferation and protein
metabolism in skin donor site wound. J Surg Res. 168:e155–e161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang XJ, Wu X, Wolf SE, Hawkins HK,
Chinkes DL and Wolfe RR: Local insulin-zinc injection accelerates
skin donor site wound healing. J Surg Res. 142:90–96. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woodley DT, Wysong A, DeClerck B, Chen M
and Li W: Keratinocyte migration and a hypothetical new role for
extracellular heat shock protein 90 alpha in orchestrating skin
wound healing. Adv Wound Care (New Rochelle). 4:203–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puddu A, Sanguineti R, Traverso CE,
Viviani GL and Nicolò M: Response to anti-VEGF-A treatment of
endothelial cells in vitro. Exp Eye Res. 146:128–136. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kryger ZB, Sisco M, Roy NK, Lu L,
Rosenberg D and Mustoe TA: Temporal expression of the transforming
growth factor-Beta pathway in the rabbit ear model of wound healing
and scarring. J Am Coll Surg. 205:78–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Sydow A Koskela, Janbaz C, Kardeby C,
Repsilber D and Ivarsson M: IL-1α counteract TGF-β regulated genes
and pathways in human fibroblasts. J Cell Biochem. 117:1622–1632.
2016. View Article : Google Scholar : PubMed/NCBI
|