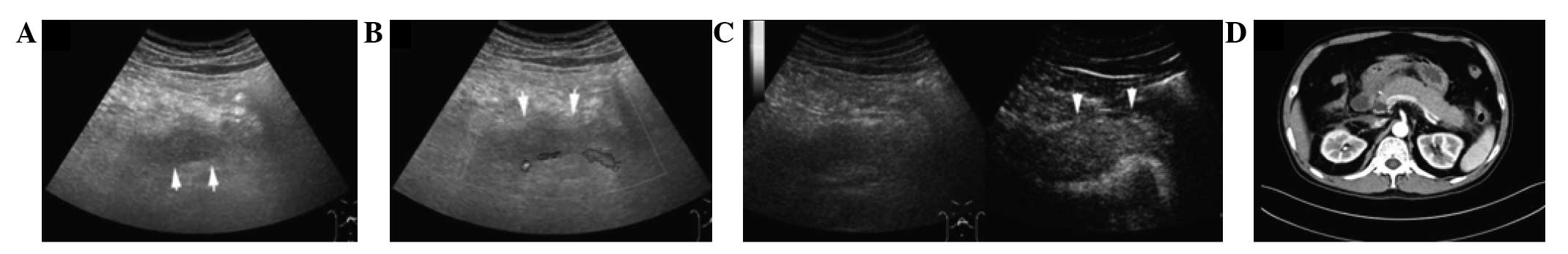
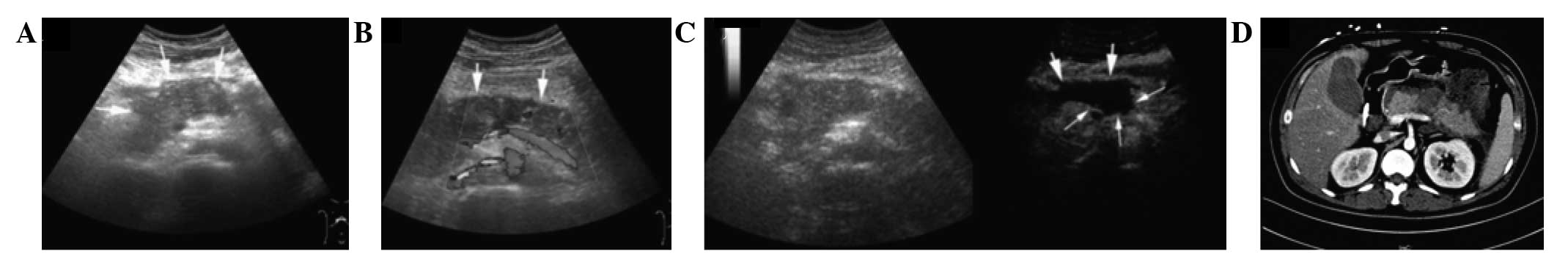
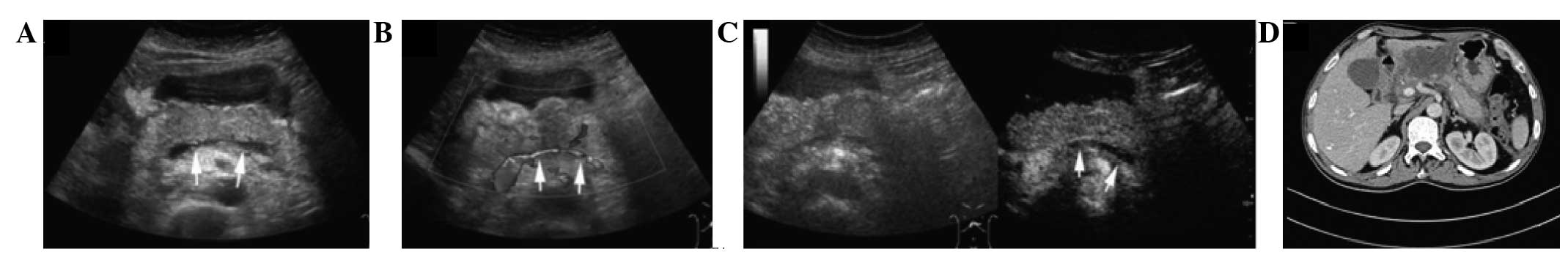
Introduction
Acute pancreatitis (AP), which can be divided into
mild acute pancreatitis (MAP) and severe acute pancreatitis (SAP),
is an inflammatory disorder of the pancreas that has been
clinically defined as a common form of acute abdominal pain
(1–3). MAP has a good prognosis and few
complications with low mortality. However, SAP accompanied by
serious complications is reported to have a high mortality rate
(4). Although SAP accounts for only
15–25% of AP cases, its mortality rate may be as high as 15–48.4%
(5). Early diagnosis and evaluation
of the severity of pancreatitis is crucial in AP management
(6,7).
Ultrasonographic examination is a medical diagnostic
imaging modality that is commonly used for AP because of its
convenience, portability, safety in terms of radiation exposure,
and low cost. Biliary tract disease, such as gallstones, is the
main etiological factor leading to AP in China (8). Conventional ultrasonography (CUS) is
considered to be a useful diagnostic imaging modality for AP
because it not only is able to detect pancreatic and peripancreatic
conditions, but also can be used to investigate whether biliary
tract disease has led to the occurrence of AP (9).
However, the diagnostic sensitivity of CUS for SAP
is reported to be low (6). It is
also unclear whether CUS is more important for diagnosing AP or
SAP. Some studies have shown that contrast-enhanced ultrasonography
(CEUS) has become an important diagnostic method for SAP because it
is able to detect necrosis of the pancreatic parenchyma without the
hepatic and renal toxicity possible with contrast-enhanced computed
tomography (CECT) (10,11). If a difference in accuracy between
CUS and CEUS for the diagnosis of AP and SAP exists, CEUS
examination should be routinely applied instead of CUS to diagnose
AP or SAP.
Pancreatic size, peripancreatic fluid collection
(PPFC), and splenic vessel complications are the variables observed
on CUS and CEUS that are accepted to be important for assessing AP.
CECT is the gold standard imaging modality for the diagnosis of AP
(12), particularly for SAP, but
some research studies have found that SAP can be successfully
diagnosed using CEUS (13). A
previous study reported that a diagnosis of SAP made using CEUS was
strongly correlated with that made using CECT (14). It has been demonstrated that CEUS has
a higher diagnostic value than CUS for AP or SAP (15,16).
However, the specific differences between CUS and
CEUS in terms of the diagnostic sensitivity for AP or SAP have not
been clarified in the literature. Therefore, the present study
aimed to investigate whether there any statistically significant
differences between CUS and CEUS, and between CECT and CEUS in
terms of diagnostic accuracy for AP or SAP. In addition, it aimed
to investigate the difference in diagnostic accuracy for AP and SAP
between CUS and CEUS in comparison with that of CECT as the gold
standard. This study focused on differences between imaging with
different types of US. The clinical and laboratory factors
associated with AP were not considered in this study.
Materials and methods
Patient data
This study was a prospective study. It was conducted
in accordance with the Declaration of Helsinki, and with approval
from the Ethics Committee of Sichuan University (Chengdu, China).
Written informed consent was obtained from all participants. In
total, 220 inpatients given a clinical diagnosis of AP at Sichuan
University from May 2011 to December 2012 were selected for this
study. The inclusion criteria were: Patients undergoing CUS, CEUS
and CECT examinations without any contraindications. The pancreas
was displayed clearly on CUS. Exclusion criteria: Any AP patients
who could not undergo the three examinations, or patients for which
the pancreas could not be distinctly shown by US. According to the
criteria, 5 patients had to be excluded from the study because
sonographic imaging of the pancreas was impaired by overlying bowel
gas prohibiting visualization of the pancreas during the US and
CEUS examinations; 3 patients were excluded because of pain that
made examination impossible; 10 patients were excluded because they
refused to undergo CEUS examination; 4 patients had a
contraindication to administration of the ultrasound contrast
medium SonoVue due to coronary heart disease; and 2 patients were
excluded because of contraindications to computed tomography (CT)
with contrast injection (renal failure or known allergy to
iodinated contrast medium). Finally, 196 patients constituted the
study population.
All patients were examined using CUS and pancreatic
CEUS in order to confirm the AP diagnosis and, if confirmed,
whether it was MAP or SAP. All ultrasonographic examinations were
performed within 72 h of admission. All CECT examinations were
performed within 4 h of the ultrasound (in all cases within 72 h
after the onset of symptoms). The sonographer and radiologist were
blinded to other laboratory or imaging findings, respectively.
Sonographic examination
The LOGIQ E9 ultrasonographic system (GE Healthcare,
Milwaukee, WI, USA) with a C1-5-MHz corresponding probe or the
PHILIPS iU22 (Philips Medical Systems, Bothell, WA, USA)
ultrasonographic system with a corresponding C5-2-MHz transducer
was used. The two ultrasonographic systems were each equipped with
harmonic contrast pulse sequencing technology. The contrast agent
used was SonoVue (Bracco SpA, Milan, Italy), which is a suspension
of stabilized sulfur hexafluoride microbubbles.
Two sonographers who had >10 years' experience
with CUS of the abdomen and >3 years' experience with CEUS
evaluation for pancreatic disease were chosen to perform the
examinations. All patients fasted for 8 h prior to undergoing the
ultrasonographic examination. First, grayscale sonography and color
Doppler ultrasonography were performed in order to observe the
pancreas volume or changes in parenchymal structure. The PPFC,
including the bursa omentalis and peripancreas interspaces,
particularly the bilateral pararenal space; the anterior pararenal
space; and the posterior pararenal space were all examined. Any
splenic vessel complications, including splenic artery embolism,
splenic artery stenosis, splenic artery aneurysms, splenic vein
embolism, and splenic vein stenosis were observed. The CUS results
were recorded.
A different sonographer then started the CEUS
program at a low mechanical index (LOGIQ E9, 0.12; PHILIPS iU22,
0.06). A 1.5–2.0 ml volume of contrast agent suspension (SonoVue)
was administered as a bolus injection through the antecubital vein,
followed by flushing with 5 ml saline solution. After an 8–15 sec
delay, the pancreatic parenchyma began to undergo enhancement. The
real-time contrast-enhanced image obtained after contrast agent
injection was recorded on a hard disk, and the injection times were
calculated simultaneously. In the entirely arterial pancreatic and
splenic systems, the contrast phases were identified as the artery
phase (0–30 sec after contrast agent injection) and venous phase
(starting at 31 sec after contrast agent injection). The results of
the pancreatic CEUS with pancreas, PPFC, and splenic vessel
complications were recorded.
CT examination
For this study, 64-slice spiral CT (Philips
Brilliance; Philips Medical Systems, Cleveland, OH, USA) or
18-slice spiral CT (Somatom Sensation 16; Siemens, Erlangen,
Germany) was used. The contrast agent was iopamidol (Iopamiro;
Bracco SpA) or iopromide (Ultravist; Bayer Schering Pharma AG,
Leverkusen, Germany) at a concentration of 37 gI/100 ml.
The range of CECT scanning was from the chest to the
pelvic floor. A total amount of 90–120 ml iopamidol or iopromide
was injected into each patient via the antecubital vein at a rate
of 3 ml/sec using a power injector.
Standard of diagnosis
Physicians with >5 years of experience with
abdominal imaging readings interpreted the results. The physicians
were blinded to the ultrasonographic imaging results. First, an
increase in pancreas volume and changes in parenchymal structure
were assessed using CECT, which was considered as the standard
diagnostic imaging modality for AP. The PPFC and splenic vessel
complications were then assessed. Finally, the pancreatic
parenchyma was assessed for pancreatic necrotic lesions, the
Balthazar grade was determined (17), and a diagnosis of SAP was made
accordingly. The standard indicators for SAP diagnosis included the
presence of a non-enhancing area in the pancreas and/or a Balthazar
severity grade D or E. The minimum size of heterogeneity or
decreased enhancement area with a pancreatic necrotic lesion was 1
cm.
The sonographic films were read by two sonographers.
First, the CUS films were read by two experienced sonographers with
>10 years' experience with abdominal ultrasonography who were
blinded to other laboratory or imaging findings. They evaluated the
images for an increase in pancreas volume, changes in the
parenchymal structure with a hypoechoic appearance (compared with
the echo of the surrounding liver) and whether the echogenicity was
uniform.
The criteria for a normal pancreas on
ultrasonography were that the head of pancreas was not >2.5 cm
and the body or tail of the pancreas was not >2.0 cm. On a
transverse section, the pancreas anteroposterior diameter was used
as the size of the pancreatic head, which was measured adjacent to
the portal vein. The body of the pancreas was measured at the
position of the anterior of the aorta abdominalis on a transverse
section. The tail of the pancreas was measured at the hilus lienis
on an oblique transverse section.
The sonographers made a diagnosis of SAP according
to peripancreatic or retroperitoneal space effusion, the change in
pancreatic parenchymal echogenicity, the continuous status of the
pancreatic capsule, the pancreatic parenchyma necrosis, and splenic
vessel complications observed with color Doppler technology. All
results were recorded in the computer.
The sonographers then read the CEUS films to search
for signs of pancreatic swelling, pancreatic parenchyma necrosis,
PPFC including the bursa omentalis, retroperitoneal space effusion,
and/or local complications such as necrotic tissue, abscess and
pancreatic pseudocysts. The CEUS diagnostic standard indicators of
SAP were the same criteria with a Balthazar CT score to measure the
severity of AP, and included the presence of pancreatic necrosis
and/or one or more PPFCs and/or complications. Splenic vessel
complications were observed and recorded using CEUS.
Statistical analysis
The disease severity was established according to
the aforementioned CECT criteria. P<0.05 was considered to
indicate a statistically significant result. SPSS version 18.0
software (SPSS, Inc., Chicago, IL, USA) with the Pearson
χ2 test used to analyze the observations, including the
pancreatic size, PPFC and splenic vessel complications, with
pairwise comparisons between CECT, US and CEUS. The χ2
test was used to analyze the diagnostic accuracy of CUS and CEUS
for AP and SAP.
Results
Results obtained using CECT, US, and
CEUS
There were 196 patients [129 men and 67 women; age
(mean ± standard deviation), 48.1±13.9 years; range, 18–79 years].
included in the study. All cases were inpatients at West China
Hospital, Sichuan University.
According to the design of experiments, the gold
standard is CECT at 72 h for SAP with Balthazar's severity grade.
CUS criteria for AP were: The echogenicity was hypoechoic, the
pancreatic volume was enlarged, which was indicative of swelling
(the head of the pancreas was >2.5 cm or the body or tail was
>2.0 cm) and PPFC was found surrounding the pancreas or the
retroperitoneal space effusion. The CUS criteria for SAP were: In
addition to the AP criteria, pancreatic parenchymal necrosis or
splenic vessel complications detected using color Doppler. The CEUS
criteria for AP were: Pancreatic volume was enlarged (the head of
the pancreas was >2.5 cm, the body or tail of the pancreas was
>2.0 cm), or the continuous status of the pancreatic capsule was
interrupted. PPFC was found in the peripancreatic or
retroperitoneal space effusion. The CEUS criteria for SAP were:
Necrosis of the pancreatic parenchyma was detected and splenic
vessel complications were observed. The degree of necrosis was
evaluated, and categorized as <30%, 30–50% or >50%.
By CECT, 122 and 74 patients were diagnosed with and
without pancreatic swelling, respectively. In addition, PPFC was
detected in 178 patients, and splenic vessel complications were
found in 51 patients. By CUS, 148 patients were diagnosed with
pancreatic swelling, 151 with PPFC, and 12 with splenic vessel
complications. By CEUS, 110 patients were diagnosed with pancreas
swelling, 172 with PPFC, and 30 with splenic vessel complications
(Table I).
 | Table I.Results of observations by CECT, CUS
and CEUS and the rate of diagnosis in the total study
population. |
Table I.
Results of observations by CECT, CUS
and CEUS and the rate of diagnosis in the total study
population.
| Observations | Examination
results | CECT, n (%) | CUS, n (%) | CEUS, n (%) |
|---|
| Parenchyma with
enlarged pancreas | Homogeneous | 77 (39.3) | 83 (42.3) | 71 (36.2) |
|
| Inhomogeneous | 45 (23.0) | 65 (33.2) | 39 (19.9) |
| Parenchyma with no
enlarged pancreas | Homogeneous | 12 (6.1) | 29 (14.8) | 20 (10.2) |
|
| Inhomogeneous | 62 (31.6) | 19 (9.7) | 66 (33.7) |
| Peripancreatic
fluid collection | Positive | 178 (90.8) | 151 (77.0) | 172 (87.8) |
|
| Negative | 18 (9.2) | 45 (23.0) | 24 (12.2) |
| Splenic vessel
complications | Positive | 51 (26.0) | 12 (6.1) | 30 (15.3) |
|
| Negative | 145 (74.0) | 181 (92.3) | 170 (86.7) |
Statistical data for pancreatic size
obtained using the three modalities
For pancreatic volume in the same section determined
using the three methods, statistically significant differences were
observed among the three methods [χ2=43.227, degrees of
freedom (df)=2, P=0.0000001). CECT and CEUS significantly differed
from CUS (CECT vs. CUS: χ2=33.737, df=1, P=0.0000002;
CEUS vs. CUS: χ2=35.076, df=1, P=0.0000001). However, no
significant difference was observed between CECT and CEUS
(χ2=2.797, df=1, P=0.424; Fig. 1). P<0.05 was considered to
indicate a statistically significant difference.
Statistical data for PPFC obtained
using the three modalities
For PPFC, statistically significant differences were
observed among the three methods (χ2=16.269, df=2,
P=0.0003). CECT and CEUS significantly differed from CUS (CECT vs.
CUS: χ2=13.787, df=1, P=0.0002; CEUS vs. CUS:
χ2=7.757, df=1, P=0.005). However, no significant
difference was observed between CECT and CEUS (χ2=0.960,
df=1, P=0.327; Fig. 2).
Statistical data for splenic vessel
complications obtained using the three modalities
For splenic vessel complications, including splenic
artery and vein complications such as splenic artery embolism,
splenic vein embolism, splenic artery stenosis, splenic vein
stenosis, and splenic artery aneurysms, statistically significant
differences were observed among the three methods
(χ2=29.199, df=2, P=0.0000004). CECT significantly
differed between CEUS and CUS (CECT vs. CUS: χ2=28.766,
df=1, P=0.00000008, CECT vs. CEUS: χ2=6.862, df=1,
P=0.009). CEUS significantly differed from CUS
(χ2=8.640, df=1, P=0.003; Fig. 3).
Statistical data for AP and SAP
diagnosis using the three modalities
Among the 196 patients, 132 were diagnosed with SAP
by CECT and 64 patients were diagnosed with MAP. By CUS, 167 were
diagnosed with AP and 63 were diagnosed with SAP. By CEUS, 184
patients were diagnosed with AP and 103 with SAP (Tables II and III). Analysis using χ2 tests,
revealed a significant difference between CEUS and CUS in terms of
AP (χ2=7.872, df=1, P=0.005) and SAP diagnoses
(χ2=25.965, df=1, P=0.0000003).
 | Table II.Diagnosis of AP by CUS and CEUS. |
Table II.
Diagnosis of AP by CUS and CEUS.
| Imaging | Positive | Negative | Total |
|---|
| CUS | 167 | 29 | 196 |
| CEUS | 184 | 12 | 196 |
| Total | 351 | 41 | 392 |
 | Table III.Diagnosis of SAP by CUS and CEUS. |
Table III.
Diagnosis of SAP by CUS and CEUS.
| Imaging | Positive | Negative | Total |
|---|
| CUS | 63 | 69 | 132 |
| CEUS | 103 | 29 | 132 |
| Total | 166 | 98 | 264 |
Discussion
Medical imaging is an important diagnostic method
for AP. Ultrasonography is widely used in AP diagnosis (18). In the present study, among the 196
patients, there were 167 and 184 patients diagnosed with AP using
CUS and CEUS, respectively. The results for diagnosis by CEUS were
closer than those of CUS to the diagnosis results obtained using
the gold standard of CECT. This demonstrates that CEUS is a more
convincing method than US for AP and SAP diagnosis. The results
conformed with those reported in previous studies, that CEUS
produces excellent results in the staging of acute pancreatitis
severity (10,12,14–16).
Pancreatic swelling is an important feature of AP
(19). In comparison with the
results obtained using CECT, the diagnostic rates with CUS and CEUS
were 121% (148/122) and 91% (111/122), respectively. These results
indicate the CUS overestimated the pancreatic size and thus did not
reflect the real size of the pancreas. One reason for this may be
that CUS barely differentiates the boundary of the pancreas. In
particular, when PPFC appeared in the lesser peritoneal sac within
hemorrhage or abscess with hypoecho there was no boundary between
the PPFC and edema of the pancreatic parenchyma, which also appears
hypoechoic by CUS.
PPFC often occurs after the onset of AP (20). In comparison with the results
obtained using CECT, the CUS diagnostic rate of PPFC was 84.8%
(151/178). With CEUS, the diagnostic rate was 96.6% (172/178), and
the results were closer to those for CECT. Since the quality of
pancreatic fluid collection varies, echogenic features also vary.
CEUS is more specific than CUS in displaying the pancreatic
parenchyma edema, the border-capsula of the pancreas, the
collection fluid of the peripancreas, and the peripancreatic
necrosis. A previous study indicated that CUS hardly differentiated
between PPFC and pancreatic parenchymal swelling (21).
In the present study, the results confirmed that CUS
overestimated pancreatic size and underestimated pancreatic fluid
collection. By contrast, the pancreatic parenchyma was enhanced on
CEUS. An ultrasonographic finding of pancreatic parenchymal
necrosis exhibits no enhancement area following the injection of
contrast agent. This result in PPFC concurs with that reported by
Golea et al (22).
Peripancreatic vessel complications of AP have low
morbidity (23,24). They include splenic vessel, portal
vein and mesenteric vessel complications (25,26).
Most patients with splenic vessel complications in AP present with
few clinical symptoms, but such complications may lead to severe,
sometimes fatal, outcomes, such as splenic artery aneurysm rupture
(27). In this study, splenic vessel
complications were examined; splenic artery and vein complications
such as splenic artery embolism, splenic vein embolism, splenic
artery stenosis, splenic vein stenosis, and splenic artery
aneurysms are examples of the complications (28–30).
In the present study, all patients with splenic
vessel complications were from the SAP group. Studies concerning
the use of CUS in the diagnosis of splenic vessel complications are
few (31). Since the peripancreatic
vessels are located in the posterior peritoneum, echo attenuation
is the main factor that affects diagnosis; however, the results of
grayscale sonography are greatly affected by meteorism in the
gastrointestinal tract, and diagnosis is sometimes difficult
(32). In the present study, the
diagnostic rates with CUS and CEUS were 23.5% (12/51) and 58.8%
(30/51), respectively. The diagnostic yield of CUS is lower than
that of CEUS (23.5% vs. 58.8%). The reasons why ultrasonography
often resulted in missed diagnoses of splenic vessel complications
require consideration. The ultrasonographic result would have been
greatly affected by meteorism in the gastrointestinal tract,
particularly the location of the pancreatic tail, which was the
predilection site of splenic artery complications. The difference
between CUS and CEUS may be attributed to the fact that CEUS is
able to display the vessels with contrast agent injection (33,34).
Early diagnosis of SAP is important in optimizing
the monitoring and treatment of patients as early as possible
(35–37). CECT is considered to be the standard
diagnostic imaging modality for SAP (12). Diagnosis of SAP using CUS is based on
indicators such as pancreatic size, PPFC, and the quality of
echogenicity of the pancreatic parenchyma or fluid collection.
However, CUS is unable to detect pancreatic parenchyma (38). Although the diagnosis of SAP using
CUS has indirect objectives, these are not widely accepted
(39,40). This is because the necrosis
pancreatic parenchyma can not be shown by CUS directly, and the
pancreatic size, PPFC and the quality of echogenicity of the
pancreatic parenchyma or fluid collection are regarded as
indicators of SAP by CUS indirectly.
Diagnosis was more accurate with CEUS than with CUS.
The diagnostic accuracy rates of US and CEUS for SAP in the present
study were 47.7% (63/132) and 78.0% (103/132), respectively. As
demonstrated in previous studies, in terms of the assessment of
pancreatic size and PPFC, the diagnostic accuracy rate for splenic
vessel complications, and statistical results, CEUS was superior to
US in the diagnosis of SAP (10,14,22).
Significant differences were observed between the results for CEUS
and CUS. Since contrast agents can clearly show the
microcirculation of the pancreatic parenchyma, necrosis of the
pancreatic parenchyma will be clearly displayed on CEUS. Thus,
pancreatic parenchymal necrosis should display no enhanced area
following contrast agent injection (32).
In conclusion, CEUS is a reliable method for the
diagnosis and monitoring of AP and SAP, and may serve as a
substitute for CECT.
Acknowledgements
The authors are indebted to Professors Xia Q, Huang
ZW and Tang WF (Department of Western and Chinese Integrated
Medicine of West China Hospital) for providing the cases in this
study. This study was supported by the nurses Lan L and Feng XY who
were the operators of contrast agent injection.
References
|
1
|
Harvey C, Hart JL and Lloyd CR: Ultrasound
in the acute abdomen. Br J Hosp Med (Lond). 69:M116–M119. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pach R, Kulig P, Kołodziejczyk P,
Szczepanik A and Sierżęga M: Ultrasonography in the diagnosis of
acute abdominal disorders. Pol Przegl Chir. 84:590–600.
2012.PubMed/NCBI
|
|
3
|
Ćwik G: Standards of the Polish Ultrasound
Society - update. Pancreas examination. J Ultrason. 13:167–177.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frossard JL, Steer ML and Pastor CM: Acute
pancreatitis. Lancet. 371:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dellinger EP, Forsmark CE, Layer P, Lévy
P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G,
Whitcomb DC, et al: Determinant-based classification of acute
pancreatitis severity: An international multidisciplinary
consultation. Ann Surg. 256:875–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Triester SL and Kowdley KV: Prognostic
factors in acute pancreatitis. J Clin Gastroenterol. 34:167–176.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bollen TL, Singh VK, Maurer R, Repas K,
van Es HW, Banks PA and Mortele KJ: A comparative evaluation of
radiologic and clinical scoring systems in the early prediction of
severity in acute pancreatitis. Am J Gastroenterol. 107:612–619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai Y, Liu Y, Jia L, Jiang H, Ji M, Lv N,
Huang K, Zou X, Li Y, Tang C, et al: Severe acute pancreatitis in
China: Etiology and mortality in 1976 patients. Pancreas.
35:232–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gandolfi L, Torresan F, Solmi L and
Puccetti A: The role of ultrasound in biliary and pancreatic
diseases. Eur J Ultrasound. 16:141–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rickes S, Uhle C, Kahl S, Kolfenbach S,
Monkemuller K, Effenberger O and Malfertheiner P: Echo enhanced
ultrasound: A new valid initial imaging approach for severe acute
pancreatitis. Gut. 55:74–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ardelean M, Şirli R, Sporea I, Bota S,
Martie A, Popescu A, Dănila M, Timar B, Buzas R and Lighezan D:
Contrast enhanced ultrasound in the pathology of the pancreas - a
monocentric experience. Med Ultrason. 16:325–331. 2014.PubMed/NCBI
|
|
12
|
Balthazar EJ: Acute pancreatitis:
Assessment of severity with clinical and CT evaluation. Radiology.
223:603–613. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faccioli N, Crippa S, Bassi C and
D'Onofrio M: Contrast-enhanced ultrasonography of the pancreas.
Pancreatology. 9:560–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Q, Zhong Y, Wen XR, Huang ZW, Fan YT,
Xia Q and Luo Y: Can contrast-enhanced ultrasound evaluate the
severity of acute pancreatitis? Dig Dis Sci. 56:1578–1584. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rickes S, Rauh P, Uhle C, Ensberg D,
Mönkemüller K and Malfertheiner P: Contrast-enhanced sonography in
pancreatic diseases. Eur J Radiol. 64:183–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rickes S, Mönkemüller K and Malfertheiner
P: Acute severe pancreatitis: Contrast-enhanced sonography. Abdom
Imaging. 32:362–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murphy KP, O'Connor OJ and Maher MM:
Updated imaging nomenclature for acute pancreatitis. AJR Am J
Roentgenol. 203:W464–W469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Windsor JA and Petrov MS: Acute
pancreatitis reclassified. Gut. 62:4–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shanaman MM, Schwarz T, Gal A and O'Brien
RT: Comparison between survey radiography, B-mode ultrasonography,
contrast-enhanced ultrasonography and contrast-enhanced
multi-detector computed tomography findings in dogs with acute
abdominal signs. Vet Radiol Ultrasound. 54:591–604. 2013.PubMed/NCBI
|
|
20
|
Dhaka N, Samanta J, Kochhar S, Kalra N,
Appasani S, Manrai M and Kochhar R: Pancreatic fluid collections:
What is the ideal imaging technique? World J Gastroenterol.
21:13403–13410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bollen TL, van Santvoort HC, Besselink MG,
van Es WH, Gooszen HG and van Leeuwen MS: Update on acute
pancreatitis: Ultrasound, computed tomography and magnetic
resonance imaging features. Semin Ultrasound CT MR. 28:371–383.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Golea A, Badea R, Socaciu M, Diaconu B and
Iacob D: Quantitative analysis of tissue perfusion using
contrast-enhanced transabdominal ultrasound (CEUS) in the
evaluation of the severity of acute pancreatitis. Med Ultrason.
12:198–204. 2010.PubMed/NCBI
|
|
23
|
Patil PV, Khalil A and Thaha MA: Splenic
parenchymal complications in pancreatitis. JOP. 12:287–291.
2011.PubMed/NCBI
|
|
24
|
Hayashi H, Beppu T, Okabe K, Masuda T,
Okabe H and Baba H: Risk factors for complications after partial
splenic embolization for liver cirrhosis. Br J Surg. 95:744–750.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Liu GJ, Chen YX, Dong HP and Wang
LX: Sinistral portal hypertension: Clinical features and surgical
treatment of chronic splenic vein occlusion. Med Princ Pract.
21:20–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heider R and Behrns KE: Pancreatic
pseudocysts complicated by splenic parenchymal involvement: Results
of operative and percutaneous management. Pancreas. 23:20–25. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carr SC, Mahvi DM, Hoch JR, Archer CW and
Turnipseed WD: Visceral artery aneurysm rupture. J Vasc Surg.
33:806–811. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malka D, Hammel P, Lévy P, Sauvanet A,
Ruszniewski P, Belghiti J and Bernades P: Splenic complications in
chronic pancreatitis: Prevalence and risk factors in a
medical-surgical series of 500 patients. Br J Surg. 85:1645–1649.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mortelé KJ, Mergo PJ, Taylor HM, Ernst MD
and Ros PR: Splenic and perisplenic involvement in acute
pancreatitis: Determination of prevalence and morphologic helical
CT features. J Comput Assist Tomogr. 25:50–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madoff DC, Denys A, Wallace MJ, Murthy R,
Gupta S, Pillsbury EP, Ahrar K, Bessoud B and Hicks ME: Splenic
arterial interventions: Anatomy, indications, technical
considerations, and potential complications. Radiographics.
25(Suppl 1): S191–S211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koito K, Namieno T, Nagakawa T and Morita
K: Splenic artery prior to rupture in the pancreatic pseudocyst:
Detection by endoscopic color Doppler ultrasonography. J Ultrasound
Med. 15:721–724. 1996.PubMed/NCBI
|
|
32
|
Dietrich CF: Comments and illustrations
regarding the guidelines and good clinical practice recommendations
for contrast-enhanced ultrasound (CEUS)-update 2008. Ultraschall
Med. 29(Suppl 4): S188–S202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai DM, Parajuly SS, Ling WW, Li YZ and
Luo Y: Diagnostic value of contrast enhanced ultrasound for splenic
artery complications following acute pancreatitis. World J
Gastroenterol. 20:1088–1094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai DM, Luo Y, Li YZ, Tang WF, Huang ZW
and Song B: Assessing splenic vein complications in patients with
acute pancreatitis using color Doppler ultrasound and contrast
enhanced ultrasound. Sichuan Da Xue Xue Bao Yi Xue Ban. 45:850–853.
2014.(In Chinese). PubMed/NCBI
|
|
35
|
Garcea G, Jackson B, Pattenden CJ, Sutton
CD, Neal CP, Dennison AR and Berry DP: Early warning scores predict
outcome in acute pancreatitis. J Gastrointest Surg. 10:1008–1015.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruiz R, Pozo E and Gonzáles M: Early
prognostic risk factors in acute pancreatitis: Study of a
representative population from Peru. Rev Gastroenterol Peru.
12:5–11. 1992.(In Spanish). PubMed/NCBI
|
|
37
|
Horzić M, Bunoza D and Marić K: Meaning of
prognostic factors in patients with acute pancreatitis. Acta Med
Croatica. 51:49–52. 1997.PubMed/NCBI
|
|
38
|
Topal NB, Kaya E, Ercan I, Pourbagher MA
and Topal U: The role of Doppler sonography in predicting severity
of acute pancreatitis. J Clin Ultrasound. 36:141–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo Y, Yuan CX, Peng YL, Wei PL, Zhang ZD,
Jiang JM, Dai L and Hu YK: Can ultrasound predict the severity of
acute pancreatitis early by observing acute fluid collection? World
J Gastroenterol. 7:293–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ripollés T, Martínez MJ, López E, Castelló
I and Delgado F: Contrast-enhanced ultrasound in the staging of
acute pancreatitis. Eur Radiol. 20:2518–2523. 2010. View Article : Google Scholar : PubMed/NCBI
|