Introduction
Lung cancer, as one of the most malignant tumors,
has a huge social and economic impact on human health in China and
the world (1). According to
statistics from the National Office on Tumor Cure and Prevention of
China, 700,000 people die of lung cancer annually (2). Despite notable advances in the
diagnosis and treatment of lung cancer, many of the
chemotherapeutic drugs currently used to treat lung cancer are
either not highly effective or may lose their efficacies due to the
development of drug resistance (3).
Hence, it is important to discover and develop novel drugs for lung
cancer treatment.
Natural chemicals have much more chemical diversity
than synthetic ones, and have long been recognized as privileged
scaffolds to develop drugs due to their evolved biological target
specificities, and their proven biological targets are
predominantly diverse functional proteins of organisms (4,5). Natural
chemical library screenings typically yield higher hit rates of
drug-like active compounds than ones that are acquired from
synthetic molecule library screenings (6). Previous studies have demonstrated that
phytochemical extracts or mixtures from several medicinal herbs
exhibit anticancer activities in vitro or in vivo and
are valuable natural sources for drug-like active natural compound
screenings (7–10).
Chenopodium album Linne is a fast-growing
annual weedy plant, belonging to the Chenopodium family,
which is widely distributed in hot sub-tropical and tropical
climates, as well as temperate regions of the world. Studies on
various phytochemical constituents of C. album have
indicated that the plant contains phytochemicals with various
pharmacological effects, including antiviral, antifungal,
antioxidant, anti-inflammatory, antiallergic and antiseptic
activities (11–13). However, to date little research
pertaining to the possible anticancer phytochemical constituents of
this plant has been performed. Khoobchandani et al (14) reported that the ethyl acetate and
methanol extracts of C. album prevented the cell growth of
human breast cancer MCF-7 cells. Although folk medical usage of
C. album L. in China has been documented, there are no
reports of its phytochemical extracts on the possible activity
against lung cancer. The present study used medicinal plant
phytochemical extract library screening to identify the petroleum
ether (PE) extract of C. album L. in order to investigate
its effects on the proliferation and cell cycle progression of A549
cells. The present results may provide data to support the use of
phytochemicals from C. album L. to develop novel cancer
therapies.
Materials and methods
Preparation of the extracts of
plants
Medicinal plant materials were acquired from the
wild in Kunming (Yunnan, China) during the summer of 2014 to
prepare a phytochemical extract library, which was identified by
Dr. Haizhou Li from the Faculty of Life Science and Technology of
Kunming University of Science and Technology (Kunming, China). For
the preparation of the phytochemical extracts, the plant materials,
including branches and leaves, were washed, dried, and finely
chopped and grinded. The samples were first extracted with 95%
ethanol by an ultrasonic method (15), and were subsequently evaporated using
a rotary evaporator (EYELA, Tokyo, Japan). Following this, the
dried material was successively extracted using PE, and was
subsequently treated with chloroform, ethyl acetate, n-butyl
alcohol in a Soxhlet extractor (EYELA). Extracts were filtered and
concentrated using a rotary evaporator to evaporate until they were
dry. All the dried extracts were weighed and solved with 99.9%
(v/v) DMSO (Beyotime Institute of Biotechnology, Haimen, China) to
prepare stock solutions at concentration of 100 mg/ml.
Subsequently, 100 µl of each phytochemical stock solution was
allotted into each well of a 96-well microplate to form a
phytochemical extract screening library.
Cell lines and culture
Human non-small cell lung cancer A549 cell line was
purchased from the Kunming Institute of Zoology, Chinese Academy of
Sciences (Kunming, China). A549 cells were maintained in RPMI 1640
medium supplemented with 10% (v/v) fetal calf serum (ScienCell
Research Laboratories, Inc., Carlsbad, CA, USA) and 100 U/ml
penicillin and streptomycin (Solarbio Science & Technology Co.,
Ltd., Beijing, China), asnd were incubated at 37°C in a humidified
incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
5% CO2 supplementation.
Anticancer phytochemical extract
screening and IC50s determination
A549 cells in 100 µl medium were seeded in a 96-well
plate at a density of 5×103 cells/well. Following 24 h,
the cells were either treated with phytochemical extracts at
different concentrations (3.91, 7.81, 15.63, 31.5, 62.5,125, 250
and 500 µg/ml) for 24, 48 and 72 h, respectively, or treated with
0.5% DMSO as controls. Subsequently, 5 mg/ml MTT (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) solution was added into each
well and incubated for 4 h. Following this, the supernatant in each
well was discarded and 100 µl DMSO was added. Optical density of
each culture was measured at 490 nm using a microplate reader
(Infinte-M200 Pro; Thermo Fisher Scientific, Inc.). The percentage
of cell growth inhibition was calculated using the following
formula: Percentage of cell growth inhibition = (C-T) / C × 100,
where C denotes absorbance of control cells and T denotes
absorbance of treatment cells. Data were presented in percentages
of cell inhibition relative to the control. Percentage of cell
growth inhibition was used to determine the IC50 values
of the anticancer activity of phytochemical extracts using Probit
analysis with GraphPad Prism 5.0 software (GraphPad Software, San
Diego, CA, USA).
Colony formation assay
A549 cells were plated in 6-well plates at a density
of 200 cells/well. Each culture was mixed with a PE extract at
concentrations of 0, 16.5, 31.5, 62.5, 125 and 250 µg/ml
respectively. Following 12 days of incubation, the cell colonies
formed in each well were stained with crystal violet (Beyotime
Institute of Biotechnology) after fixation with formaldehyde, and
the number of colony formed in each well was manually counted.
Morphological observation of A549
cells treated with a PE extract
Morphology of A549 cells treated with a PE extract
concentrations of 62.5 or 31.25 µg/ml, or with 0.5% DMSO control
for 72 h was observed under a bright field using an inverted
fluorescence microscope (Olympus Corp., Tokyo, Japan) at ×200
magnification.
Briefly, A549 cells were cultured in 24-well plates
at 1×104 cells/well and were analyzed following 24-h
treatment with PE extract (31.25 and 62.5 µg/ml, respectively).
Treated cells were fixed with cold 4.0% formaldehyde for 10 min,
washed with phosphate-buffered saline (PBS), and incubated with 10
µM Hoechst 33342 (Sigma-Aldrich; Merck Millipore) at 37°C for 15
min. Subsequently, the cells were washed with PBS and the cell
nuclei were observed under a fluorescence microscope (Olympus
Corp.).
Cell cycle analysis
A549 cells at 50–60% confluence were treated with a
phytochemical extract at concentrations of 31.25 and 62.5 µg/ml,
respectively, for 24 h. Cells were subsequently harvested by
trypsinization and washed twice with PBS. Afterwards, the cells
were fixed with cold 70% ethanol for 24 h at 4°C and centrifuged at
1,000 × g for 5 min. Cell pellets were collected and washed
with cold PBS. Finally, the cells were suspended in 500 µl staining
buffer of 50 µg/ml propidium iodide (PI) with 100 µg/ml RNaseA
(Beyotime Institute of Biotechnology), and incubated at 37°C for 30
min in the dark. Cell cycle progression was then analyzed by a flow
cytometer (BD Biosciences, San Jose, CA, USA). A minimum of 10,000
cells were used for each assay and DNA content histograms were
further analyzed by FlowJo 7.6 software (Tree Star, Inc., Ashland,
OR, USA) for cell cycle analysis.
Cell apoptosis assays
Prepared A549 cells were cultured in 6-well plates
at a density of 5×105 cells/ml and were treated with a
PE extract at concentrations of 31.25 and 62.5 µg/ml, respectively,
for 24 h. Following treatment, the cells were collected and washed
with 1 ml cold PBS, and were resuspended with 250 µl staining
buffer (Beyotime Institute of Biotechnology) with Annexin
V/fluorescein isothiocyanate (5 µl) and PI (10 µl, 20 µg/ml). Cells
were incubated at 37°C in the dark for 15 min. Finally, the stained
cells were analyzed using a flow cytometer. Data were analyzed by
FlowJo 7.6 software.
Statistical analysis
All data were presented as the mean ± standard
deviation. Student's t-tests were performed to analyze the
significant difference between treatment and control data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Growth inhibitory effects of C. album
L. extracts on A549 cells
Cytotoxic activities against A549 cell growth
following treatment with phytochemical extracts of PE, chloroform,
ethyl acetate, and n-butyl alcohol of C. album L. at
concentrations of 3.91, 7.81, 15.63, 31.25, 62.5,125, 250 and 500
µg/ml were screened and measured respectively for 72 h. Gemcitabine
treatment was used as a positive control for cytotoxicity (Table I). The IC50 values of
these extracts of C. album L. toward A549 cell growth were
calculated and the PE extract of C. album L. exhibited the
strongest cell growth inhibitory effect with the lowest
IC50 value of 33.31±2.79 µg/ml among the extracts
screened (Table I). Dose effect
assays showed the PE extract of C. album L. repressed A549
cell growth in a dose-dependent manner (Fig. 1A). Time effect assays demonstrated
that the PE extract of C. album L. inhibited A549 cell
growth in a time-dependent manner at the various extract
concentrations tested (Fig. 1B).
These results demonstrated the PE extract of C. album L. had
a potent and specific growth inhibitory effect on A549 cells.
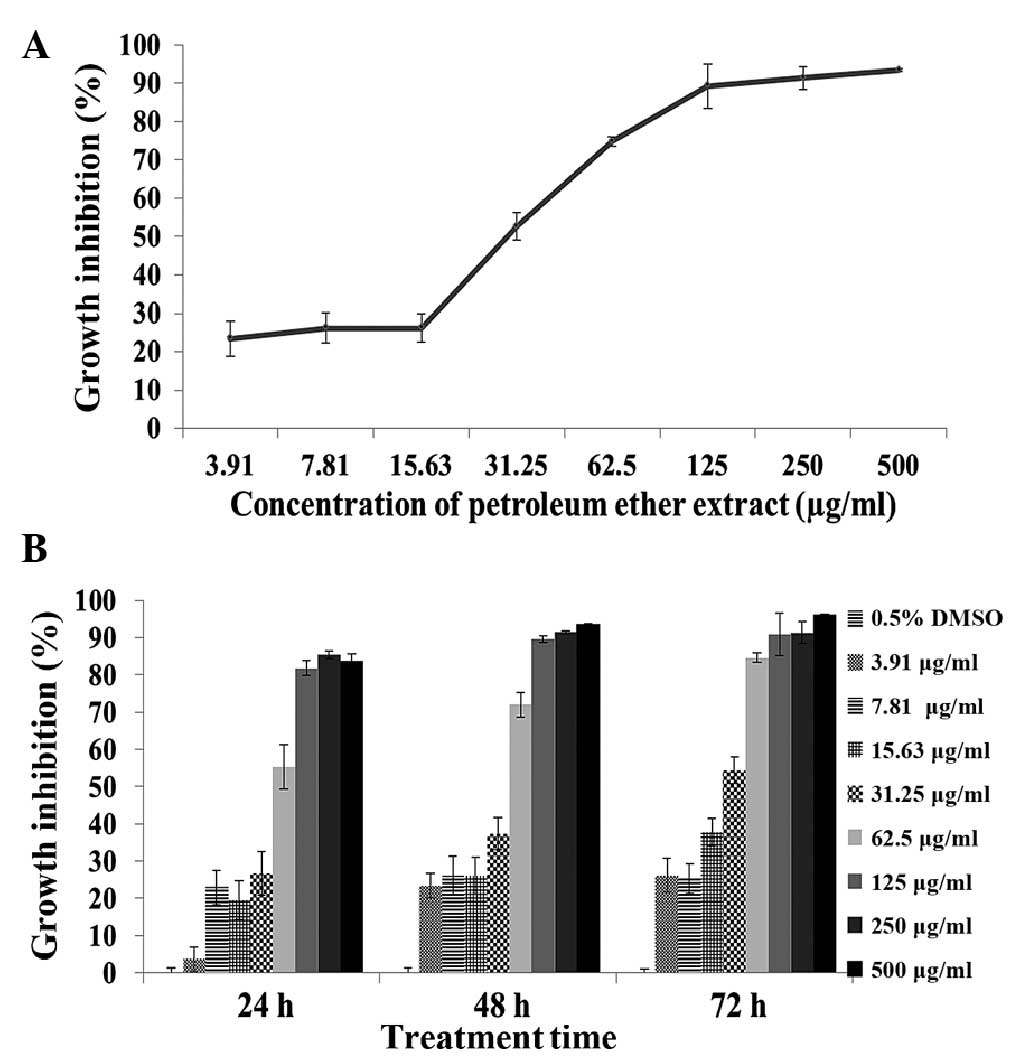 | Figure 1.Growth inhibitory effects of the PE
extract of Chenopodium album L. on A549 cells. (A) Growth
inhibition percentages of A549 cells treated with the PE extract at
7.81, 15.63, 31.25, 62.5, 125, 250 and 500 µg/ml, respectively, for
72 h. (B) Growth inhibition percentages of A549 cells treated with
the PE extract at 3.91, 7.81, 15.63, 31.25, 62.5, 125, 250 and 500
µg/ml, respectively, for 24, 48 and 72 h. Data presented as the
mean ± standard deviation of at least three experiments. PE,
petroleum ether. |
 | Table I.IC50values of extracts of
Chenopodium album L. and gemcitabine on A549 cell
growth. |
Table I.
IC50values of extracts of
Chenopodium album L. and gemcitabine on A549 cell
growth.
| Samples | IC50
values (µg/ml) |
|---|
| Petroleum ether
extract | 33.31±2.79 |
| Chloroform
extract | 84.96±5.43 |
| Ethyl acetate
extract | 304.79±3.92 |
| N-butyl alcohol
extract | ND |
| Gemcitabine | 0.45±1.28 |
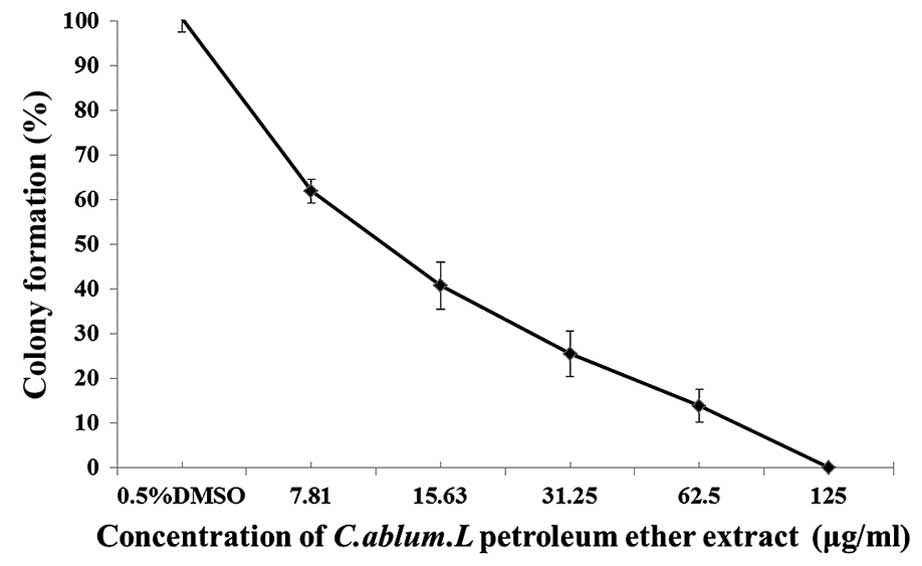
Inhibitory effects of the PE extract
of C. album L. on colony formation in A549 cells
The capability of cell colony formation may
represent cell viability after cell inoculation and indicate how
cell growth depends on the cell population and the ability of cell
propagation. When A549 cells were treated with increasing
concentrations of the PE extract of C. album L. from 7.81,
15.63, 31.25 and 62.5 to 125 µg/ml, the number of the cell colonies
formed was reduced in a dose-dependent manner (Fig. 2). These results demonstrated that the
colony formation and cell propagation abilities of A549 cells were
sensitive to the treatment of PE extract of C. album L.
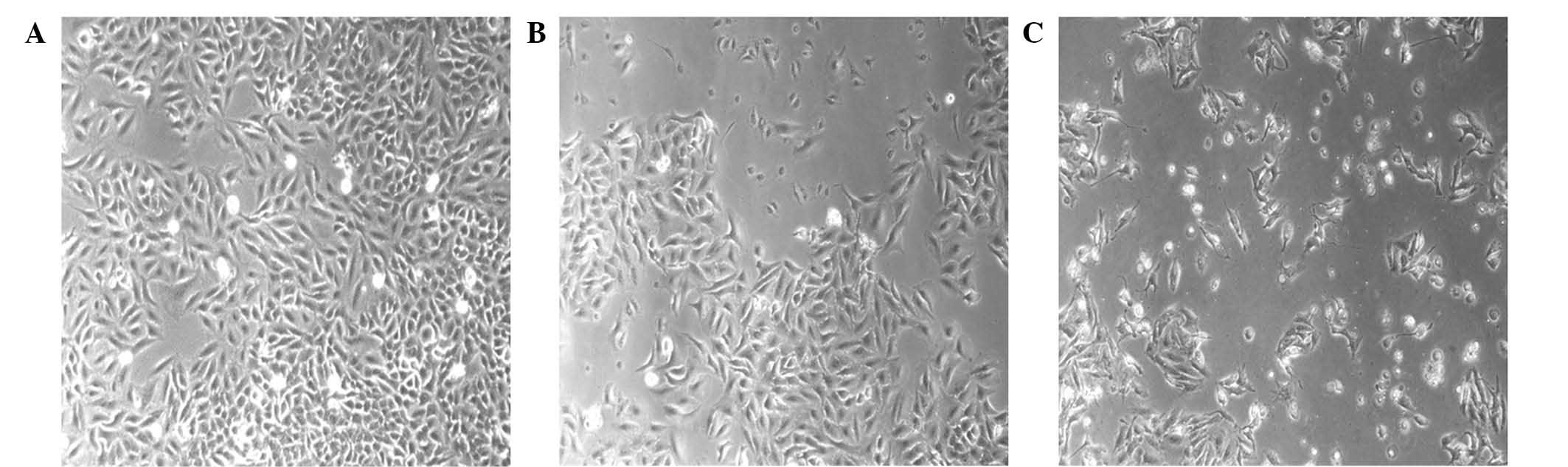
Morphological changes of A549 cells
treated with the PE extract of C. album L
When comparing the morphological properties of
control A549 cells treated with 0.5% DMSO and the PE-treated A549
cells, the morphologies of A549 cells treated with the PE extract
of C. album L. at concentrations of 31.25 and 62.5 µg/ml for
24 h exhibited apoptotic-associated cellular phenotypes, including
cell roundness and shrinkage (Fig.
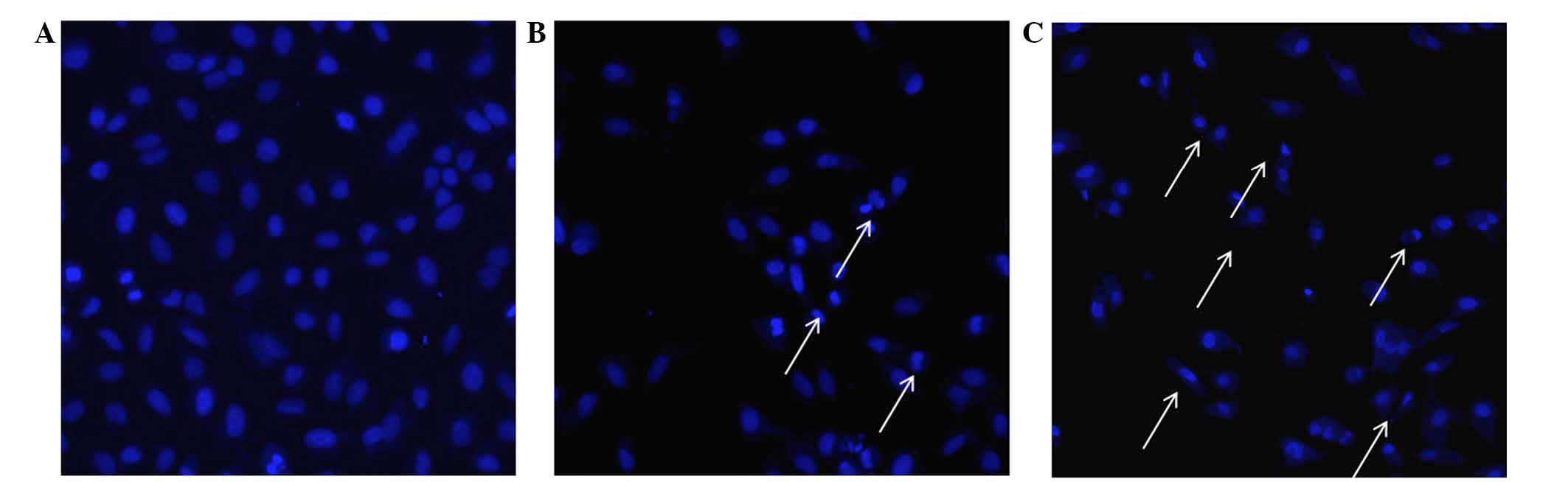
3). PE extract treatment induced the nuclear compaction of A549
cells, whereas the control cells treated with 0.5% DMSO showed
normal nuclear morphology (Fig. 4).
These cellular phonotypical results indicated that treatment with
the PE extract of C. album L. may have induced A549 cell
apoptosis.
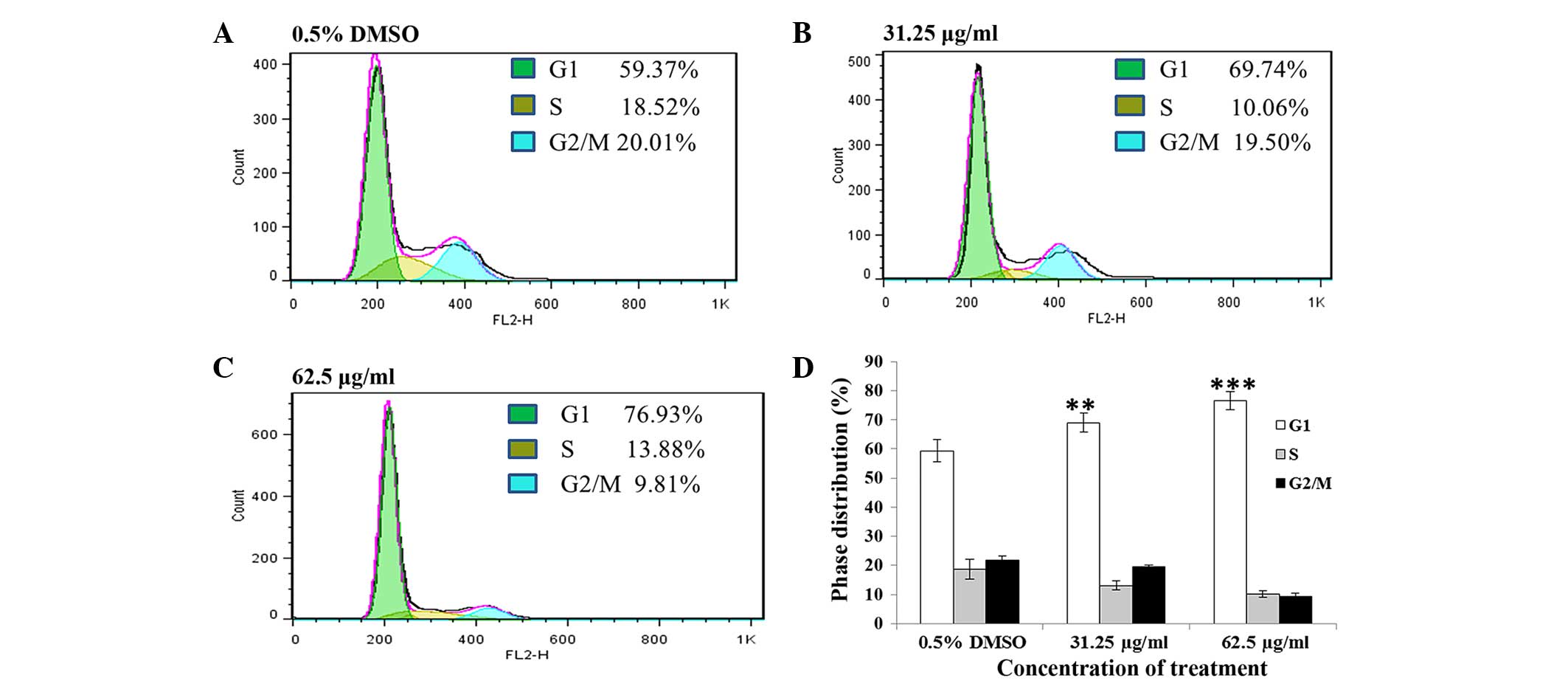
A549 cells exhibited G1 phase arrest
and apoptosis after treatment with the PE extract of C. album
L
To investigate the mechanism of the cell growth
inhibitory effect induced by the PE extract on A549, the cell cycle
of A549 cells was assessed following treatment with 31.25 and 62.5
µg/ml PE extract for 24 h. The results showed that these
phytochemical treatments significantly increased the ratio of the
G1 population of the cells (Fig. 5)
in a concentration-dependent manner (untreated, 59.37%; 31.25
µg/ml, 69.74%, P<0.01; 62.5 µg/ml, 76.93%, P<0.001).
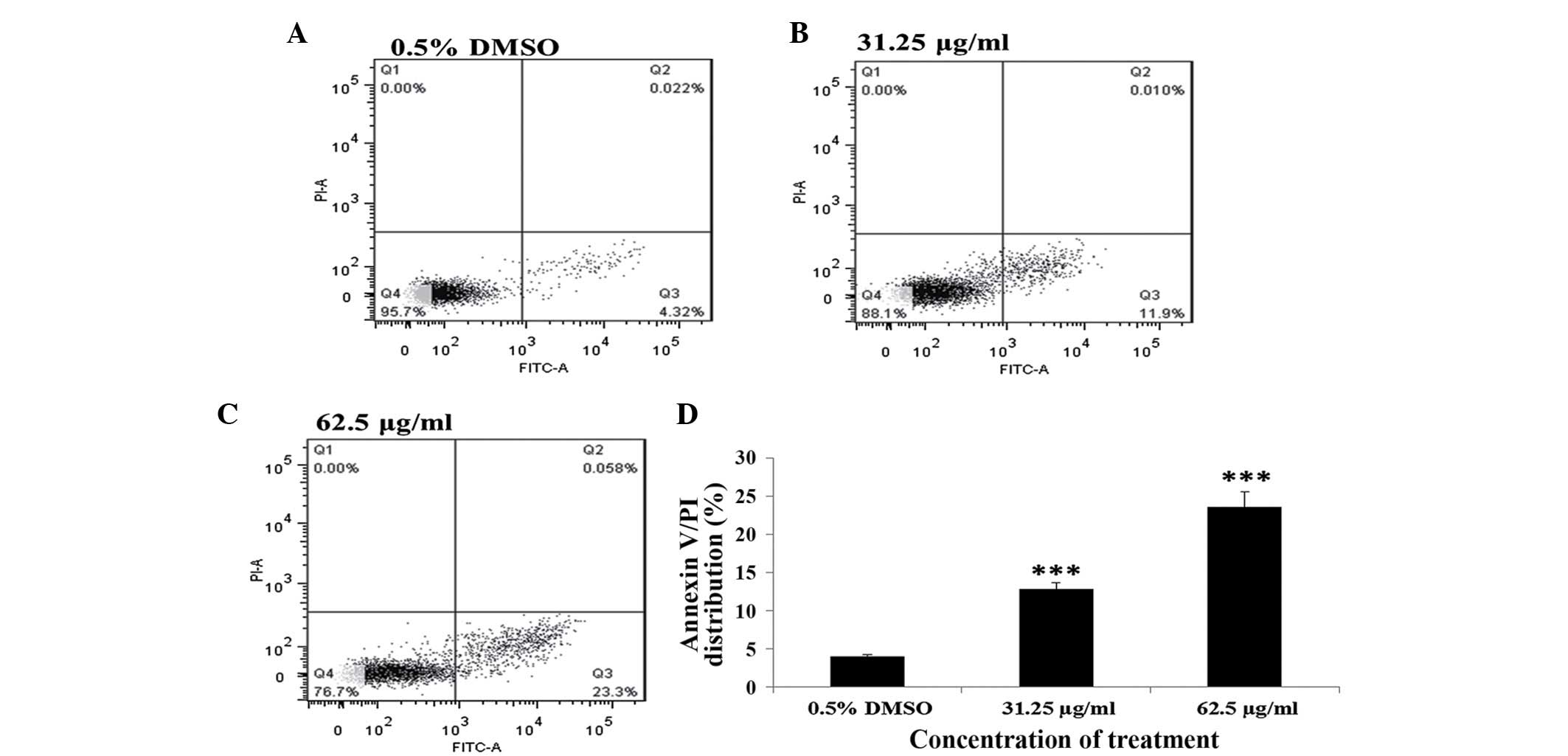
In addition, the effect of PE extract of C.
album L. on cell apoptosis was assessed by measuring the ratio
of apoptotic cells in the cell population following different PE
extract treatments. PE extract-treated A549 cells were subjected to
cell apoptosis analysis using a flow cytometer after the cells were
stained by Annexin V-FICT/PI. The results showed that A549 cells
treated with either 31.25 or 62.5 µg/ml of the PE extract for 24 h
exhibited significant increases in the ratio of apoptotic cells in
the cell population (untreated, 0.775%; 31.25 µg/ml, 11.9%,
P<0.001; 62.5 µg/ml, 22.3%, P<0.001; Fig. 6). These findings indicated that A549
cell growth inhibition following treatment with the PE extract of
C. album L. may be associated with the induction of cell
cycle G1 phase arrest and apoptosis.
Discussion
Chinese medicinal herbs have been widely been used
as a folk medicine for centuries in China and southeast Asia
(16–18). However, empirical studies related to
the action mechanisms of the phytochemicals from these widely used
Chinese medicinal herbs remain insufficient. Therefore, the present
pilot study was initiated by building a small phytochemical extract
library from >50 Chinese medicinal herbs, which was subsequently
used as a platform to screen plant constituents of possible novel
anticancer activities on an array of in vitro human cancer
cell lines. This study specifically focused on the phytochemical
extracts from C. album L. and explored their possible
anticancer activities against human non-small cell lung cancer A549
cells. The present findings demonstrated for the first time that
the PE extract of C. album L. significantly inhibited A549
cell growth in a time- and dose-dependent manner, as determined via
MTT and colony formation assays.
Cancer cells generally evade the programmed cell
death regulatory pathways of normal tissues to support their
malignant growth (19,20) and uncontrolled proliferation, thus
the suppression of apoptosis has a key role in cancer development
(21,22). To date, various anti-cancer drugs
targeting cancer cell apoptosis have been developed from natural
chemicals (23,16). The present study demonstrated that
the PE extract of C. album L. affected the cellular
morphology of human non-small cell lung cancer A549 cells, and
their proliferative abilities. The present findings also showed
that the phytochemical extract induced cellular apoptosis and G1
cell cycle arrest, which may provide important information to
develop novel cancer therapies. To evaluate how the PE extract
induced A549 cell apoptosis, the nuclear morphology of A549 cells
treated with the extract was analyzed using Hoechst 33342
immunofluorescent staining, and the externalization of
phosphatidylserine (PS) of A549 cells treated with the extract
using the binding assay of Annexin V to PS followed by cell sorting
with a flow cytometer (24,25). The results indicated that the PE
extract caused A549 cells to undergo chromatin condensation and
externalization of PS, which are typical apoptotic phenotypes.
Cell cycle progress is crucial for cell
proliferation (26,27). G1 phase arrest of the cell cycle
provides an opportunity for cells to either undergo repair or
follow an apoptotic pathway (28,29).
Many chemicals developed as anti-tumor agents were designed to
target cellular components involved in promoting G1/S transition
(30,31). The present results have shown that
the PE extract of C. ambrosioides L. significantly induced
G1 phase arrest of A549 cells, which may be one of the mechanisms
to trigger the cell apoptosis. This provides an important base and
opportunity to further characterize the natural molecule(s)
associated with this significant biological activity in future
research.
At present, herbal medicines have been shown to be a
promising approach for curing lung cancer (32,33).
Since our crude extract is an unfractionated plant extraction, it
is possible that the components mediating cell death of different
tumor cell are not identical. For a specific plant, different
extraction processes may produce a variety of compounds with
different concentrations and various bioactivities (34). The present results suggested that
there may be valuable active compound(s) against human non-small
cell lung cancer A549 cell in the PE extract of C. album L.
At this stage, it is not possible to elucidate whether these
effects on A549 cell growth are induced by specific compounds or
are the result of the combined action of multiple compounds in the
extract. As an edible Chinese medicinal herb, C. album L.
has no toxicity and few side effects. C. album L is a wild
neglected herb which has various pharmacological properties, such
as antiviral, antifungal, anti-inflammatory, antiallergic,
antiseptic and immunomodulating activities. However it has some
side effects since it contains porphyrin. Any plant that contains
such a substance belongs to the ling sensitivity plants; after
people eat it and are then exposed to sunlight, they are prone to
developing a skin disease called phytophotodermatitis (35). therefore, the plant can be consumed
as a human food and is expected to benefit individuals with lung
cancer (36).
In conclusion, the present study, for the first
time, screened different phytochemical extracts from C.
album L. against non-small cell lung cancer A549 cell to
explore their anticancer activities, and demonstrated that the PE
extract of C. album L. specifically inhibited A549 cell
growth by inducing cell cycle G1 phase arrest and cell apoptosis.
These results may provide valuable data for assessing the possible
usage of phytochemicals from C. album L. in exploring and
developing novel cancer therapies and healthcare products.
Acknowledgements
The present study was supported by the Key Subject
Project Foundation for Natural Product and New Drug Research of
Kunming University of Science and Technology (grant no. 14078183),
the Personnel Training Project of Yunnan Province (grant nos.
KKSY201226096 and KKSY201226097).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. Cancer J Clin. 62:10–29. 2013. View Article : Google Scholar
|
|
2
|
She J, Yang P, Hong QY and Bai CX: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verdeccchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000-02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clardy J and Walsh C: Lesson from natural
molecules. Nature. 432:829–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piggott AM and Karuso P: Quality, not
quantity: The role of natural products and chemical proteomics in
modern drug discovery. Comb Chem High Throughput Screen. 7:607–630.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koch MA, Schuffenhauer A, Scheck M, Wetzel
S, Casaulta S, Odermatt A, Ertl P and Waldmann H: Charting
biologically relevant chemical space: A structural classification
of natural products (SCONP). Proc Natl Acad Sci USA.
102:17272–17277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hao XN, Chan SW and Chen SL: Detection of
Puerarin and Danshensu in traditional Chinese medicinal preparation
containing Pueraria lobata and Salvia Miltiorrhiza by
high-performance liquid chromatography. J Liq Chromatogr Relat
Technol. 30:2779–2787. 2007. View Article : Google Scholar
|
|
8
|
Hong JY, Nam JW, Seo EK and Lee SK:
Daphnane diterpene esters with anti-proliferative activities
against human lung cancer cells from Daphne genkwa. Chem Pharm Bull
(Tokyo). 58:234–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toyang NJ, Ateh EN, Davis H, Tane P,
Sondengam LB, Bryant J and Verpoorte R: In vivo antiprostate tumor
potential of Vernonia guineensis Benth.(Asteraceae) tuber extract
(VGDE) and the cytotoxicity of its major compound pentaisovaleryl
sucrose. J Ethnopharmacol. 150:724–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang DS, Rizwani GH, Guo H, Ahmed M, Ahmed
M, Hassan SZ, Hassan A, Chen ZS and Xu RH: Annona squamosa Linn:
Cytotoxic activity found in leaf extract against human tumor cell
lines. Pak J Pharm Sci. 27:1559–1563. 2014.PubMed/NCBI
|
|
11
|
Kumar R, Mishra AK, Dubey NK and Tripathi
YB: Evaluation of Chenopodium ambrosioides oil as a potential
source of antifungal, antiaflatoxigenic and antioxidant activity.
Int J Food Microbial. 115:159–164. 2007. View Article : Google Scholar
|
|
12
|
Kaur C and Kapoor HC: Antioxidant activity
and total phenolic content of some Asian vegetables. J Food Sci
Technol. 37:153–161. 2002. View Article : Google Scholar
|
|
13
|
Dai Y, Ye WC, Wang ZT, Matsuda H, Kubo M
and But PPH: Antipruritic and antinociceptive effects of
Chenopodium album L. in mice. J Ethnopharmacol. 81:245–250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khoobchandani M, Ojeswi BK, Sharma B and
Srivastava MM: Chenopodium album prevents progression of cell
growth and enhances cell toxicity in human breast cancer cell
lines. Oxid Med Cell Longev. 2:160–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Pan H, Feng Y, Li HZ and Zhao Y:
Bioactivity-guided isolation of anticancer constituents from Hedera
nepalensis K. S Afr J Bot. 100:87–93. 2015. View Article : Google Scholar
|
|
16
|
Cheng YL, Lee SC, Harn HJ, Huang HC and
Chang WL: The extract of Hibiscus syriacus inducing apoptosis by
activating p53 and AIF in human lung cancer cells. Am J Chin Med.
36:171–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li WY, Chan SW, Guo DJ, Chung MK, Leung TY
and Yu PH: Water extract of Rheum officinale Baill. induces
apoptosis in human lung adenocarcinoma A549 and human breast cancer
MCF-7 cell lines. J Ethnopharmacol. 124:251–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo-or radio-therapy for cancer. Biosci Trends. 4:297–307.
2010.PubMed/NCBI
|
|
19
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu YL, Jahangiri A, Delay M and Aghi MK:
Tumor cell autophagy as an adaptiveresponse mediating resistance to
treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu H, Ahn NS, Yang X, Lee YS and Kang KS:
Ganoderma lucidum extract induces cell cycle arrest and apoptosis
in MCF-7 human breast cancer cell. Int J Cancer. 102:250–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson VL, Ko SC, Holmstrom TH, Eriksson
JE and Chow SC: Effector caspases are dispensable for the early
nuclear morphological changes during chemical-induced apoptosis. J
Cell Sci. 113:2941–2953. 2000.PubMed/NCBI
|
|
25
|
van Engeland M, Ramaekers FC, Schutte B
and Reutelingsperger CP: A novel assay to measure loss of plasma
membrane asymmetry during apoptosis of adherent cells in culture.
Cytometry. 24:131–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schultz DR and Harringto WJ Jr: Apoptosis:
Programmed cell death at molecular level. Semin Arthritis Rheum.
32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ong CS, Zhou J, Ong CN and Shen HM:
Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells
via the Akt-GSK-3β-Cyclin D1 pathway. Cancer Lett. 298:167–175.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitchakarn P, Suzuki S, Ogawa K, Pompimon
W, Takahashi S, Asamoto M, Limtrakul P and Shirai T: Induction of
G1 arrest and apoptosis in androgen-dependent human prostate cancer
by Kuguacin J, a triterpenoid from Momordica charantia leaf. Cancer
Lett. 306:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yano H, Mizoguchi A, Fukuda K, Haramaki M,
Ogasawara S, Momosaki S and Kojiro M: The herbal medicine
sho-saiko-to inhibits proliferation of cancer cell lines by
inducing apoptosis and arrest at the G0/G1 phase. Cancer Res.
54:448–454. 1994.PubMed/NCBI
|
|
31
|
Li Y, Ma HL, Han L, Liu WY, Zhao BX, Zhang
SL and Miao JY: Novel ferrocenyl derivatives exert anti-cancer
effect in human lung cancer cells in vitro via inducing G1-phase
arrest and senescence. Acta Pharmacol Sin. 34:960–968. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao J, Morgan WA, Sanchez-Medina A and
Corcoran O: The ethanol extract of Scutellaria baicalensis and the
active compounds induce cell cycle arrest and apoptosis including
upregulation of p53 and Bax in human lung cancer cells. Toxicol
Appl Pharmacol. 254:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai JC, Tsai S and Chang WC: Effect of
ethanol extracts of three Chinese medicinal plants with laxative
properties on ion transport of the rat intestinal epithelia. Biol
Pharm Bull. 27:162–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martins S, Mussatto SI, Martínez-Avila G,
Montañez-Saenz J, Aguilar CN and Teixeira JA: Bioactive phenolic
compounds: Production and extraction by solid-state fermentation. A
review. Biotechnol Adv. 29:365–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bilgili SG, Akdeniz N, Akbayram S, Ceylan
A, Çalka Ö and Karaman K: Phototoxic dermatitis due to Chenopodium
album in a child. Pediatr Dermatol. 28:647–676. 2011. View Article : Google Scholar
|
|
36
|
Sun CH, Li Y, He HY, Du W and Cheng XF:
Nutritive compositions of Chenopodium album and the evaluation as a
vegetable resource. Guihaia. 25:589–601. 2005.
|