Introduction
Moyamoya disease (MMD) is a progressive
cerebrovascular steno-occlusive disorder. It typically involves the
distal internal carotid arteries (ICAs), the proximal anterior
cerebral arteries (ACAs) and the middle cerebral arteries (MCAs),
accompanied by the development of an abnormal collateral vascular
network at the base of the brain (1–3). Thus
far, the majority of MMD cases have been reported in Eastern Asia
(4); however, even in that region,
the coexistence of MMD and Graves' disease (GD) is rare. In 1991,
Kushima et al first reported two cases of females suffering
from MMD and GD simultaneously (5).
To the best of our knowledge, only ~50 cases of simultaneous MMD
and GD have been described in the literature available in the
MEDLINE database to date. The patients were predominantly women,
and their ages ranged between 10 and 54 years (mean, 29.3 years).
Transient ischemic attacks and cerebral infarction were the most
common symptoms in these patients. The majority of the cases showed
thyrotoxicity when the cerebral ischemic event occurred and
eventually recovered from the neurological symptoms after medical
and/or surgical treatment (6).
Although the association between MMD and GD remains unclear, a
number of previous studies have suggested that GD could induce the
development of MMD through ‘thyroid storm’ (7,8),
‘cerebrovascular hemodynamic changes’ (9) and ‘autoimmune arteritis’ (10–13).
Von Willebrand factor (vWF) is a useful marker of
endothelial activation or dysfunction (14). Increasing evidence has demonstrated
that vWF is involved in cardiovascular (15) and ischemic cerebrovascular diseases
(16,17). In addition, coagulation factor VIII
(FVIII) is involved in the process of thrombosis, and elevated
FVIII levels are a prothrombotic risk factor for thromboembolism
(18). The correlation of
hyperthyroidism with elevated FVIII and vWF activities has been
previously reported (19).
The present study reported the case of a 12-year-old
male with significant overactivation of vWF and FVIII. The patient
suffered from the rare coexistence of GD with MMD. Thus, a
comprehensive investigation of a potential correlation between GD
and the overactivation of vWF/FVIII and MMD was performed. Written
informed consent was obtained from the family.
Case report
A 12-year-old Chinese male was admitted to the
Beijing Tian Tan Hospital (Beijing, China) in May 2013 with a
rapidly progressive mild quadriplegia, aphasia and urinary
incontinence. At 5 days prior to admission, the patient experienced
several episodes of vomiting, paroxysmal aphasia and mild
hemiplegia on his left side, along with recurrent brief
convulsions. The condition of the patient rapidly deteriorated
during the subsequent 5 days. The patient had previously suffered
irritability, tremors, excessive sweating and frequent palpitations
following moderate physical activity for approximately 1 year. He
had no other disease history and a relevant family disease
history.
On admission, the patient was dysphoric with slurred
speech, and had a high blood pressure (165/95 mmHg), and rapid and
regular heart rate (110–140 bpm). In addition, the patient
presented facial paralysis (skewing of the mouth to the right side)
and mild quadriplegia with globally increased muscle tone.
Hyperactive deep tendon reflexes and positive bilateral Babinski
signs were observed. A moderately enlarged thyroid gland was also
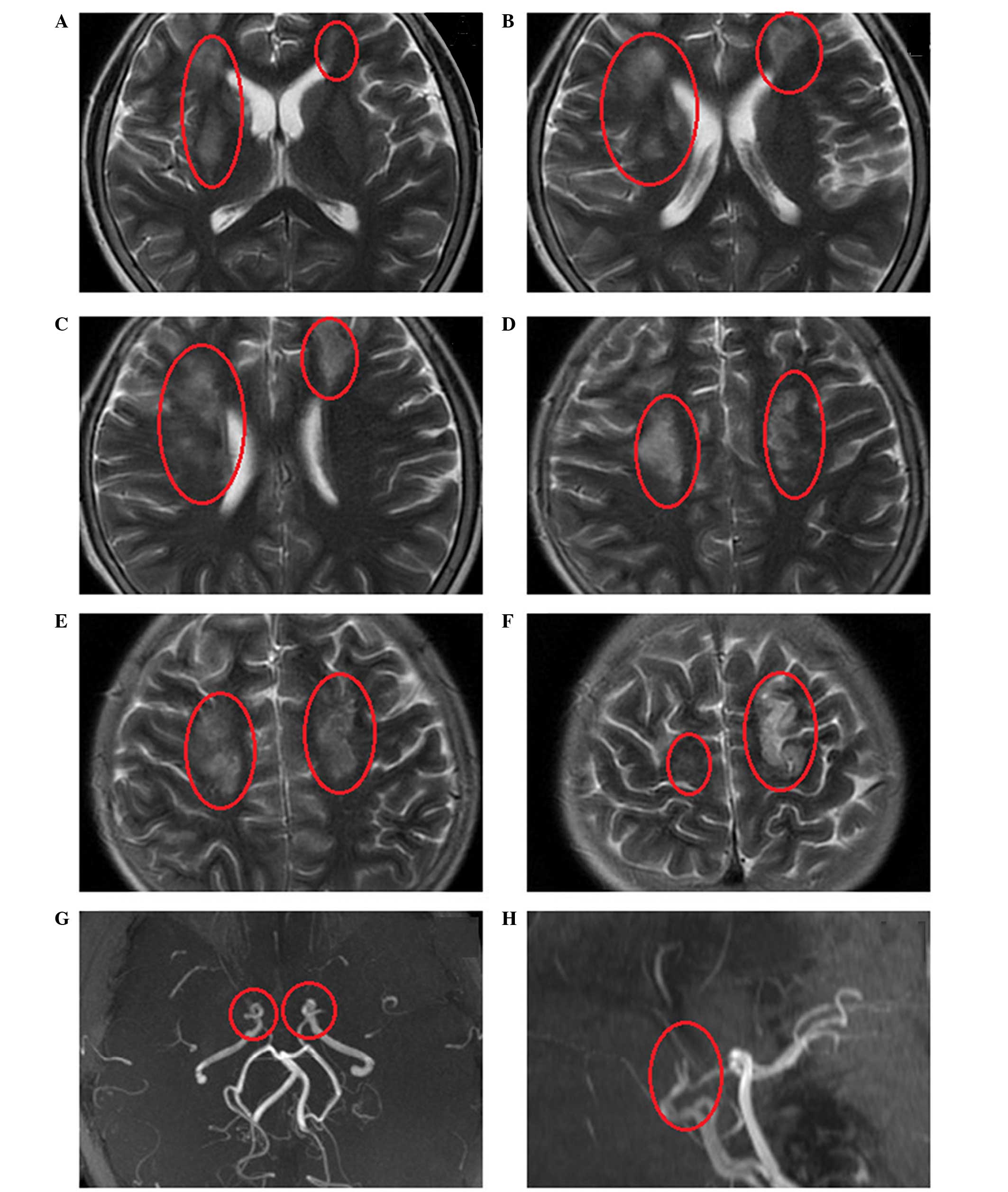
identified. Emergency head magnetic resonance imaging (MRI) scans
revealed multiple regions of cerebral infarction that could not be
attributed to a single vascular territory (Fig. 1). In addition, magnetic resonance
angiography (MRA) revealed severe stenosis at the bilateral
terminal portion of the ICAs, while the bilateral MCAs and ACAs had
almost disappeared (Fig. 1).
Accordingly, combined with the patient's symptom of progressive
mild quadriplegia, aphasia, recurrent convulsions and urinary
incontinence that appeared 5 days prior to the first admission, and
the signs of globally increased muscle tone, hyperactive deep
tendon reflexes and positive bilateral Babinski signs on admission
and the results of the head MRI, a diagnosis of multiple cerebral
infarction was made definitely. Blood examination showed the
following results: Thyroid-stimulating hormone (TSH), <0.001
µU/ml (reference range, 0.35–4.94 µU/ml); and free triiodothyronine
(FT3), 29.17 pmol/l (reference range, 2.63–5.7 pmol/l). Positive
thyrotropin receptor antibody (TR-Ab), as well as elevated levels
of thyroid peroxidase antibody (TPO-Ab; 396.44 U/ml; reference
range, <12 U/ml) and thyroglobulin antibody (TG-Ab; 134.56 U/ml;
reference range, <34 U/ml), were also detected in the blood
(Table I). Due to the above symptoms
of irritability, tremors, excessive sweating, frequent palpitations
and enlarged thyriod gland with elevated TPO-Ab, elevated TG-Ab and
positive TR-Ab in blood, GD was suspected firstly. A thyroid gland
needle biopsy revealed thyroid follicular epithelial cell
proliferation, which confirmed the diagnosis of GD. In addition,
quantification of coagulation factor activity demonstrated that
FVIII activity was 261.6% (reference range, 50–150%) and vWF
activity was 324.2% (reference range, 40–120%), while other
coagulation parameters were all within the normal ranges, as shown
in Table I.
 | Table I.Examination results of thyroid
function, coagulation parameters and thyroid autoantibodies on
admission and during follow-up. |
Table I.
Examination results of thyroid
function, coagulation parameters and thyroid autoantibodies on
admission and during follow-up.
| Parameter | Reference range | Admission | After 3 months | After 6 months | After 9 months | After 12 months | After 18 months |
|---|
| TT3 (nmol/l) | 0.89–2.44 | 7.04 | 0.97 | 3.48 | 1.17 | 4.39 | 1.85 |
| TT4 (nmol/l) | 62.68–150.84 | 210.73 | 64.06 | 171.65 | 52.12 | 220.58 | 113.50 |
| TSH (µU/ml) | 0.35–4.94 | <0.001 | 0.027 | 0.001 | 3.803 | 0.048 | 0.007 |
| FT3 (pmol/l) | 2.63–5.70 | 29.17 | 3.21 | 10.63 | 3.26 | 15.32 | 5.45 |
| FT4 (pmol/l) | 9–19.04 | 35.99 | 11.31 | 27.63 | 10.26 | 34.16 | 19.06 |
| FVIII (%) | 50–150 | 261.60 | 122.40 | 175.90 | 115.60 | 185.60 | 151.80 |
| vWF (%) | 40–120 | 324.20 | 117.10 | 128.00 | 119.50 | 146.50 | 125.40 |
| FVII (%) | 50–150 | 125.00 | 75.00 | 113.00 | 55.00 | 59.00 | 60.0 |
| FIX (%) | 50–150 | 148.20 | 138.60 | 137.20 | 129.70 | 115.80 | 66.0 |
| PT-S (sec) | 9.4–12.5 | 12.60 | 11.30 | 11.90 | 11.50 | 9.68 | 10.80 |
| PT (%) | 70–120 | 100.70 | 80.00 | 89.00 | 112.00 | 108.00 | 94.00 |
| PT-INR | 0.9–1.2 | 1.04 | 1.09 | 1.14 | 0.98 | 1.05 | 1.01 |
| FIB-C (mg/l) | 200–400 | 251 | 230 | 381 | 418 | 352 | 228 |
| APTT (sec) | 25.5–38.4 | 24.70 | 25.50 | 27.80 | 24.50 | 29.50 | 29.20 |
| APTT-R | 0.91–1.38 | 0.89 | 0.89 | 0.98 | 0.93 | 1.12 | 1.02 |
| TPO-Ab (U/ml) | 0–12 | 396.44 | 205.17 | 111.21 | 221.3 | 252.1 | 594.9 |
| TG-Ab (U/ml) | 0–34 | 134.56 | 71.31 | 65.63 | 85.20 | 74.50 | 255.30 |
| TR-Ab | − | + | + | +/− | + | + | + |
The patient was managed with standard antithyroid
treatment (20 mg/day thiamazole, Merck Serono, Darmstadt, Germany;
100 mg/day metoprolol, AstraZeneca Co., Ltd., Wuxi, China) until
the submission of this manuscript, in combination with a low iodine
diet. In beginning of the treatment, other management included 30
mg/day Adalat for 2 weeks (Bayer Schering Pharma AG) for control of
blood pressure, sedative administration (5 mg diazepam, Zhejiang
Medicine Co., Ltd., Zhejiang, China) lasting until the calming of
irritability after 1 week. However, no anticoagulants or
thrombolytic agents were administered to the patient. After 2
months of treatment, the neurological symptoms had improved
significantly; the patient had recovered from paralysis, walked
with a steady gait and no slurred speech was observed. Further
blood tests showed that the patient's thyroid function had almost
returned to normal (TSH, 0.027 µU/ml; FT3, 3.21 pmol/l), with
depressed vWF and FVIII activity (117.10 and 122.40%,
respectively), although the thyroid autoantibodies persisted at
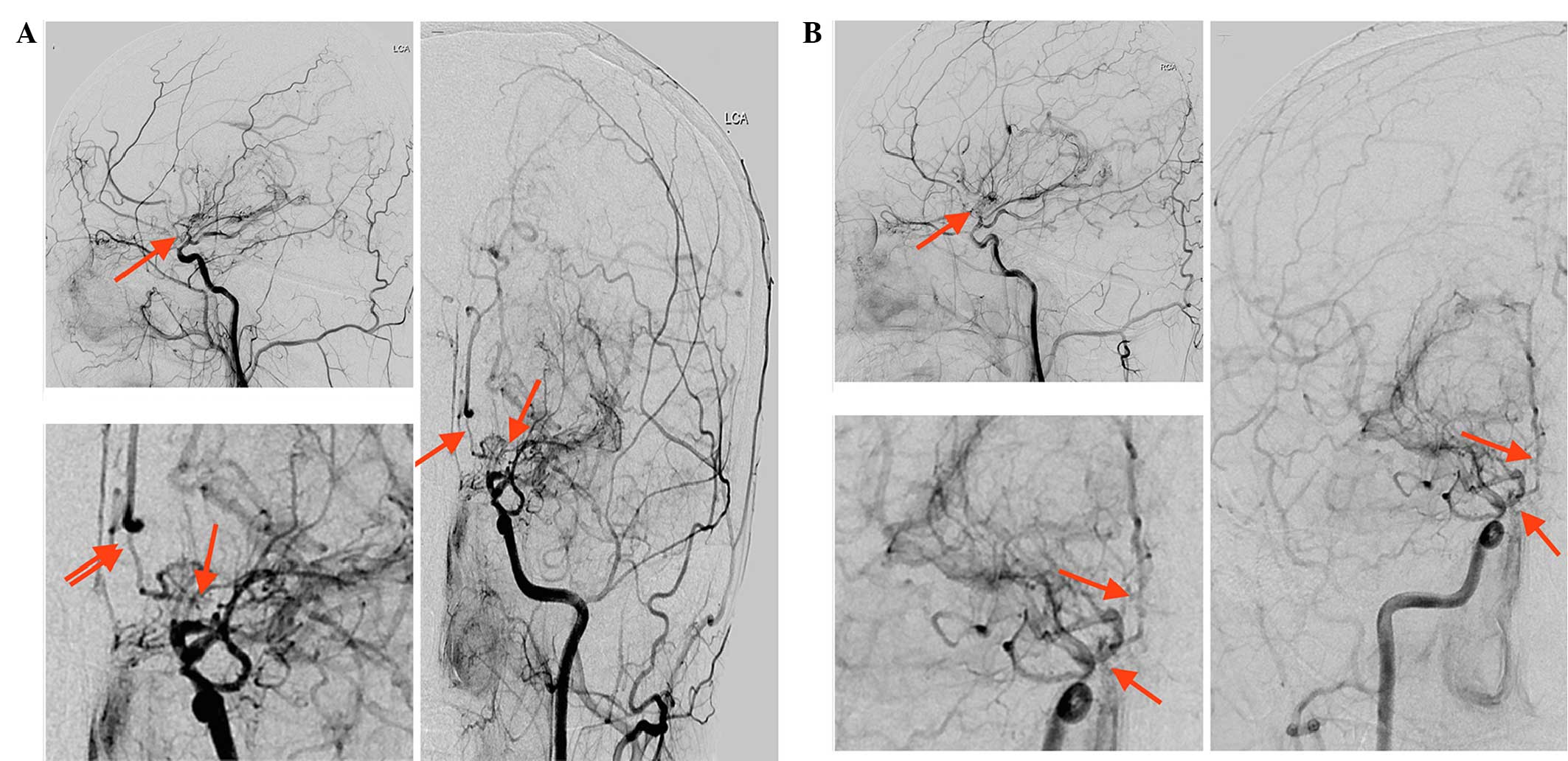
high levels (Table I). Digital
subtraction angiography (DSA) was then performed, which
demonstrated severe stenosis of the bilateral distal segments of
the ICAs, and of the ACAs and MCAs. In addition, only minimal
antegrade flow of ACA and MCA territories was observed. A network
of collateral vessels was also visible at the base of the brain and
in the bilateral basal ganglia areas (Fig. 2). To prevent further attacks,
revascularization surgery was performed initially on the right side
superficial temporal artery binding to the middle meningeal artery
at 3 months after the first admission. This results of DSA
associated with the symptoms of gradually emerged quadriplegia,
aphasia, convulsions and the head MRI results which showed
bilateral basal ganglia and subcortical multiple infarction,
together, could meet the diagnostic criteria for MMD (20). After a further 3 months (6 months
after the first admission), an encephalo-duro-arterio-synangiosis
procedure was performed successfully on the left side superficial
temporal artery attaching to the endocranium and arachnoid.
During the 20-month follow-up period, a continuous
20 mg/day thiamazole and 100 mg/day metoprolol was administrated,
the thyroid function, thyroid autoantibodies and coagulation
parameters were measured every 3 months. During this period, a
correlation was observed between the patient's thyroid function and
vWF/FVIII activity. After 3 months, following the administration of
propranolol and metoprolol, his thyroid function (FT3 and FT4)
recovered to the normal range. Notably, a decline in FVIII/vWF
activity was observed simultaneously. After a further 3 months,
while FT3 and FT4 in the patient's blood were higher than the
normal range, a homologous elevation of the patient's FVIII/vWF
activity in the blood was observed. A similar phenomenon was
observed during the next 12-month follow-up period (Table I). When the thyroid function
recovered to the normal range, a corresponding decline in FVIII/vWF
activity was observed (Table I). At
the same time, the thyroid autoantibodies persisted at high levels,
whereas other coagulation parameters were consistently around the
normal ranges.
Discussion
The case reported in the present study was affected
by GD and MMD successively. The manifestations observed were mainly
due to cerebral infarction, while the clinical features of
thyrotoxicosis had appeared approximately 1 year prior to
admission. This is consistent with the features of previously
reported cases, which suggest that transient ischemic attacks and
cerebral infarction were the common symptoms and thyrotoxicity was
showed when the cerebral ischemic event occurred (6).
Due to the limited incidence of co-existing GD and
MMD, extensive studies have not been possible, and no specific
mechanism underlying this association has been elucidated to date.
Previous hypotheses on the underlying mechanism have been proposed
that presume the existence of a pathoetiological association
between GD and MMD, such as genetic background, autoimmunization
(10,11), thyrotoxic states (9,21) and
significant hemodynamic changes (9).
There are certain evidence-based medicine practices and
epidemiological data that support these theories. For instance, Kim
et al concluded that elevated thyroid autoantibodies are
associated with MMD, based on a prospective study (11). This theory was supported by a
case-control study conducted by Li et al, which demonstrated
that increased thyroid function and elevated thyroid autoantibodies
are associated with MMD, particularly in pediatric patients
(10). Furthermore, improvement in
the ischemic symptoms without recurrence has been reported
following antithyroid drug administration alone in two patients
affected by hyperthyroidism combined with bilateral internal
carotid artery stenosis (21). In
another study, two patients had recurrent stroke subsequent to
antithyroid drug withdrawal for 6 months, while the majority of
patients in the same study did not present recurrence of stroke
with regular use of antithyroid drugs (9). These studies indicate that GD is an
underlying risk factor for MMD. However, the mechanism by which
elevated thyroid autoantibodies or increased thyroid function evoke
the development of stenosis of the intracranial arteries, as seen
in MMD, remains to be elucidated.
The patient in the present study was diagnosed with
MMD associated with GD, based on the symptoms of irritability,
tremors, excessive sweating, frequent palpitations, the enlarged
thyroid gland and the examination results of elevated TPO-Ab,
elevated TG-Ab and positive TR-Ab in blood, along with thyroid
gland pathology by needle biopsy. Furthermore, his multiple
cerebral infarction and the results of DSA that showed a serious
stenosis at the bilateral terminal portion of the ICAs, the
bilateral MCAs and ACAs (Fig. 1).
Notably, significant overactivation of FVIII and vWF were
identified in the plasma on admission, which are considered to
serve an important role in vascular diseases (15–18). The
correlation of high plasma levels of vWF/FVIII with cardiovascular
disease (15), cerebral sinus and
deep venous thrombosis (18), as
well as ischemic stroke (16,17), has
been extensively investigated. Certain studies have concluded that
high plasma levels of vWF are predictive of ischemic stroke
(22,23), and may even be an independent risk
factor for the prediction of ischemic stroke (23).
The pathophysiologic role of FVIII- or
vWF-associated processes in ischemic brain injury is unclear. It is
well-known that vWF and FVIII serve crucial roles in primary
thrombosis. In addition, vWF mediates the initial adhesion of
platelets at sites of vascular injury, and this subsequently leads
to fibrin coagulation and the formation of a platelet thrombus via
a complex coagulation cascade, platelets adhesion mediated by vWF
is a prerequisite for normal hemostasis (24–27). vWF
is synthesized and stored in endothelial cells, and plasma levels
are increased in response to different states of endothelial
damage; therefore, vWF has been proposed as a useful marker of
endothelial activation or dysfunction (14). Earlier studies have identified that
concentric and eccentric fibrocellular thickening of the intima,
which induce stenosis of the vascular lumen, are typical
pathological changes in the terminal of the internal carotid artery
in MMD (28,29). Recent studies using high-resolution
3T MRI of a cross-section of the MCA showed no plaque or
enhancement in the MCA wall (9,30), The
artery blood vessel image under 3T MRI from MMD is at variance with
atherosclerotic features. This phenomenon was also observed in the
patient of the present study. Furthermore, in another study,
thromboemboli in the internal wall of the Moyamoya artery were
observed in ~50% of cases at autopsy (31). This indicates that abnormal
thrombogenesis serves an important role in the etiology of MMD.
Previous studies have demonstrated that vascular
endothelium is a specific target of thyroid hormones (32), and that hyperthyroidism tends to be
associated with endothelial dysfunction or damage. This endothelial
dysfunction depends not only on the cause but also on the degree of
hyperthyroidism (19).
Hyperthyroidism is known to result in high sympathetic nerve
activity, thus plasma adrenaline levels are increased, acting as a
strong stimulus of vWF secretion by endothelial cells (33). Clinical studies have also
demonstrated that the elevated vWF levels in hyperthyroid patients
returned to the normal range following administration of
propranolol (34,35). In accordance with the observations of
the current study, hyperthyroidism has a significant correlation
with the elevation of FVIII and vWF activity. Considering the
pivotal role of vWF and FVIII in thrombogenesis, GD could be
expected to be involved in the mechanism underlying the development
of MMD, which is mediated by vWF/FVIII overactivation. GD is such a
complex disease that may influence multiple aspects associated with
the pathophysiologic process of MMD, including cerebral hemodynamic
changes (9), autoimmune inflammation
(10,11) and endothelial dysfunction (19). In addition, thrombogenesis is
mediated by vWF and FVIII activities, which may be due to injury to
the intima or vessel wall, while the injury of intima or vessel
wall could be a result of cerebral hemodynamic changes or
autoimmune inflammation. Further evidence of vWF and FVIII
mediating the development of MMD is the varying trend of vWF and
FVIII activities during the follow-up period in the present study.
Upon admission, the current patient had significantly elevated vWF
and FVIII activities, and presented with rapid neurological
deterioration. Following the administration of thiamazole and
metoprolol, the patient's thyroid function returned to normal,
while vWF and FVIII activities were simultaneously suppressed, with
a correlation observed in their fluctuations (Table I). During the follow-up period, the
thyroid autoantibodies TR-Ab, TG-Ab and TPO-Ab persisted at high
levels in the patient's blood, whereas other coagulation parameters
were consistently within or close to the normal range. Therefore,
the effect of thyroid autoantibodies on the development of MMD was
unclear in the present patient.
However, an evident limitation of the present study
is that only one case was included. Nevertheless, during the
20-month follow-up period, correlations were identified among GD,
overactivation of vWF/FVIII and intracranial arterial stenosis,
which require further confirmation in more cases and in-depth
studies in the future. Moyamoya disease associated Graves' disease
has a tendency to present with coagulation dysfunction.
Overactivation of vWF/FVIII due to thyrotoxicosis might contribute
to the ischemic accidents. Future research is warranted to
investigate novel therapeutic methods in prevention of ischemic
attack.
Acknowledgements
The present study was supported by a grant from the
National Youth Science Fund (grant no. 81301118). The authors thank
Dr Rong Wang and Dr Wei-Qing Wan and their colleagues (Center of
Cerebrovascular Disease, Beijing Tian Tan Hospital, Capital Medical
University, Beijing, China) for their excellent vascular surgical
procedures on the patient. In addition, we also thank the patient
and his parents for their best collaboration during the
follow-up.
References
|
1
|
Houkin K, Ito M, Sugiyama T, Shichonohe H,
Nakayama N, Kazumata K and Kuroda S: Review of past research and
current concepts on the etiology of moyamoya disease. Neurol Med
Chir (Tokyo). 52:267–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kudo T: Spontaneous occlusion of the
circle of Willis. A disease apparently confined to Japanese.
Neurology. 18:485–496. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki J and Kodama N: Cerebrovascular
“Moyamoya” disease. 2. Collateral routes to forebrain via ethmoid
sinus and superior nasal meatus. Angiology. 22:223–236. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen PC, Yang SH, Chien KL, Tsai IJ and
Kuo MF: Epidemiology of moyamoya disease in Taiwan: A nationwide
population-based study. Stroke. 45:1258–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kushima K, Satoh Y, Ban Y, Taniyama M, Ito
K and Sugita K: Graves' thyrotoxicosis and Moyamoya disease. Can J
Neurol Sci. 18:140–142. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohba S, Nakagawa T and Murakami H:
Concurrent Graves' disease and intracranial arterial
stenosis/occlusion: special considerations regarding the state of
thyroid function, etiology, and treatment. Neurosurg Rev.
34:297–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu SW, Chaloupka JC and Fattal D: Rapidly
progressive fatal bihemispheric infarction secondary to Moyamoya
syndrome in association with Graves thyrotoxicosis. AJNR Am J
Neuroradiol. 27:643–647. 2006.PubMed/NCBI
|
|
8
|
Inaba M, Henmi Y, Kumeda Y, Ueda M, Nagata
M, Emoto M, Ishikawa T, Ishimura E and Nishizawa Y: Increased
stiffness in common carotid artery in hyperthyroid Graves' disease
patients. Biomed Pharmacother. 56:241–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni J, Zhou LX, Wei YP, Li ML, Xu WH, Gao S
and Cui LY: Moyamoya syndrome associated with Graves' disease: A
case series study. Ann Transl Med. 2:772014.PubMed/NCBI
|
|
10
|
Li H, Zhang ZS, Dong ZN, Ma MJ, Yang WZ,
Han C, Du MM, Liu YX, Yang H, Liu W, et al: Increased thyroid
function and elevated thyroid autoantibodies in pediatric patients
with moyamoya disease: A case-control study. Stroke. 42:1138–1139.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SJ, Heo KG, Shin HY, Bang OY, Kim GM,
Chung CS, Kim KH, Jeon P, Kim JS, Hong SC and Lee KH: Association
of thyroid autoantibodies with moyamoya-type cerebrovascular
disease: A prospective study. Stroke. 41:173–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panegyres PK, Morris JG, O'Neill PJ and
Balleine R: Moyamoya-like disease with inflammation. Eur Neurol.
33:260–263. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tendler BE, Shoukri K, Malchoff C,
MacGillivray D, Duckrow R, Talmadge T and Ramsby GR: Concurrence of
Graves' disease and dysplastic cerebral blood vessels of the
moyamoya variety. Thyroid. 7:625–629. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lip GY and Blann A: von Willebrand factor:
A marker of endothelial dysfunction in vascular disorders?
Cardiovasc Res. 34:255–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vischer UM: von Willebrand factor,
endothelial dysfunction, and cardiovascular disease. J Thromb
Haemost. 4:1186–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samai A, Monlezun D, Shaban A, George A,
Dowell L, Kruse-Jarres R, Schluter L, El Khoury R and Martin-Schild
S: Von Willebrand factor drives the association between elevated
factor VIII and poor outcomes in patients with ischemic stroke.
Stroke. 45:2789–2791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wieberdink RG, van Schie MC, Koudstaal PJ,
Hofman A, Witteman JC, de Maat MP, Leebeek FW and Breteler MM: High
von Willebrand factor levels increase the risk of stroke: The
Rotterdam study. Stroke. 41:2151–2156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koster T, Blann AD, Briët E, Vandenbroucke
JP and Rosendaal FR: Role of clotting factor VIII in effect of von
Willebrand factor on occurrence of deep-vein thrombosis. Lancet.
345:152–155. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Popławska-Kita A, Szelachowska M,
Modzelewska A, Siewko K, Dzięcioł J, Klimiuk PA and Górska M:
Endothelial dysfunction in Graves' disease. Adv Med Sci. 58:31–37.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Research Committee on the Pathology and
Treatment of Spontaneous Occlusion of the Circle of Willis; Health
Labour Sciences Research Grant for Research on Measures for
Infractable Diseases, . Guidelines for diagnosis and treatment of
moyamoya disease (spontaneous occlusion of the circle of Willis).
Neurol Med Chir (Tokyo). 52:245–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamasaki H, Takeuchi T, Mikami T, Komeichi
K and Tsutsumi H: A case of graves' disease diagnosed in the course
of bilateral carotid artery stenoses (moyamoya disease); a case
report and review of the literature. Clin Pediatr Endocrinol.
22:39–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conway DS, Pearce LA, Chin BS, Hart RG and
Lip GY: Prognostic value of plasma von Willebrand factor and
soluble P-selectin as indices of endothelial damage and platelet
activation in 994 patients with nonvalvular atrial fibrillation.
Circulation. 107:3141–3145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carter AM, Catto AJ, Mansfield MW, Bamford
JM and Grant PJ: Predictive variables for mortality after acute
ischemic stroke. Stroke. 38:1873–1880. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadler JE: Biochemistry and genetics of
von Willebrand factor. Annu Rev Biochem. 67:395–424. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lenting PJ, Casari C, Christophe OD and
Denis CV: von Willebrand factor: The old, the new and the unknown.
J ThrombHaemost. 10:2428–2437. 2012. View Article : Google Scholar
|
|
26
|
Müller G: [Structure and function of the
factor VIII/von Willebrand factor complex]. Z Gesamte Inn Med.
45:65–68. 1990.(In German). PubMed/NCBI
|
|
27
|
Hoyer LW: The factor VIII complex:
Structure and function. Blood. 58:1–13. 1981.PubMed/NCBI
|
|
28
|
Rao M, Zhang H, Liu Q, Zhang S, Hu L and
Deng F: Clinical and experimental pathology of Moyamoya disease.
Chin Med J (Engl). 116:1845–1849. 2003.PubMed/NCBI
|
|
29
|
Ikeda E and Hosoda Y: Distribution of
thrombotic lesions in the cerebral arteries in spontaneous
occlusion of the circle of Willis: Cerebrovascular moyamoya
disease. Clin Neuropathol. 12:44–48. 1993.PubMed/NCBI
|
|
30
|
Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC,
Kim JS and Kwon SU: High resolution MRI difference between moyamoya
disease and intracranial atherosclerosis. Eur J Neurol.
20:1311–1318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hosoda Y, Ikeda E and Hirose S:
Histopathological studies on spontaneous occlusion of the circle of
Willis (cerebrovascular moyamoya disease). Clin Neurol Neurosurg.
99(Suppl 2): S203–S208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Napoli R, Biondi B, Guardasole V,
Matarazzo M, Pardo F, Angelini V, Fazio S and Saccà L: Impact of
hyperthyroidism and its correction on vascular reactivity in
humans. Circulation. 104:3076–3080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vischer UM, Lang U and Wollheim CB:
Autocrine regulation of endothelial exocytosis: Von Willebrand
factor release is induced by prostacyclin in cultured endothelial
cells. FEBS Lett. 424:211–215. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Wang X, Lin Z and Wu H: Elevated
plasma levels of VWF: Ag in hyperthyroidism are mediated through
beta-adrenergic receptors. Endocr Res. 19:123–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burggraaf J, Lalezari S, Emeis JJ, Vischer
UM, de Meyer PH, Pijl H and Cohen AF: Endothelial function in
patients with hyperthyroidism before and after treatment with
propranolol and thiamazol. Thyroid. 11:153–160. 2001. View Article : Google Scholar : PubMed/NCBI
|