Introduction
It has previously been demonstrated that
brain-derived neurotrophic factor (BDNF), which is a neurotrophic
factor, has a key role in depression. In depressive patients with
serum reduced levels of BDNF, BNDF increased after treatment with
antidepressant agents (1,2). A postmortem study reported a
significant decrease in BDNF levels in the hippocampi of
depression-induced suicide subjects, but not in suicide subjects
treated with antidepressants (3).
Further studies have demonstrated that downstream components of the
BDNF/tyrosine receptor kinase B (TrkB) signaling pathway, such as
the phosphatidylinositol-3-kinase
(PI3K)/phosphatidylinositol-3-kinase (Akt) pathway, were inhibited
in depressive patients (3–6). The phospho-cAMP response
element-binding protein (pCREB) may regulate the transcription of
downstream genes, including BDNF, potentially by binding to
response elements in the promoter regions of gene transcription,
thus regulating neurotrophy, differentiation, cell viability and
recognition functions (7–10).
Kai-Xin-San (KXS) is a well-known traditional
Chinese medicine first recorded in Bei Ji Qian Jin Yao Fang
(Thousand Formulae for Emergency Thousand Formulae for Emergency)
by Sun Si-Miao (11,12). KXS consists of ginseng (Panax
ginseng C.A. Meyer), hoelen (Poria cocos F.A. Wolf),
polygala (Polygala tenaifolia Willd) and acorus (Acorus
tatarinowii Schott) at the ratio of 3:3:2:2 (13). It has been widely used in the
treatment of depression, as well as in learning and memory deficits
for thousands years (14). In our
previous studies, we have demonstrated the anti-depressive effect
of KXS in rats by utilizing chronic unpredictable mild stress
(CMS), the tail suspension test, the forced swim test and chronic
fatigue syndrome; in addition, increased levels of BDNF and pCREB
were detected, suggesting that they may have roles in the
anti-depressive effect of KXS (15–18). The
present study aimed to explore the mechanism of KXS via the
regulation of the BDNF and pCREB pathways in rats and neuronal
cells.
Materials and methods
KXS preparation
Herbs for KXS (ginseng, hoelen, polygala, and
acorus) were purchased from LvYe Medicinal Material Company
(Beijing, China), and the quality met the requirements outlined in
the Chinese Pharmacopoeia (2010) (19). The voucher specimen was registered
and deposited in the Herbarium of the Traditional Chinese Medicinal
pharmacy. KXS was supplied in powder form derived from a mixture of
the aqueous extract as described previously (20). Briefly, 1.5 kg ginseng, 1.5 kg
hoelen, 1 kg polygala and 1 kg acorus gramineus was soaked in 50 l
water for 3 h and was subsequently extracted three times using a
circumfluence extraction method. The aqueous extracts were filtered
and evaporated under reduced pressure. The obtained concentrates
were freeze-dried to powder form and stored at 4°C. Average yield
was 1 g KXS powder/4.83 g total herbs. KXS powder was standardized
using a high-performance liquid chromatography-fingerprint method,
as previously described (21).
Animals
In total, 24 male Wistar rats, weighing 180±10 g at
6 weeks, were obtained from the Animal Breeding Center of the
People's Liberation Army general hospital (Beijing, China). All
rats were housed in a temperature- (23±2°C) and humidity-controlled
(60±10%) facility with a 12-h light/dark cycle with free access to
food and water. All animal experimental protocols were approved by
the Animal Experimentation Ethics Committee of General Hospital of
Chinese PLA (X5-2013-04). All animal handling procedures were
performed in accordance with the Principles of Laboratory Animal
Care and Chinese legislation for the use and care of laboratory
animals.
Induction of CMS
CMS was induced in 16 rats following the established
protocol (22). Rats received 4
weeks of stress stimulation, which included high-speed agitation
(10 min), deprivation of food or water (24 h), immobilization (2
h), continuous illumination (24 h), tilted cage (12 h) and forced
swimming in ice water (5 min). Rats were randomly assigned one
stimulation daily at 3–5 p.m. for four weeks. The remaining eight
rats were housed undisturbed without contact with the stressed
animals as the control group.
Drug administration
The 16 CMS-induced rats were randomly divided into
two groups treated with either water (n=8; CMS group) or KXS at 370
mg/kg orally at 9–10 am daily for three weeks (n=8; KXS group).
Sucrose-preference test
Sucrose-preference test was performed to define
anhedonia prior to surgery (baseline) and after CMS induction. Rats
were food-and water-deprived for 18 h, and subsequently fed with
pre-weighted bottles containing 1% sucrose solution and water for 1
h. Intake was measured by weighing the bottles before and after
each test. All tests were carried out in the home cage to minimize
extraneous novelty and disturbance. Sucrose preference was
calculated as: Sucrose preference = sucrose intake / (sucrose
intake + water intake). Anhedonia was defined as a reduction in
sucrose preference relative to baseline levels.
Open-field test
Locomotor activity was assessed to detect immobility
or changes in motor activity in an open-field test. A metallic
cubic open field arena (80×80×80 cm) was used. The floor of the box
was 25 squares (5 squares long; 5 squares wide). Rats were
individually placed into the center of the arena and allowed to
explore freely for 3 min. The floor of the open-field apparatus was
wiped cleaned with 70% ethanol between tests. The behavior,
including the number of square lines crossed with all four paws,
and rearing, including the number of times each rat stood on its
hind limbs, were recorded.
Western blot
At the end of experiment, all rats were sacrificed
with 10% chloral hydrate solution (3.5 ml/kg; i.p.). Hippocampus
and prefrontal cortex tissues were collected and lysed with
radioimmunoprecipitation assay buffer (Applygen Technologies, Inc.,
Beijing, China), schizolysised for 20 min on ice and centrifuged at
12,000 × g for 10 min at 4°C. Protein samples were heated at
95°C for 8 min, separated by 12% SDS-PAGE and transferred to
nitrocellulose membranes. Membrane were blocked for 2 h in TBST (25
mM Tris, 140 mM NaCl, 27 mM KCl and 0.02% Tween 20) containing 5%
bovine serum albumin and incubated with primary antibodies specific
for BDNF (ab108319, 1:200)(Abcam, Cambridge, MA, USA), TrkB
(BS1431, 1:500), ERK (AP0491, 1:500), pERK (BS4621, 1:500), Akt
(BS1502, 1:500), PI3K (BS3678, 1:500), pGSK3β (BS4084, 1:500) and
GSK3β (BS1402, 1:500; Bioworld Technology, Inc., St. Louis Park,
MN, USA), pCREB (#9198, 1:1,000) and CREB (#9197, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C over night.
Following three washes with TBST, membranes were incubated for 1 h
at room temperature with horseradish peroxidase-labeled secondary
antibodies (BS13278, 1:5,000; Bioworld Technology, Inc.), washed
with TBST three times. Blots were developed using a
electrochemiluminescence system (UVP LLC, Upland, CA, USA).
Primary hippocampal neurons
culture
Primary hippocampal neuronal cultures were prepared
as described previously (23), with
a few modifications. A total of 36 Newborn Wistar rats (age, <24
h) were purchased from the Peking University Health Science Center
(Beijing, China). Hippocampi were dissected on ice, minced and
digested with 0.25% trypsin for 15 min at 37°C. Susequently, the
samples were supplemented with 5 ml fetal bovine serum (FBS) to
terminate the digestion procedure. Cell suspension was passed
through a 200 mesh (diameter, 74 µm) cell strainer and separated by
density gradient centrifugation for 20 min at 300 × g at
room temperature. The suspension was centrifuged for 5 min at 225 ×
g at room temperature and the pellet was resuspended in 10
ml Dulbecco's modified Eagle's medium (DMEM). Following the
measurement of cell density using a hemacytometer, the neuronal
cells were plated into 6-well plates at 5×105 cell/ml
for culture in DMEM supplemented with 10% FBS, 1 mmol/l glutamine,
20 mmol/l sodium pyruvate, and 10 ng/ml nerve growth factor (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). 200 nM K252a
(Sigma Aldrich; Merck Millipore, Darmstadt, Germany) was added to
cells 30 min before the exposure to KXS as an inhibitor of TrkB.
Cells were used for assay analysis at day 7 when the percentage was
~85%, as assessed by microtubule-associated protein 2 (MAP-2)
immunostaining.
Cell survival and proliferation
assays
Cell viability was evaluated via MTT assay (24). Briefly, 20 µl MTT solution (2 mg/ml
in PBS) was added at a final concentration of 0.5 mg/ml and
incubated at 37°C for 4 h. Following removal of the medium, 150 µl
DMSO was added. Optical densities were subsequently read using a
microplate reader at 570 nm (1420 Vitor 3; Perkin-Elmer, Waltham,
MA, USA). Viability was expressed as a percentage of the
KXS-treated cells according to the control cells.
Statistical analysis
All data were expressed as the mean ± standard
error. Differences between groups were analyzed by one-way analysis
of variance followed by Dunnett's test. Statistically analyses were
performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Behavioral index
Table I shows the
results from the open-field and sucrose-preference tests. The index
of sucrose preference in the CMS group was significantly lower than
that of the control group (P<0.01) and the KXS group
(P<0.05). Similarly, the number of lines crossed and rearing
values in the CMS group were significantly lower than that of the
control group (P<0.01) and the KXS group (P<0.01, P<0.05),
suggested that KXS could improve the depressive symptom in rats
induced by CMS.
 | Table I.Effects of KXS on behavioral indices
in the CMS-treated rats (n=8/group). |
Table I.
Effects of KXS on behavioral indices
in the CMS-treated rats (n=8/group).
| Group | Lines crossed
(n) | Rearing count
(n) | Sucrose intake
(ml) | Body weight
(g) |
|---|
| Control | 68.9±8.5 | 9.0±2.1 | 90.54±3.98 | 422.3±24.4 |
| CMS |
15.4±6.2a |
3.8±2.0a |
70.94±7.09a |
352.4±31.1a |
| KXS |
36.8±8.6c |
6.6±2.5b |
91.16±8.49b |
388.3±17.4b |
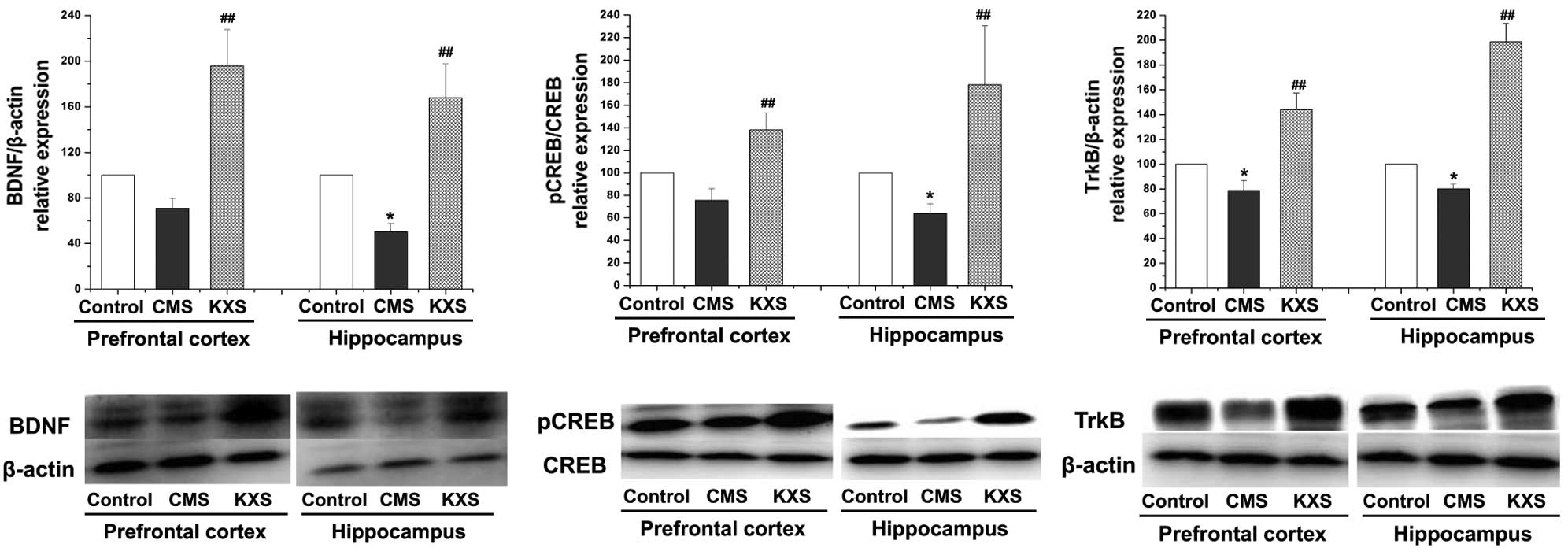
Protein expression levels of BDNF,
CREB and TrkB
Western blot analysis demonstrated that the protein
expression levels of BDNF, pCREB and TrkB in the hippocampus and
TrkB in the prefrontal cortex were significantly reduced in the CMS
group compared with the control group (P<0.05). Furthermore, the
protein expression levels of BDNF, pCREB and TrkB in the
hippocampus and prefrontal cortex were increased in the KXS group
compared with the CMS group (P<0.01; Fig. 1), suggested that the antidepressive
effect of KXS might be involved in its regulating on BDNF and TrkB
through promoting the pCREB level.
Protein expression levels of pERK/ERK,
PI3K, Akt and pGSK3β/GSK3β
Fig. 2 demonstrates
that the protein expression levels of pERK/ERK (P<0.01), PI3K
(hippocampus, P<0.01; prefrontal cortex, P<0.05), and Akt
(hippocampus, P<0.01; prefrontal cortex, P<0.05) in the
hippocampus and prefrontal cortex, and pGSK3β/GSK3β (P<0.05) in
the hippocampus of the KXS group were significantly elevated,
compared with the CMS group. The results suggest that the
regulation effect of KXS on BDNF and pCREB might be related to its
activation on the upstream signaling pathway of CREB.
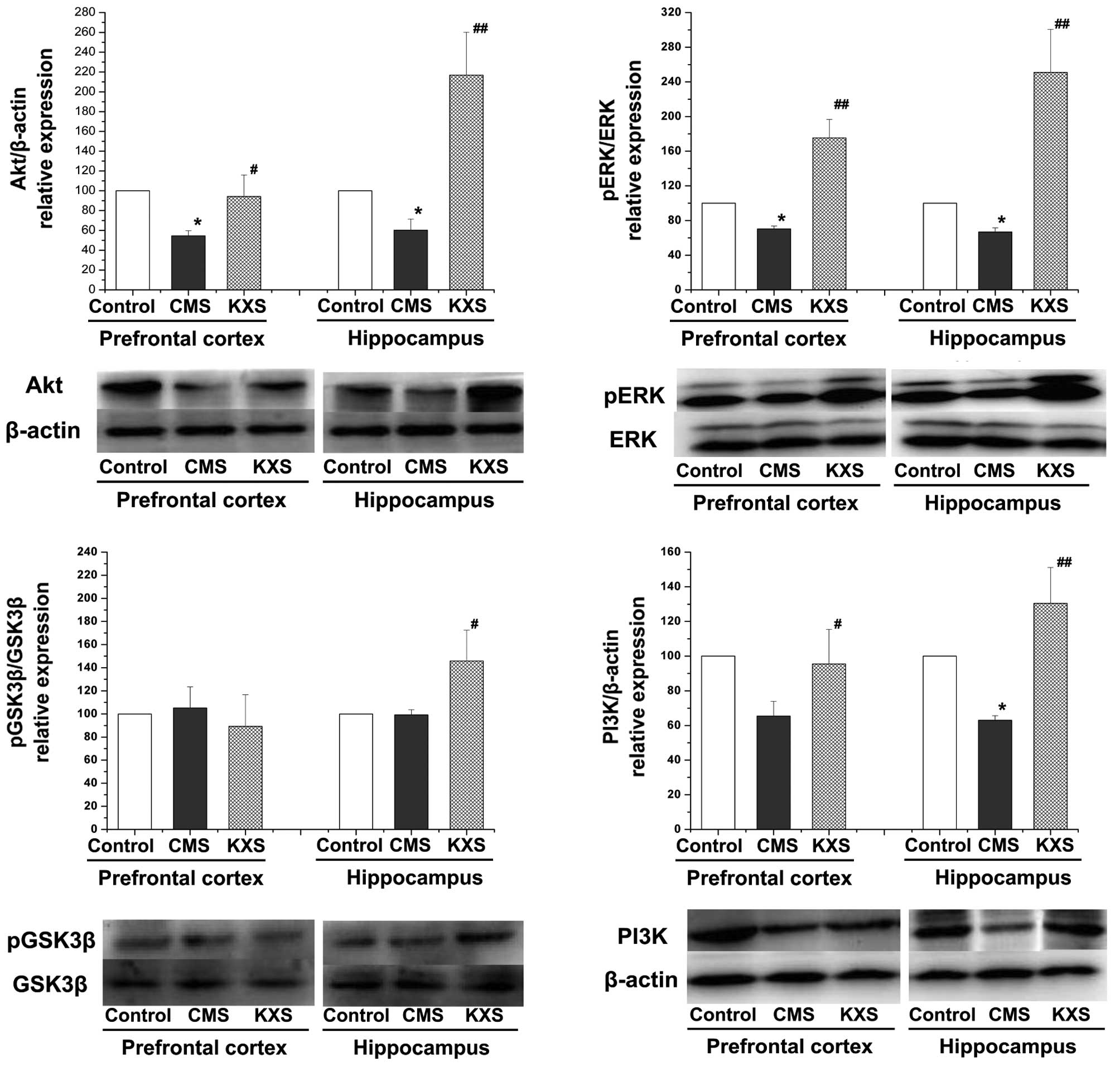 | Figure 2.Effects of KXS on the TrkB/BDNF/ERK
and TrkB/BDNF/PI3K signaling pathway in CMS rats. KXS (370 mg/kg)
was administered daily for three weeks. Data are presented as the
mean ± standard deviation, *P<0.05 vs. the control group;
##P<0.01 and #P<0.05 vs. the CMS group.
CMS, chronic unpredictable mild stress; KXS, Kai-Xin-San; BDNF,
brain-derived neurotrophic factor; Akt, protein kinase B; p,
phospho; ERK, extracellular signal-regulated kinase; TrkB, tyrosine
receptor kinase B; GSK3β, glycogen synthase kinase 3β; PI3K,
phosphatidylinositol-3-kinase. |
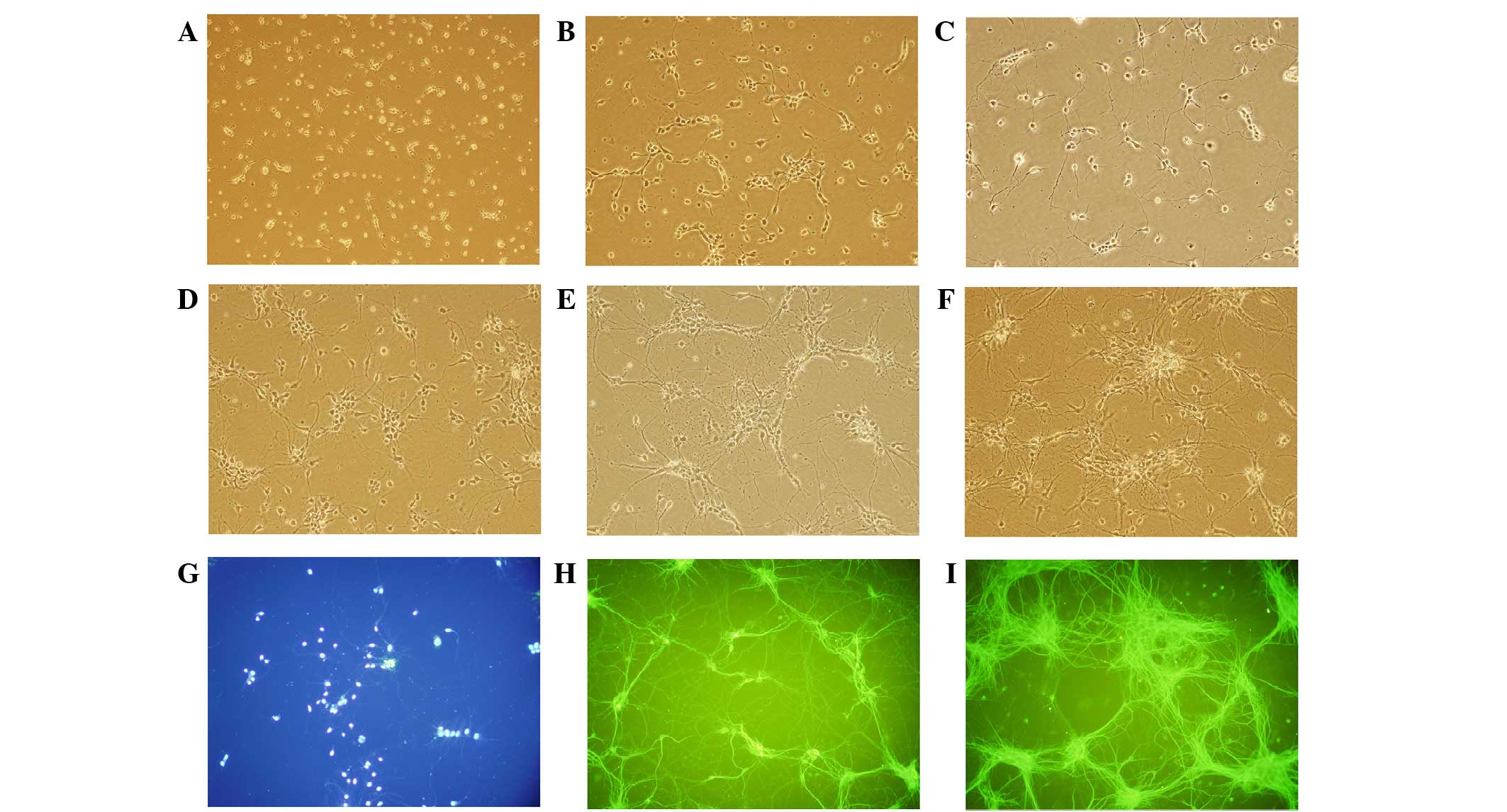
Cell viability and BDNF
expression
Fig. 3 shows MAP-2
immunostaining of neuronal cells. The morphous and purity of the
neuronal cells was suitable for the experiment. In cells treated
with KXS, cell viability was significantly increased, as compared
with the control cells (P<0.01). The level of BDNF release
increased in a time-dependent manner, peaking at 6 h post-treatment
(Fig. 4). KXS could increase the
nerve cell viability which might be involved in the increasing
level of BDNF.
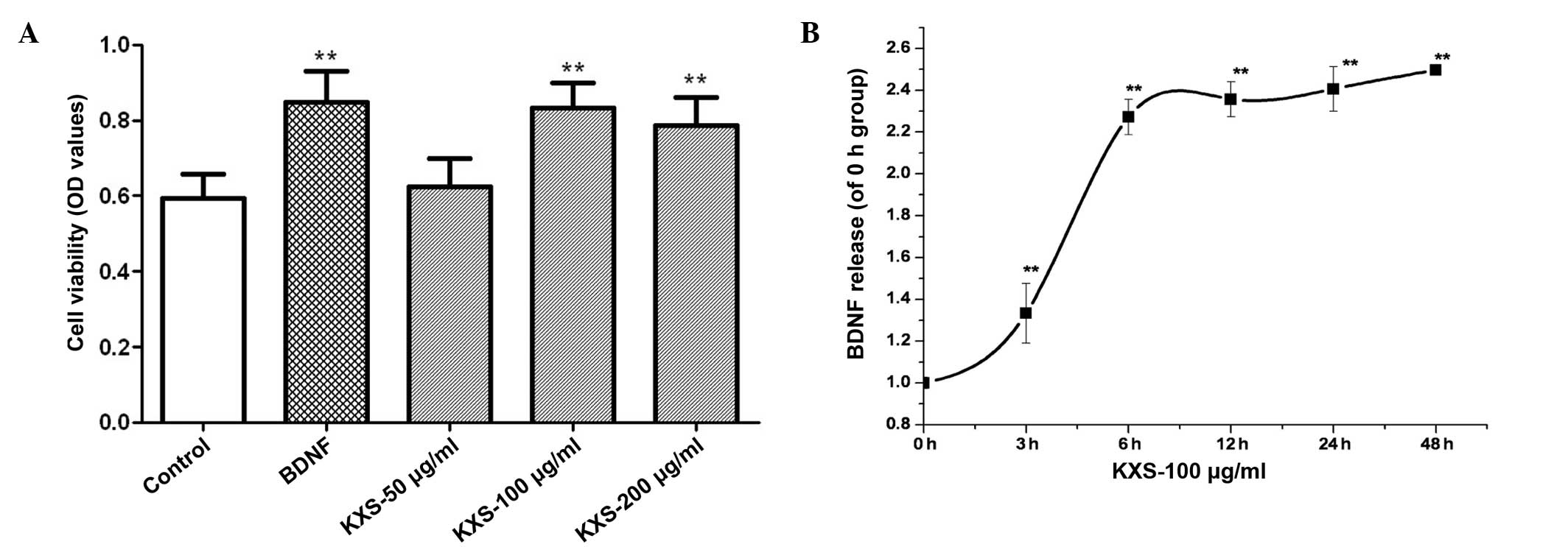 | Figure 4.Effect of KXS on cell survial and
BDNF release from primary hippocampal neurons. (A) For cell
viability assay, cells were treated with 25 pg/l BDNF or 50, 100
and 200 µg/ml of KXS for 48 h. **P<0.01 vs. the control group.
(B) In the BDNF release test, cultured cells were treated with 100
µg/ml KXS for 0, 3, 6, 12, 24 and 48 h. **P<0.01 vs. the 0 h
group. Data are presented as the mean ± standard deviation. KXS,
Kai-Xin-San; BDNF, brain-derived neurotrophic factor; OD, optical
density. |
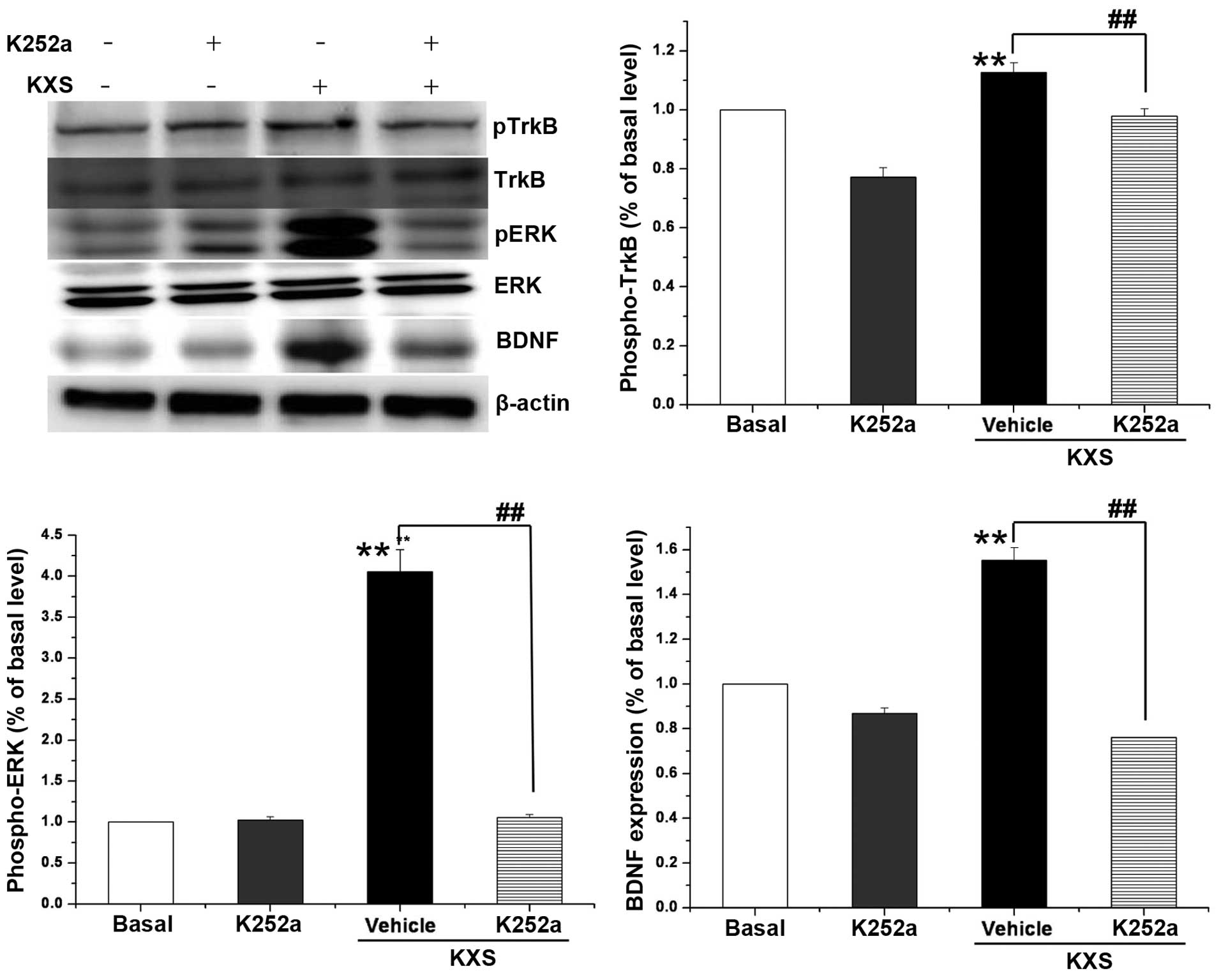
Protein expression levels of TrkB/ERK
in neuronal cells
Fig. 5 indicates that
the protein expression levels of BDNF, pERK and pTrkB in the KXS
group were significantly higher than that of the control group
(P<0.01). Such an increase was not observed when cells were
treated with K252a, which is an inhibitor of TrkB. The effects of
KXS on the pathway components in cells coincided with those of
animal experiments.
Discussion
In CMS rats treated with KXS, increased protein
expression levels of pCREB, BDNF and TrkB were detected in the
hippocampus and prefrontal cortex. Consistently, in primary
hippocampal neurons, an increased level of upstream components,
including ERK, pERK, PI3K, Akt and GSK3β, was observed when cells
were treated with KXS. These results suggest that pCREB and
upstream components may be associated with the antidepressive
effect of KXS.
Depression is a complex mental disorder involving
multiple factors. Impaired release of BDNF, which is a small
protein utilized by the brain for development and nerve cell growth
regulation and function (25), has
been demonstrated to have a key role in major depression (26). The present finding of increased BDNF
levels in CMS rats treated with KXS is consistent with previous
reports (22). In previous animal
and human studies, circulating levels of BDNF were very low and
could be increased with treatment of antidepressants (27–30),
which may account for its efficacy in depressive behaviors
(31,32).
It has been demonstrated that ERK and PI3K pathway
are the two most important pathways altered through the BDNF
mediated-TrkB activation, resulting in neuroprotection effect
(33). ERKs are able to activate and
transfer signals to the nucleus quickly, in order to induce CREB
phosphorylation and activate the nuclear transcription factor,
CREB. Enhanced CREB ultimately results in increased expression of
BDNF (34). These data suggest a
positive feedback loop between BDNF and CREB, which may be
associated with nerve cell nutrition and nerve injury repair
(35). In this study, increased pERK
was observed in CMS rats treated with KXS; however, such an
increase was not observed when primary neurons cells were treated
with K252a, which is an inhibitor of TrkB. This suggests that the
KXS-induced increase of BNDF may be mediated by the TrkB-dependent
ERK pathway. Activated CREB may further regulate the transcription
of genes involved in neuronal cell survival.
GSK-3β is an essential pro-apoptotic factor for
neuronal the apoptosis cascade and PI3K-mediated neuronal survival
pathway (36). In its active form,
GSK-3β is able to enhance apoptosis; however, once inactivated by
phorphorylation, it cannot trigger apoptotic pathways (37). In the present study, inactivated
GSK-3β by phosphorylation and an PI3K/AKT-mediated increase in BDNF
was observed when rats were treated with KXS. This finding is
consistent with a previous report, demonstrating that BDNF may
provide neuroprotection via the activation of the PI3K/Akt pathway
(38).
In conclusion, increased protein expression levels
of pCREB, BDNF and TrkB were detected in the hippocampus and
prefrontal cortex. Consistently, in primary hippocampal neurons,
increased levels of upstream components, including ERK, pERK, PI3K,
Akt, and GSK3β, were observed when cells were treated with KXS.
These results suggest that pCREB and its upstream components may be
associated with the antidepressive effect of KXS. Subsequent
studies in other cells and animal models are required to confirm
the present findings and conclusions.
Acknowledgments
This work was supported by grants from the National
Natural Science Foundation (grant nos. 81302909 and 81173430).
Glossary
Abbreviations
Abbreviations:
|
KXS
|
Kai-Xin-San
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
CREB
|
cAMP response element-binding
|
|
p-CREB
|
phospho-cAMP response element-binding
protein
|
|
CMS
|
chronic mild stress
|
|
TrkB
|
receptor tyrosine kinase B
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
pERK
|
phospho-extracellular signal-regulated
kinase
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
Akt
|
protein kinase B (PKB)
|
|
GSK3β
|
glycogen synthase kinase 3β
|
|
pGSK3β
|
phospho-glycogen synthase kinase
3β
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
OD
|
optical density
|
|
PBS
|
phosphate-buffered saline
|
|
NGF
|
nerve growth factor
|
|
MAP-2
|
microtubule-associated protein 2
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Voleti B and Duman RS: The roles of
neurotrophic factor and Wnt signaling in depression. Clin Pharmacol
Ther. 91:333–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sen S, Duman R and Sanacora G: Serum
brain-derived neurotrophic factor, depression and antidepressant
medications: Meta-analyses and implications. Biol Psychiatry.
64:527–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karege F, Vaudan G, Schwald M, Perroud N
and La Harpe R: Neurotrophin levels in postmortem brains of suicide
victims and the effects of antemortem diagnosis and psychotropic
drugs. Brain Res Mol Brain Res. 136:29–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsiung SC, Adlersberg M, Arango V, Mann
JJ, Tamir H and Liu KP: Attenuated 5-HT1A receptor signaling in
brains of suicide victims: Involvement of adenylyl cyclase,
phosphatidylinositol 3-kinase, Akt and mitogen-activated protein
kinase. J Neurochem. 87:182–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jernigan CS, Goswami DB, Austin MC, Iyo
AH, Chandran A, Stockmeier CA and Karolewicz B: The mTOR signaling
pathway in the prefrontal cortex is compromised in major depressive
disorder. Prog Neuropsychopharmacol Biol Psychiatry. 35:1774–1779.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dwivedi Y, Rizavi HS, Zhang H, Roberts RC,
Conley RR and Pandey GN: Modulation in activation and expression of
phosphatase and tensin homolog on chromosome ten, Akt1 and
3-phosphoinositide-dependent kinase 1: Further evidence
demonstrating altered phosphoinositide 3-kinase signaling in
postmortem brain of suicide subjects. Biol Psychiatry.
67:1017–1025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molteni R, Calabrese F, Racagni G,
Fumagalli F and Riva MA: Antipsychotic drug actions on gene
modulation and signaling mechanisms. Pharmacol Ther. 124:74–85.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fishback JA, Robson MJ, Xu YT and
Matsumoto RR: Sigma receptors: Potential targets for a new class of
antidepressant drug. Pharmacol Ther. 127:271–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blendy JA: The role of CREB in depression
and antidepressant treatment. Biol Psychiatry. 59:1144–1150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milnerwood AJ and Raymond LA: Early
synaptic pathophysiology in neurodegeneration: Insights from
Huntington's disease. Trends Neurosci. 33:513–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Y, Duan X, Huang F, Cheng X, Zhang L,
Liu P, Shulan S, Duan JA, Dong TT and Tsim KW: Kai-Xin-San, a
traditional Chinese medicine formula, induces neuronal
differentiation of cultured PC12 Cells: Modulating neurotransmitter
regulation enzymes and potentiating NGF inducing neurite outgrowth.
J Ethnopharmacol. 193:272–282. 2016. View Article : Google Scholar
|
|
12
|
Qiong W, Yong-Liang Z, Ying-Hui L,
Shan-Guang C, Jiang-Hui G, Yi-Xi C, Ning J and Xin-Min L: The
memory enhancement effect of Kai Xin San on cognitive deficit
induced by simulated weightlessness in rats. J Ethnopharmacol.
187:9–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu KY, Xu SL, Choi RCY, Yan AL, Dong TTX
and Tsim KWK: Kai-Xin-San, a Chinese Herbal Decoction Containing
Ginseng Radix et Rhizoma, Polygalae Radix, Acori Tatarinowii
Rhizoma, and Poria, Stimulates the Expression and Secretion of
Neurotrophic Factors in Cultured Astrocytes. Evid Based Complement
Alternat Med. 7313852013.PubMed/NCBI
|
|
14
|
Dang H, Sun L, Liu X, Peng B, Wang Q, Jia
W, Chen Y, Pan A and Xiao P: Preventive action of Kai Xin San
aqueous extract on depressive-like symptoms and cognition deficit
induced by chronic mild stress. Exp Biol Med (Maywood).
234:785–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Liu P, Guo DH, Rahman K, Wang DX,
Chen ML and Xie TT: Behavioral and biochemical effects of
Kaixin-San, a traditional Chinese medicinal empirical formula. Drug
Develop Res. 69:267–271. 2008. View Article : Google Scholar
|
|
16
|
Zhou XJ, Liu M, Yan JJ, Cao Y and Liu P:
Antidepressant-like effect of the extracted of Kai Xin San, a
traditional Chinese herbal prescription, is explained by modulation
of the central monoaminergic neurotransmitter system in mouse. J
Ethnopharmacol. 139:422–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong XZ, Li ZL, Zheng XL, Mu LH, Zhang G
and Liu P: A representative prescription for emotional disease,
Ding-Zhi-Xiao-Wan restores 5-HT system deficit through interfering
the synthesis and transshipment in chronic mild stress-induced
depressive rats. J Ethnopharmacol. 150:1053–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Y, Hu Y, Liu P, Zhao HX, Zhou XJ and
Wei YM: Effects of a Chinese traditional formula Kai Xin San (KXS)
on chronic fatigue syndrome mice induced by forced wheel running. J
Ethnopharmacol. 139:19–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Pharmacopoeia Committee, .
Pharmacopoeia of People's Republic of China [M]. Part 1. Beijing:
Chemical Industry Press; 2010, pp. 7–437
|
|
20
|
Mu LH, Huang ZX, Liu P, Hu Y and Gao Y:
Acute and subchronic oral toicity assessment of the herbal formula
Kai-Xin-San. J Ethnopharmacol. 138:351–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Cao Y, Liu M, Liu P, Cui H and
Dai-Hong G: Behavioral and biochemical effects of a formulation of
the traditional Chinese medicine, Kai-Xin-San, in fatigued rats.
Exp Ther Med. 6:973–976. 2013.PubMed/NCBI
|
|
22
|
Hu Y, Liu M, Liu P, Guo DH, Wei RB and
Rahman K: Possible mechanism of the antidepressant effect of
3,6′-disinapoyl sucrose from polygala tenuifolia willd. J Pharm
Pharmacol. 63:869–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu D, Zhang H, Gu W, Liu Y and Zhang M:
Neuroprotective effects of ginsenoside Rb1 on high glucose-induced
neurotoxicity in primary cultured rat hippocampal neurons. PLoS
One. 8:e793992013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He XL, Zhang P, Dong XZ, Yang MH, Chen SL
and Bi MG: JR6, a new compound isolated from Justicia procumbens,
induces apoptosis in human bladder cancer EJ cells through
caspase-dependent pathway. J Ethnopharmacol. 144:284–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Y, Zhou XJ, Liu P, Dong XZ, Mu LH, Chen
YB, Liu MY and Yu BY: Anti-depressant and neuroprotective effect of
the Chinese herb KaiXinSan against lentiviral shRNA Knockdown
brain-derived neurotrophic factor-induced injury in vitro and in
vivo. Neuropsychobiology. 69:129–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu H and Chen ZY: The role of BDNF in
depression on the basis of its location in the neural circuitry.
Acta Pharmacol Sin. 32:3–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sen S, Duman R and Sanacora G: Serum
brain-derived neurotrophic factor, depression and antidepressant
medications: Meta-analyses and implications. Biol Psychiatry.
64:527–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bernard R, Kerman IA, Thompson RC, Jones
EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H and
Watson SJ: Altered expression of glutamate signaling, growth factor
and glia genes in the locus coeruleus of patients with major
depression. Mol Psychiatry. 16:634–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kozicz T, Tilburg-Ouwens D, Faludi G,
Palkovits M and Roubos E: Gender-related urocortin 1 and
brain-derived neurotrophic factor expression in the adult human
midbrain of suicide victims with major depression. Neuroscience.
152:1015–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Altar CA, Whitehead RE, Chen R, Wörtwein G
and Madsen TM: Effects of electroconvulsive seizures and
antidepressant drugs on brain-derived neurotrophic factor protein
in rat brain. Biol Psychiatry. 54:703–709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shirayama Y, Chen AC, Nakagawa S, Russell
DS and Duman RS: Brain-derived neurotrophic factor produces
antidepressant effects in behavioral models of depression. J
Neurosci. 22:3251–3261. 2002.PubMed/NCBI
|
|
32
|
Deltheil T, Tanaka K, Reperant C, Hen R,
David DJ and Gardier AM: Synergistic neurochemical and behavioural
effects of acute intrahippocampal injection of brain-derived
neurotrophic factor and antidepressants in adult mice. Int J
Neuropsychopharmacol. 12:905–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jain V, Baitharu I, Prasad D and
Ilavazhagan G: Enriched Environment prevents hypobaric hypoxia
induced memory impairment and neurodegeneration: Role of
BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS One.
8:e622352013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan X, Liu J, Ye Z, Huang J, He F, Xiao W,
Hu X and Luo Z: CaMKII-Mediated CREB Phosphorylation Is Involved in
Ca2+-Induced BDNF mRNA Transcription and Neurite
Outgrowth Promoted by Electrical Stimulation. PLoS One.
11:e01627842016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fišar Z and Hroudová J: Intracellular
signalling pathways and mood disorders. Folia Biol (Praha).
56:135–148. 2010.PubMed/NCBI
|
|
36
|
Dong XZ, Huang CL, Yu BY, Hu Y, Mu LH and
Liu P: Effect of Tenuifoliside a isolated from polygala tenuifolia
on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine.
21:1178–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang JS, Herreros-Villanueva M, Koenig A,
Deng Z, de Narvajas AA, Gomez TS, Meng X, Bujanda L, Ellenrieder V,
Li XK, Kaufmann SH and Billadeau DD: Differential activity of GSK-3
isoforms regulates NF-κB and TRAIL- or TNFα induced apoptosis in
pancreatic cancer cells. Cell Death Dis. 5:e11422004. View Article : Google Scholar
|
|
38
|
Zhang L, Zhao H, Zhang X, Chen L, Zhao X,
Bai X and Zhang J: Nobiletin protects against cerebral ischemia via
activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and
ameliorating BBB permeability in rat. Brain Res Bull. 96:45–53.
2013. View Article : Google Scholar : PubMed/NCBI
|