Introduction
The diagnosis and treatment of pulmonary diseases
has markedly improved with advances in the technologies of
interventional pulmonary medicine (1–4). For
example endobronchial ablation techniques have been employed in the
treatment of intraluminal diseases of the tracheobronchial tree
(5,6). The viability of these technologies for
the curative treatment of airway obstructions caused by benign
lesions, including benign tumors, endobronchial tuberculosis and
granulomatosis, has been established (7). They are also important in the
palliative treatment of patients with late-stage lung cancers
(8). In China, the bronchoscopic
ablation techniques commonly used include neodymium-doped yttrium
aluminium garnet (Nd:Y3Al5O12, or
Nd:YAG) laser therapy, high-frequency electrocautery, argon plasma
coagulation (APC) and CO2 cryotherapy (9–11). These
techniques may be performed via rigid or flexible fiberoptic
bronchoscopy.
Among the malignant tumors, lung cancer has been
ranked first in the world in terms of its morbidity and mortality
(12). The majority of patients with
lung cancer present at the late stage, when curative surgical
resection is not an option, and 30% have obstructions in the
trachea or main bronchi (13). For
these patients, endobronchial therapy can restore airway patency,
alleviate dyspnea, preserve quality of life, improve survival rates
and allow further treatments, such as external beam radiation,
chemotherapy and surgery (14).
The detection rate for bronchogenic carcinomas in
situ and early-stage intraluminal carcinomas has improved with
technological developments (15,16).
Patients who suffer from superficial lesions, but are inoperable
due to an unfit health status, can be treated by bronchoscopic
interventions to prevent progression to invasive cancer (17). For these patients, the first choice
for palliation or treatment with curative intent is currently
photodynamic therapy (18). However,
the availability of photodynamic therapy is limited at most
institutions in China because of its expense and the cumbersomeness
of the equipment. Instead, endobronchial ablation utilizing Nd:YAG
laser therapy, electrocautery, APC or cryotherapy are typically
used because of their lower cost, portability and comparable
efficacy (11). However, long-term
observational studies and prospective randomized controlled trials
are necessary for definitive verification of these techniques
(19).
China initially lagged behind developed countries in
adopting interventional pulmonary medicine, and a disparity still
exists. In some parts of the country, the availability of ablation
technologies remains very limited and there is the question of
whether purchasing one or two of these would suffice and be
comparable to an entire set of the latest equipment. There is a
relative paucity of data to differentiate the various endobronchial
ablation technologies according to their biological effects,
efficacy and safety with specific applications. In addition, the
healing course of lesions induced by endobronchial ablation is not
known.
In a preliminary in vitro study, the authors
of the present study evaluated several endobronchial coagulation
techniques (microwave, APC, electrocautery and cryotherapy) and
determined specific values for technical parameters associated with
their safety and efficacy (20). In
the present study, the efficacy of Nd:YAG laser therapy,
electrocautery, APC and CO2 cryotherapy in dogs was
evaluated, to determine their relative merits and the optimal
technical parametric values for clinical practice.
Materials and methods
Animals and pre-tracheal ablation
procedures
The present study was approved by the Institutional
Animal Research Ethics Committee. A total of 6 healthy adult beagle
dogs (3 male and 3 female) weighing 10–12 kg were provided by the
Laboratory Animal Center at the Second Military Medical University
(Shanghai, China). The beagle dogs were bred under normal room
conditions at a temperature of 16–26°C, humidity of 40–70%, noise
level <60 dB, and 100–200 lux illumination. Adequate drinking
water was provided. The daily quantity of food was approximately
3–5% of the dog weight. The food was divided into two portions; one
was provided in the morning, and the other was provided in the
afternoon.
General anesthesia was induced using intravenous
amobarbital sodium (0.1 ml/kg; Shanghai Xinya Pharmaceutical Co.
Ltd., Shanghai, China), and 2% lidocaine (Jincheng Hayes
Pharmaceutical Co., Ltd., Jincheng, China) was administered onto
the tracheal mucosa. Following anesthetization, the dogs were
placed in the supine position with the head and limbs fixed on the
operating bench.
Using a laryngoscope blade and an intubation stylet,
with the tongue displaced to the left, the blade was introduced
with its concave surface directed ventrally and the soft palate was
displaced dorsally to reveal the rima glottidis. Subsequently, the
intubation stylet was advanced beyond the level of the vocal folds
and the trachea was intubated (7.5-mm internal diameter tracheal
tube). The mouth was kept open with a bite blocker, and the tongue
was extended and secured with a strip of gauze tied to the bite
blocker.
An Olympus T260 Fiberoptic Bronchoscope (Olympus
Corporation, Tokyo, Japan) was introduced into the trachea and the
tracheobronchial tree was examined. The middle and lower parts of
the trachea, excluding the membranous part, were selected as the
target tissue for the ablation treatments. During the bronchoscopy,
mucous secretions were removed directly through negative pressure
suction when required. Each dog underwent four endobrachial
ablation procedures, as described in the following sections.
Nd:YAG laser ablation
A LaserPro 810 Laser Probe (Collin SAS, Bagneux,
France) was inserted through the working channel of the
bronchoscope, and advanced at least 1 cm beyond the distal end of
the bronchoscope. With the aid of a pilot red light, the laser
energy was focused on the target tissue; the tip of the probe was
directed to the target tissue and was maintained at a distance of
4–10 mm from the tissue surface. Power was set at 20 W, and was
applied with a pulse of 1, 2 or 3 sec (setting on the equipment) at
three separate sites at 2-cm intervals.
High-frequency electrocautery
An electrocautery probe (VIO® 300 D; Erbe
Elektromedizin GmbH, Tübingen, Germany) was passed through the
working channel of the bronchoscope, and protruded 1–2 cm beyond
the distal end of the bronchoscope. The probe was placed in contact
with the target site, and an electric current, with the power set
at 40 W, was applied for 1, 3 or 5 sec at three separate sites at
2-cm intervals.
APC ablation
The APC probe (VIO® 300 D) was inserted
through the working channel of the bronchoscope, and advanced 1 cm
beyond the distal end of the bronchoscope, with the tip of the
probe held 4–10 mm from the target tissue. APC was performed with
an argon flow rate of 2 l/min and a power of 40 W, with a burst of
1, 3 or 5 sec at three separate sites at 2-cm intervals.
Cryotherapy ablation
Cryotherapy (K300 Cryosurgery Equipment; Beijing
Kooland Technology, Co., Ltd., Beijing, China) was performed by
passing a cryoprobe through the flexible bronchoscope until it had
advanced 1 cm beyond the distal end of the bronchoscope. The tip of
the cryoprobe was kept in contact with the tracheal mucosa. Three
freeze-thaw cycles, with the impedance set at 100 Ω, were applied
at two separate sites (at a 2-cm interval), with each freeze-thaw
cycle lasting 60 or 120 sec.
Post-ablation protocol
Two dogs were sacrificed immediately following the
endobronchial ablations to evaluate early pathological changes of
the tracheal wall. Sacrifice was conducted by the intravenous
injection of 0.1 ml/kg pentobarbital sodium, after which the
femoral artery and vein in the femoral triangle area were cut out
to induce exsanguination. The general health status of the
remaining four dogs was monitored daily, including eating and
physical activities, with special attention to respiratory
complications and accidental death, until they were randomly
sacrificed on days 3, 7, 14 or 21 postoperatively. The trachea with
the injured sites was removed, divided into tissue blocks (10×10
mm) and rinsed with normal saline. Subsequently, the specimens were
fixed in formalin for 24 h, embedded in paraffin, step-sectioned
into 3-µm slices and stained with hematoxylin and eosin for
analysis under a light microscope.
Evaluation of the biological effects
of endobronchial interventions
Assessment of the biological effects of the ablation
techniques was based on endoscopic gross findings and
histopathological examinations. During the bronchoscopic
interventions, the gross endoscopic changes within the tracheal
lumen were recorded. These involved changes in the local mucosal
color and texture, and the extent of injuries, including necrosis,
carbonization, vaporization and perforation.
The tracheal tissue sections were observed under a
light microscope to evaluate the histopathological changes of the
mucosa, submucosa and cartilage layer. The severity of injuries was
defined as mild (+), moderate (++) or severe (+++), according to
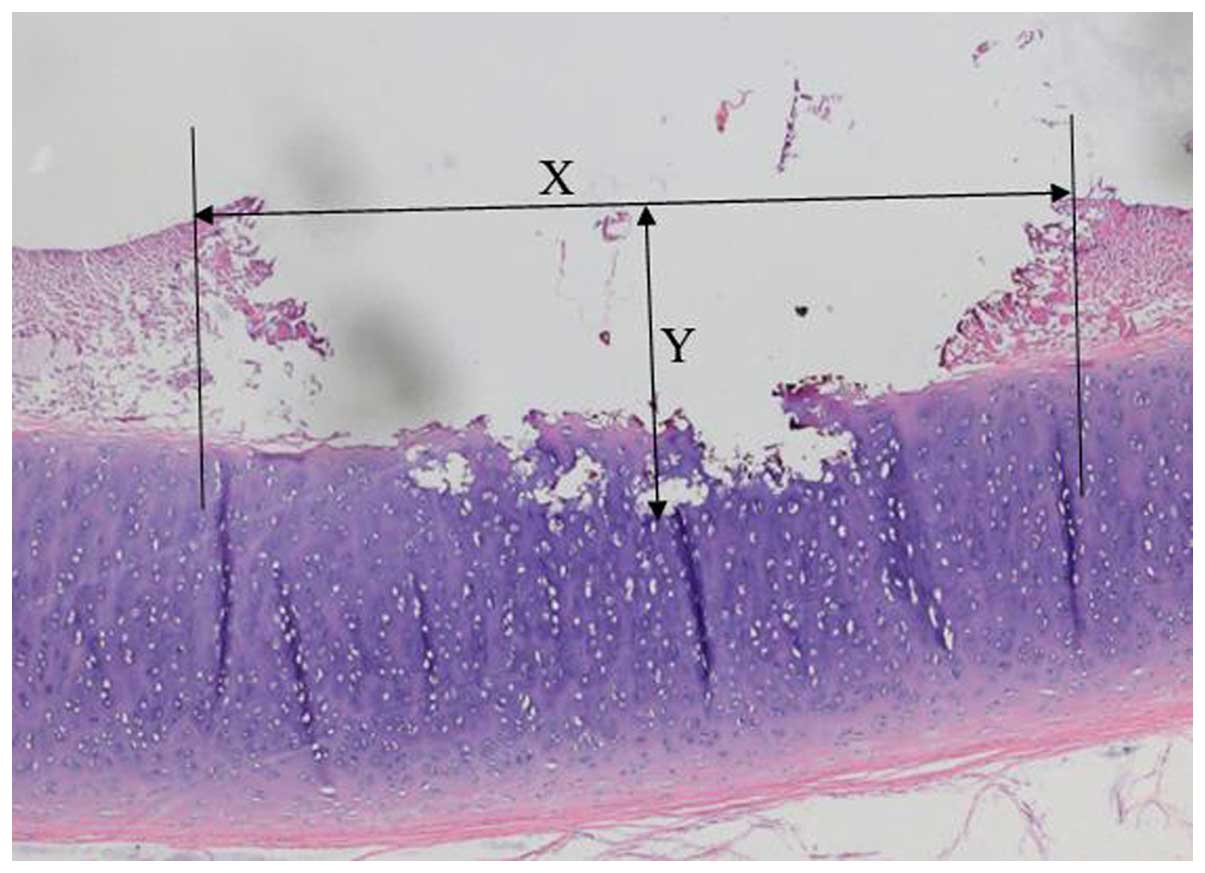
the extent of the injured area. The maximal depths and dimensions
of tissue damage were measured in the central area of the lesions
in step sections. The actual measurements were performed using a
Vernier caliper (Fig. 1).
Results
Gross appearance of the trachea
following endobronchial ablation
Gross alterations of the trachea of the dogs
following the four types of endobronchial ablations were observed
by bronchoscopy. In general, immediate alterations included
desiccation of the mucosal surface, a whitish or charred yellow
coagulation spot and hardening of the injured area. Over time, the
area of coagulation expanded to become a crater-shaped lesion. The
surrounding area and bottom of the lesion were covered with charred
eschar, sometimes with perforation.
Laser-induced tracheal injury had a sharp edge
(Fig. 2), while injuries induced by
high-frequency electrocautery or APC had no sharp boundaries
(Fig. 2). The severity of injury
positively correlated with the application time.
Cryotherapy-induced injury had markedly less obvious tissue defect
compared with the other ablation techniques. When 100 Ω impedance
was applied for 60 sec, whitish mucosa, edema and hardening of the
tissue were observed. When applied for 120 sec, these changes were
more obvious and the integrity of the mucosa was destroyed
(Fig. 2). Normal tracheal mucosa on
gross endoscopic examination showed a smooth mucosal surface,
clearly visible vessels and cartilage rings (Fig. 2).
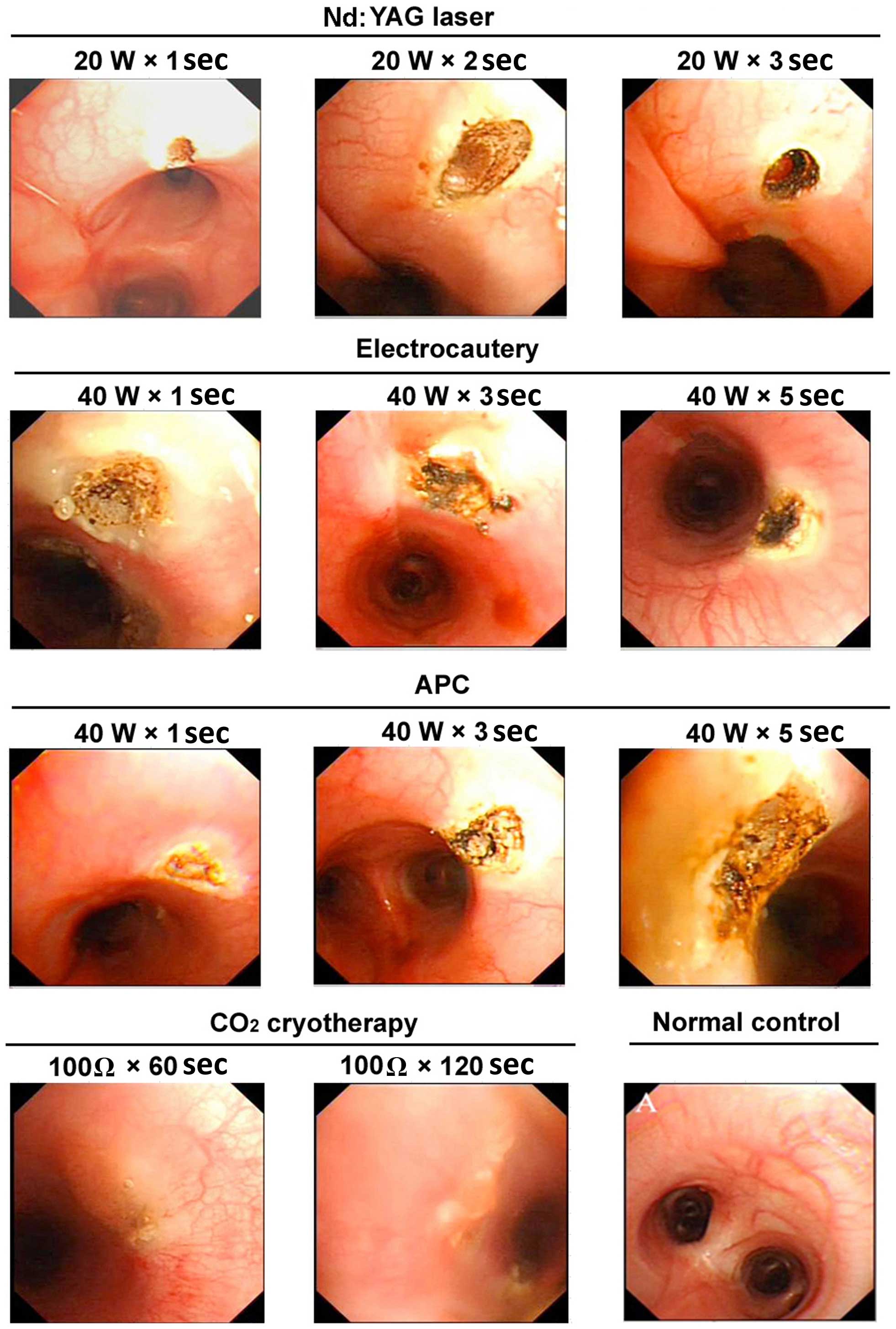 | Figure 2.Representative images of the gross
appearance of the tracheal lumen of dogs following various
endobronchial ablation therapies. Nd:YAG laser: 1 sec, mucosal
carbonation and local cavity formation; 2 sec, volcano-shaped wound
with black carbonized tissue at the bottom and surrounding areas; 3
sec, further deepening of the wound, with increased tissue
carbonization and vaporization. Electrocautery: 1 sec, tracheal
wall whitening with a slightly sunken, yellow central focus, a
small area of injury with a clear boundary and no obvious tissue
vaporization; 3 sec, increased coagulation spots around the central
region, with tissue carbonization and vaporization in the center of
the volcano-shaped injury; 5 sec, increased tissue carbonation and
vaporization, a further deepened wound, and significantly increased
black eschar. APC: 1 sec, yellow solidification spots, pale
adjacent mucosa and no obvious tissue loss; 3 sec, larger
coagulation spots, with a central depression and surface
carbonization; 5 sec, further expanded area of solidification, a
deepened central depression, and evident tissue carbonization.
Cryotherapy: 60 sec, whitened mucosa, mucosal edema and tissue
hardening; 120 sec, pale area of mucosal injury, evident mucosal
edema and loss of mucosal integrity. W, watt; Ω, ohm; Nd:YAG,
neodymium-doped yttrium aluminium garnet laser therapy; APC, argon
plasma coagulation. |
Histopathological alterations of the
trachea following endobronchial ablations
Histopathological alterations of the trachea
following all four types of endobronchial ablations in the dogs
included epithelial and submucosal necrosis and shedding, as well
as destruction and perforation of the cartilage layer (Fig. 3). The severity of injuries correlated
with the application time of ablation. Notably, the
histopathological changes were consistent with the endoscopic gross
appearances of the tracheal lumen.
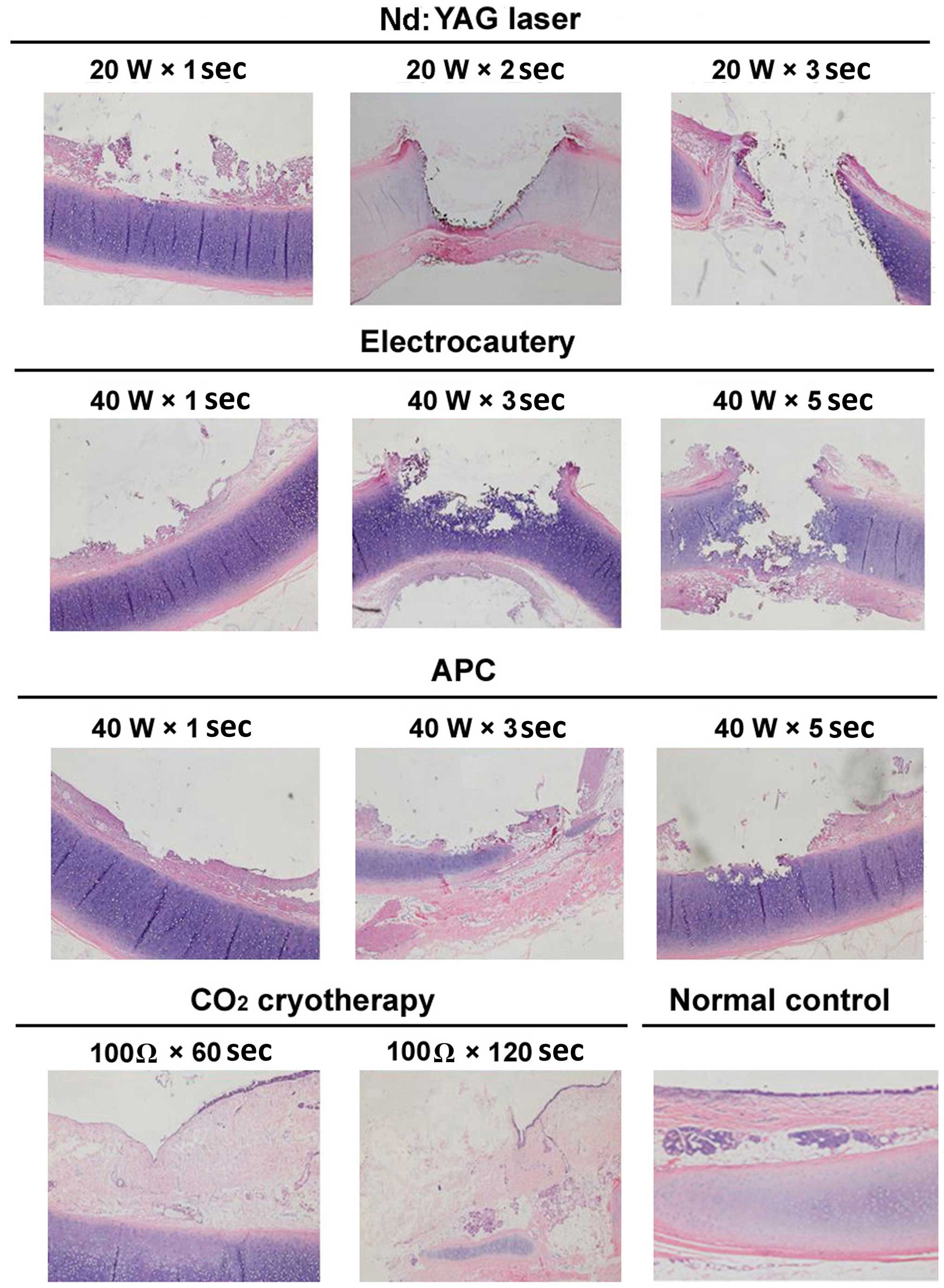 | Figure 3.Representative images of
histopathological changes of the tracheal mucosa of dogs following
various endobronchial ablation therapies (hematoxylin and eosin
staining, magnification, ×40). Nd:YAG laser: 1 sec, mucosal
necrosis and defect, with submucosal tissue necrosis at the surface
of the cartilage; 2 sec, mucosal/submucosal defect and vaporization
deeper into the cartilage, with damage affecting >2/3 of the
cartilage, and obvious surrounding tissue necrosis; 3 sec, tracheal
wall defect and perforation in the central region, with increased
necrosis of the surrounding tissue. Electrocautery: 1 sec, mucosal
epithelial necrosis, and submucosal tissue necrosis affecting the
cartilage layer; 3 sec, mucosal/submucosal necrosis deeper into the
cartilage layer, with 1/2 of the cartilage structure being
destroyed; 5 sec, increased tissue necrosis, with damage and
fracture of mucosa/submucosa and the cartilage layer, and airway
perforation. APC: 1 sec, mucosal shedding and superficial
submucosal necrosis; 3 sec, larger coagulation spots, a central
depression and surface carbonization; 5 sec. mucosal defect, with
submucosal tissue necrosis affecting the surface of the cartilage;
the cartilage layer was slightly damaged but retained its
integrity. Cryotherapy: 60 sec, epithelial cell shedding and local
thrombosis, but no obvious tissue necrosis; 120 sec, mucous layer
defect, disordered submucosal structure, and damage reaching to the
surface of the cartilage layer; the cartilage structure was intact
and local thrombosis was observed. W, watt; Ω, ohm; Nd:YAG,
neodymium-doped yttrium aluminium garnet laser therapy; APC, argon
plasma coagulation. |
To compare the safety and efficacy of the four types
of ablative techniques, the depths and dimensions of tracheal
injuries, and the severity of tissue necrosis and cartilage damage,
induced by the different methods were examined (Table I). The histopathological changes of
the tracheal wall induced by these various ablation techniques
varied according to the parametric settings and application times
(Table I; Fig. 3). The parametric settings included
the power output (W for Nd:YAG laser, APC and electrocautery) or
impedance (Ω for cryotherapy).
 | Table I.Tracheal histopathologic changes
induced by four endobronchial ablation techniques in dogs. |
Table I.
Tracheal histopathologic changes
induced by four endobronchial ablation techniques in dogs.
| Ablation type | Setting | Width (mm) | Depth (mm) | Mucosal epithelial
shedding necrosis | Stromal coagulative
necrosis | Submucosal
coagulative necrosis defect | Cartilage layer
destruction |
|---|
| Nd:YAG laser | 20 W × 1 sec | 2.0±0.1 | 1.8±0.1 | ++ | ++ | + | + |
|
| 20 W × 2 sec | 3.0±0.1 | 2.6±0.2 | ++ | ++ | ++ | ++ |
|
| 20 W × 3 sec | 4.0±0.2 | −a | +++ | +++ | ++ | +++ |
| Electrocautery | 40 W × 1 sec | 4.0±0.1 | 1.6±0.1 | ++ | ++ | + | − |
|
| 40 W × 3 sec | 5.0±0.2 | 2.7±0.1 | ++ | ++ | ++ | ++ |
|
| 40 W × 5 sec | 5.5±0.3 | 3.2±0.2 | +++ | +++ | +++ | +++ |
| APC | 40 W × 1 sec | 3.0±0.2 | 0.5±0.1 | + | + | − | − |
|
| 40 W × 3 sec | 4.5±0.2 | 1.0±0.2 | ++ | + | + | − |
|
| 40 W × 5 sec | 5.6±0.3 | 1.8±0.2 | +++ | ++ | ++ | + |
| Cryotherapy | 100 Ω × 60 sec | 2.0±0.1 | 0.9±0.1 | + | + | + | − |
|
| 100 Ω ×
120 sec | 2.5±0.2 | 1.6±0.2 | +++ | ++ | ++ | − |
When the 20-W laser was applied for 1 sec,
histopathological changes of the tracheal injury included mucosal
necrosis and submucosal coagulative necrosis, which extended deep
into the surface of the cartilage (Fig.
3). When the application time was extended to 2 sec, two-thirds
of the cartilage layer was destroyed, accompanied by apparent
coagulative necrosis in the surrounding tissue (Fig. 3). Furthermore, following application
of 20 W for 3 sec, a transmural tracheal defect was observed at the
center of the injury and coagulative necrosis in the surrounding
tissue was more severe, with perforation of the tracheal wall
(Fig. 3). The injuries induced by
high-frequency electrocautery and APC were similar to those induced
by laser therapy, and the severity of injuries also correlated with
the application time of each technique (Fig. 3).
Cryotherapy-induced injury showed no apparent
necrosis. However, mucosal and epithelial shedding and
thromboembolism formation was observed. The cartilage layer
remained intact (Fig. 3).
Histological analysis of the normal tracheal wall under an optical
microscope showed three layers of structure (from top to bottom):
mucosa, submucosa and adventitia (Fig.
3).
Parametric settings and application
times that allow equivalent efficacy of the four endobronchial
ablation techniques
To identify the settings for the four types of
endobronchial ablation techniques that achieved similar efficacies
in the dog trachea, the histopathological changes and the depths of
injuries induced by each type were compared (Table II). The following settings had
equivalent effects on the tracheal wall: 20 W for 1 sec for Nd:YAG
laser therapy; 40 W for 1 sec for electrocautery; 40 W for 5 sec
for APC; and 100 Ω for 120 sec for CO2 cryotherapy. The
histological characteristics of tracheal injuries under these
settings included mucosal necrosis and shedding, submucosal
coagulative necrosis and an intact cartilage layer. The depth of
injury was <2 mm.
 | Table II.Efficacy-equivalent settings of four
endobronchial ablation techniques applied to the trachea of
dogs. |
Table II.
Efficacy-equivalent settings of four
endobronchial ablation techniques applied to the trachea of
dogs.
| Ablation type | Setting | Onset of
effects | Depth of lesion
(mm) |
|---|
| Nd:YAG laser | 20 W × 1 sec | Immediate | 1.8±0.1 |
| Electrocautery | 40 W × 1 sec | Immediate | 1.6±0.1 |
| APC | 40 W × 5 sec | Immediate | 1.8±0.2 |
| CO2
cryotherapy | 100 Ω ×
120 sec | Delayed | 1.6±0.2 |
Tissue repair and healing following
endobronchial interventions
Changes of tracheal injuries induced by the four
endobronchial ablation techniques over time were similar, and could
be considered to occur in four phases, including acute injury,
inflammation, repair and healing (Fig.
4). The acute phase consisted of pathological changes
characterized by desiccation of the mucosal surface, a whitish or
charred yellow coagulation spot and hardening of the injured areas
(Fig. 3).
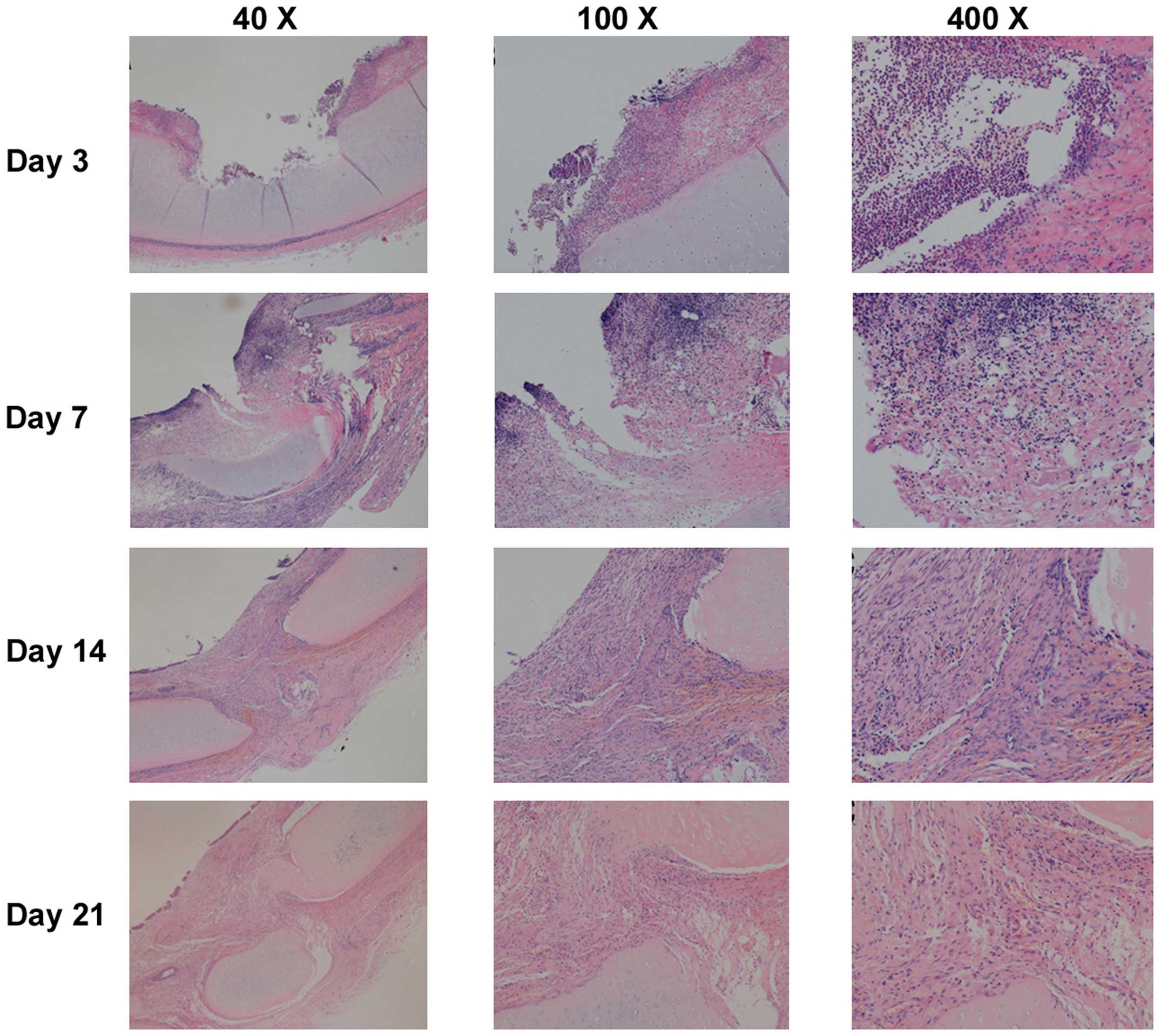 | Figure 4.Representative images of histological
changes of the tracheal mucosa on days 3, 7, 14 and 21 following
endobronchial ablation in dogs (hematoxylin and eosin staining).
The tracheal wall repair process was similar following the four
different ablation techniques, with four stages: Acute injury,
inflammation, repair and healing. On day 3 (inflammatory stage),
tissue defects were present with a small amount of necrotic tissue,
mucosal hyperemia and edema, and inflammatory cell infiltration. On
day 7 (tissue repair stage), mucosal hyperemia and edema around the
injury were decreased and the defect area was filled with
hyperplastic tissue. Pseudostratified columnar epithelium
hyperplastic tissue incompletely covered the damaged region, and
submucosal granulation tissue was rich with new capillaries. Tissue
defects were filled with granulation tissue, and inflammatory cells
infiltrated the granulation tissue. On days 14 and 21 (healing
stage), the mucosa in the injured area was similar to normal mucosa
without obvious hyperemia and edema. Pseudostratified columnar
epithelium grew well, with fibrous tissue hyperplasia, and
submucosal glands decreased significantly compared with normal
tissue. Inflammatory cell content gradually reduced, and the
destroyed submucosa and cartilage were repaired with fibrous
tissue, which maintained the integrity of the tracheal wall. |
The inflammatory phase of injury was evident on day
3 following the procedure, and was characterized by hyperemia and
edema of the mucosa surrounding the injury, with the presence of
tissue defect and necrotic debris. Furthermore, a large number of
inflammatory cells were observed to have infiltrated into the
injured areas (Fig. 4).
The repair phase was initiated on day 7 following
the ablation treatments (Fig. 4).
The hyperemia and edema of the mucosa surrounding the lesion
gradually subsided. In addition, the tissue defect was replaced by
the proliferation of a pseudostratified, columnar epithelium in the
mucosa, granulation tissue with the infiltration of inflammatory
cells and newly forming capillaries in the submucosa. The gap
caused by tissue disruption was filled with granulation tissue.
The healing phase of injuries could be seen 2–3
weeks postoperatively (Fig. 4). The
gross appearance of the lesion was similar to that of normal
tissue, with no hyperemia and edema. Histopathological analyses
showed a well-developed, pseudostratified columnar epithelium and
proliferation of fibrous tissue, with fewer inflammatory cells in
the submucosa. Fibrous tissue had replaced the damaged submucosa
and cartilage to maintain the integrity of the tracheal wall.
Discussion
The present study evaluated the safety and efficacy
of four commonly used endobronchial ablation techniques, which were
applied via fiberoptic bronchoscopy to beagle dogs. The ablation
techniques included Nd:YAG laser therapy, high-frequency
electrocautery, APC and CO2 cryotherapy. The results of
the study indicated that the biological effects on the trachea
induced by each technique differed according to parametric settings
(power or impedance) and application times. However, it was
possible to achieve comparable efficacy and safety when these tools
were applied at optimal settings and application times, which were
specific to each technique. Furthermore, the repair and healing
processes in the damaged trachea following these interventions were
observed and described.
Ablation may be categorized as hot or cold therapy.
The hot therapies, which include Nd:YAG laser therapy,
high-frequency electrocautery and APC, produce and apply thermal
energy to a tissue, causing immediate coagulative necrosis and
tissue vaporization (21,22). Conversely, the effect of cold
therapies, including cryotherapy, is relatively delayed, with
tissue necrosis appearing 2–3 days following the procedure
(23–26). In the present study, the most common
histopathological changes of the trachea wall among the various
endobronchial ablation techniques were tissue coagulative necrosis
and vaporization. However, the extent of tissue damage varied
depending on the settings for power output (Nd:YAGlaser, APC and
electrocautery) or impedance (cryotherapy), and application
time.
Nd:YAG laser therapy was the most efficient ablation
technique and resulted in immediate tissue coagulation,
penetration, vaporization and transmural tracheal damage during
treatment. The effect of high-frequency electrocautery was similar
to Nd:YAG laser therapy. Electrocautery applied at 40 W for 3 sec
caused tissue vaporization that extended into half of the cartilage
layer; after 5 sec of application, the transmural tracheal wall was
destroyed. APC induced more superficial coagulative necrosis and
less vaporization compared with Nd:YAG laser therapy and
electrocautery; when applied at 40 W for 5 sec, the cartilage layer
was superficially damaged, but most of the deep structures in the
cartilage remained intact. Although cryotherapy was superior in
terms of safety and toleration by the bronchial wall, its effect
was delayed. Cryotherapy applied at 100 Ω for either 60 or 120 sec
did not affect the cartilage layer. These features were consistent
with the results of previous studies (5,6,23,24,26–31).
Based on our previous study (20) and clinical experience, the current
study chose to assess Nd:YAG laser therapy at 20 W, electrocautery
and APC at 40 W, and cryotherapy at 100 Ω. The results indicated
that, at any power (or impedance) held constant, tracheal injury
was exacerbated with application time. Mucosal coagulative necrosis
can infiltrate into the submucosa and subsequently extend into the
cartilage layer, resulting in airway perforation. This suggests
that ablation may achieve the desired level of tissue damage if the
duration of application is adjusted, which is useful, especially
when treating carcinomas in situ, to reduce the risk of
airway perforation. The results of the present study indicated that
the ablation techniques can be applied safely when the following
parameters are used: Nd:YAG laser therapy, 20 W for ≤1 sec;
electrocautery, 40 W for ≤3 sec; APC, 40 W for ≤5 sec; and
cryotherapy, 100 Ω for ≤120 sec.
Nd:YAG laser therapy is the most commonly used
endobronchial ablation technique because of the excellent
coherence, monochromaticity and collimation of the laser (5,28).
However, Nd:YAG laser therapy should be performed with caution in
clinical practice. To minimize the risk of airway perforation, the
laser beam must always be parallel to the wall of the airway and
not perpendicular to it, and the minimum effective power and
shortest duration of application should be selected. Lai et
al (27) found that the serum
levels of interleukin-2 and natural killer cell activity were
increased following Nd:YAG laser therapy, suggesting that laser
therapy may enhance immunity, in addition to its thermal effect.
Long-term studies of patients with late-stage lung cancer have
shown that Nd:YAG laser therapy is able to effectively relieve
symptoms, improve the quality of life and prolong survival
(28,32).
As a thermal ablation technique, high-frequency
electrocautery transforms electrical energy into thermal energy to
remove lesions. It generally results in coagulative necrosis at low
temperatures, and tissue vaporization and carbonization at high
temperatures, with the focus on damaged tissue at the surface of
contact (29,31). In the present study, the extent of
tracheal damage caused by electrocautery applied at 40 W was
positively associated with the duration of application. The
biological effects of electrocautery on tissue are dependent on
various factors, including the nature of lesions, the current
waveform, the power output, the duration of contact, the mode of
application and the type of probe (29,31,33).
Local blood flow and mucosal secretion are also thought to
influence the efficacy of electrocautery (34). In the present study, the influence of
the local mucosa was excluded, since normal trachea was selected as
the target tissue and mucosal secretion could be easily and rapidly
removed during the procedure; Our results indicated that the extent
of tissue damage was primarily associated with the duration of
ablation. Considering the risk of airway perforation as a
complication, we recommend that electrocautery be applied with a
power setting of 40 W for <3 sec to ensure safety.
In the present study, it was observed that tissue
penetration was limited using APC and, thus, the risk of airway
perforation associated with this technique was less compared with
the other techniques. The advantages of APC include the ability to
reach lesions located lateral to the probe or around bends and
corners that are not suitable for Nd:YAG laser therapy and
electrocautery, as well as adequate hemostasis of the lesion
(35–37). The complications associated with APC
are similar to electrocautery, although a case of fatal air
embolism has previously been reported (38). APC is not the best choice for removal
of a bulky lesion, since it is less precise compared with other
ablation techniques (30).
Cryotherapy causes cellular injury and death by
exposing biological tissues to cycles of freezing and thawing.
Multiple factors can influence the efficacy of cryotherapy,
including the speed of freeze-thawing and the lowest temperature
that the cryoprobe can achieve to destroy live tissue. Cryotherapy
does not affect cartilage because of its low water content
(39). Cryotherapy is used in the
treatment of endobronchial tumors. Its advantages include a lower
cost, fewer precautions and superior safety. Furthermore, it is
less likely to cause complications such as perforation, malacia and
cicatricial stricture (25).
However, cryotherapy cannot be used to achieve immediate airway
patency in patients with severe airway stenosis, owing to its
delayed effect (40). In a previous
study, the application of cryotherapy was extended by a technique
that allowed immediate recanalization of obstructed airways
affected by the extraction of large pieces of tumors (38). This involved using a probe that was
able to rapidly freeze tissue and remove the entire tissue around
the probe before the frozen tissue thawed. However, airway bleeding
with the potential for massive hemorrhage is an important
consideration, such that the safety of this new technique requires
further investigation.
The present study demonstrated that the use of these
ablation techniques at specific settings could cause similar
biological effects. Nd:YAG laser therapy at 20 W for 1 sec,
electrocautery at 40 W for 3 sec, APC at 40 W for 5 sec and
cryotherapy at 100 Ω for 120 sec resulted in identical pathological
changes in the tracheal wall. These alterations included shedding
necrotic mucosa, partial tracheal defects, submucosal coagulative
necrosis and destruction of the superficial cartilage layer, with
similar infiltration depths of tissue damage. Therefore, we propose
that these specific settings are efficacy-equivalent values for
these ablation techniques. This is important information for a
number of institutes where only one or two endobronchial ablation
modalities are available; most endobronchial therapies can be
performed to achieve the desired results, even if the available
equipment is limited.
It is important to note that fibrous scarring tissue
is retractile and, once it loses cartilaginous support, iatrogenic
secondary stenosis can develop (30). Verkindre et al (30) found that, after setting
electrocautery to deliver 40 or 120 W, coagulative necrosis and
intense inflammation of the mucosa of early lesions extended deep
into the cartilage layer, followed by formation of transmural
fibrosis and the destruction of cartilage, which resulted in
iatrogenic secondary stenosis. By mentioning this possibility, the
authors do not question the clinical application of ablation
techniques, but only want to alert physicians to the potential for
iatrogenic secondary stenosis caused by extensive damage to the
tracheal wall.
In conclusion, the present study demonstrated that
the biological effects of various endobronchial ablation techniques
on the trachea were a function of the power or impedance settings
and application period, and that these techniques may be equally
efficacious when applied using settings and application durations
specific to each technique. Specifically, this study determined
that the safe parametric values for endobronchial ablation with
these techniques were 20 W for ≤1 sec for Nd:YAG laser therapy, 40
W for ≤3 sec for high-frequency electrocautery, 40 W at ≤5 sec for
APC and 100 Ω for ≤120 sec for CO2 cryotherapy. The
results of the present study may serve as a reference for the
clinical application of endobronchial ablation techniques.
Acknowledgements
The authors would like to thank Medjaden Bioscience
for assisting in the preparation of this manuscript. This work was
supported by Science and Technology Commission of Shanghai
Municipality (grant no. 134119a0302).
References
|
1
|
Bhamra-Ariza P, Keogh AM and Muller DW:
Percutaneous interventional therapies for the treatment of patients
with severe pulmonary hypertension. J Am Coll Cardiol. 63:611–618.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersen PE and Kjeldsen AD:
Interventional treatment of pulmonary arteriovenous malformations.
World J Radiol. 2:339–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keogh AM, Mayer E, Benza RL, Corris P,
Dartevelle PG, Frost AE, Kim NH, Lang IM, Pepke-Zaba J and Sandoval
J: Interventional and surgical modalities of treatment in pulmonary
hypertension. J Am Coll Cardiol. 54:(Suppl 1). S67–S77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gudausky TM and Beekman RH III: Current
options and long-term results for interventional treatment of
pulmonary valvar stenosis. Cardiol Young. 16:418–427. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantovani G, Astara G, Manca G, Versace R,
Contu P and Carai A: Endoscopic laser ablation as palliative
treatment of endobronchial, nonresectable, or recurrent lung
cancer: Assessment of its impact on quality of life. Clin Lung
Cancer. 1:277–285; discussion 286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taber SW, Buschemeyer WC III, Fingar VH
and Wieman TJ: The treatment of malignant endobronchial obstruction
with laser ablation. Surgery. 126:730–733; discussion 733–735.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chua AP and Mehta AC: Barotrauma from
novel endobronchial ablation techniques. J Bronchology Interv
Pulmonol. 16:75–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manali ED, Stathopoulos GT, Gildea TR,
Fleming P, Thornton J, Xu M, Papiris SA, Mehta AC and Mughal MM:
High dose-rate endobronchial radiotherapy for proximal airway
obstruction due to lung cancer: 8-year experience of a referral
center. Cancer Biother Radiopharm. 25:207–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Wang WJ and Wang HF: Therapeutic
effect of tracheal anastomosis versus interventional bronchoscopy
in the treatment of airway stenosis. Nan Fang Yi Ke Da Xue Xue Bao.
30:1359–1362. 2010.(In Chinese). PubMed/NCBI
|
|
10
|
Li Y, Yao XP, Bai C, Huang Y, Wang Q, Zhao
LJ, Dong YC, Teng HY and Li Q: Therapeutic efficacy analysis of
bronchoscopic interventional therapy on severe tuberculous main
bronchial stenosis complicated with unilateral atelectasis.
Zhonghua Jie He He Hu Xi Za Zhi. 34:454–458. 2011.(In Chinese).
PubMed/NCBI
|
|
11
|
Zhang J, Wang J, Wang T, Xu M, Dang BW,
Pei YH and Zhang CY: A pilot study on interventional bronchoscopy
in the management of airway stenosis with benign hyperplasia.
Zhonghua Jie He He Hu Xi Za Zhi. 34:334–338. 2011.(In Chinese).
PubMed/NCBI
|
|
12
|
Ost DE, Ernst A, Grosu HB, Lei X,
Diaz-Mendoza J, Slade M, Gildea TR, Machuzak M, Jimenez CA, Toth J,
et al: Therapeutic bronchoscopy for malignant central airway
obstruction: success rates and impact on dyspnea and quality of
life. Chest. 147:1282–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saji H, Furukawa K, Tsutsui H, Tsuboi M,
Ichinose S, Usuda J, Ohira T and Ikeda N: Outcomes of airway
stenting for advanced lung cancer with central airway obstruction.
Interact Cardiovasc Thorac Surg. 11:425–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beamis JF Jr: Interventional pulmonology
techniques for treating malignant large airway obstruction: An
update. Curr Opin Pulm Med. 11:292–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones GS and Baldwin DR: Lung cancer
screening and management. Minerva Med. 106:339–354. 2015.PubMed/NCBI
|
|
16
|
Colt HG and Murgu SD: Interventional
bronchoscopy from bench to bedside: New techniques for early lung
cancer detection. Clin Chest Med. 31:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasic A, Brokx HAP, Noordegraaf AV, Paul
RMA, Postmus PE and Sutedja TG: Cost-effectiveness of early
intervention: Comparison between intraluminal bronchoscopic
treatment and surgical resection for T1N0 lung cancer patients.
Respiration. 71:391–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morrison SA, Hill SL, Rogers GS and Graham
RA: Efficacy and safety of continuous low-irradiance photodynamic
therapy in the treatment of chest wall progression of breast
cancer. J Surg Res. 192:235–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simone CB II, Friedberg JS, Glatstein E,
Stevenson JP, Sterman DH, Hahn SM and Cengel KA: Photodynamic
therapy for the treatment of non-small cell lung cancer. J Thorac
Dis. 4:63–75. 2012.PubMed/NCBI
|
|
20
|
Bai C, Dong YC, Song XL, Huang Y, Shi H,
Hu ZL and Li Q: In vitro study of safety and co-efficiency of the
transbronchial coagulation techniques. Chin Med J (Engl).
126:124–128. 2013.PubMed/NCBI
|
|
21
|
Zhikai Z, Lizhi N, Liang Z, Jianying Z,
Fei Y, Jibing C, Jialiang L and Kecheng X: Treatment of central
type lung cancer by combined cryotherapy: Experiences of 47
patients. Cryobiology. 67:225–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schumann C, Hetzel M, Babiak AJ, Hetzel J,
Merk T, Wibmer T, Lepper PM and Krüger S: Endobronchial tumor
debulking with a flexible cryoprobe for immediate treatment of
malignant stenosis. J Thorac Cardiovasc Surg. 139:997–1000. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rojas-Tula DG, Gómez-Fernández M,
García-López JJ, Cobos-Ceballos MJ, Gil-Fuentes A, Pérez-Laya JM,
Serrano-Rebollo JC, Ortega-González A, Vargas-Hidalgo T, de
Oña-Lacasta JM Ruíz and Celdrán-Gil J: Endobronchial cryotherapy
for a mycetoma. J Bronchology Interv Pulmonol. 20:330–332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mu D, Nan D, Li W, Fu E, Xie Y, Liu T and
Jin F: Efficacy and safety of bronchoscopic cryotherapy for
granular endobronchial tuberculosis. Respiration. 82:268–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Choi WJ, Sung SW, Kim YK, Kim CH,
Zo JI and Park KJ: Endoscopic cryotherapy of lung and bronchial
tumors: A systematic review. Korean J Intern Med. 26:137–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fitzmaurice GJ, Redmond KC, Fitzpatrick DA
and Bartosik W: Endobronchial cryotherapy facilitates end-stage
treatment options in patients with bronchial stenosis: A case
series. Ann Thorac Med. 9:120–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai JP, Tao ZD, Xiao JY, Chen XH, Zhao SP,
Tian YQ and Betz CS: Microinvasive Nd:YAG laser therapy of early
glottic carcinoma and its effect on soluble interleukin-2 receptor,
interleukin-2 and natural killer cells. Laryngoscope.
111:1585–1588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hermes A, Heigener D, Gatzemeier U, Schatz
J and Reck M: Efficacy and safety of bronchoscopic laser therapy in
patients with tracheal and bronchial obstruction: A retrospective
single institution report. Clin Respir J. 6:67–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wahidi MM, Unroe MA, Adlakha N, Beyea M
and Shofer SL: The use of electrocautery as the primary ablation
modality for malignant and benign airway obstruction. J Thorac
Oncol. 6:1516–1520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verkindre C, Brichet A, Maurage CA, Ramon
P, Homasson JP and Marquette CH: Morphological changes induced by
extensive endobronchial electrocautery. Eur Respir J. 14:796–799.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sindhwani G, Rawat J and Keserwani V: Role
of endobronchial electrocautery in management of neoplastic central
airway obstruction: Initial experience with seven cases. Indian J
Chest Dis Allied Sci. 54:165–168. 2012.PubMed/NCBI
|
|
32
|
Rolle A, Pereszlenyi A, Koch R, Richard M
and Baier B: Is surgery for multiple lung metastases reasonable? A
total of 328 consecutive patients with multiple-laser
metastasectomies with a new 1318-nm Nd:YAG laser. J Thoracic
Cardiovasc Surg. 131:1236–1242. 2006. View Article : Google Scholar
|
|
33
|
Seaman JC and Musani AI: Endobronchial
ablative therapies. Clin Chest Med. 34:417–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nikfarjam M, Muralidharan V,
Malcontenti-Wilson C, McLaren W and Christophi C: Impact of blood
flow occlusion on liver necrosis following thermal ablation. ANZ J
Surg. 76:84–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reddy C, Majid A, Michaud G, Feller-Kopman
D, Eberhardt R, Herth F and Ernst A: Gas embolism following
bronchoscopic argon plasma coagulation: A case series. Chest.
134:1066–1069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin F, Mu D, Xie Y, Fu E and Guo Y:
Application of bronchoscopic argon plasma coagulation in the
treatment of tumorous endobronchial tuberculosis: Historical
controlled trial. J Thorac Cardiovasc Surg. 145:1650–1653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bolliger CT, Sutedja TG, Strausz J and
Freitag L: Therapeutic bronchoscopy with immediate effect: Laser,
electrocautery, argon plasma coagulation and stents. Eur Respir J.
27:1258–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boujaoude Z, Young D, Lotano R and
Abouzgheib W: Cryosurgery for the immediate treatment of acute
central airway obstruction. J Bronchology Interv Pulmonol.
20:45–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu CH, Lin HP, Cheng SJ, Sun A and Chen
HM: Cryotherapy for oral precancers and cancers. J Formos Med
Assoc. 113:272–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vergnon JM, Huber RM and Moghissi K: Place
of cryotherapy, brachytherapy and photodynamic therapy in
therapeutic bronchoscopy of lung cancers. Eur Respir J. 28:200–218.
2006. View Article : Google Scholar : PubMed/NCBI
|