Introduction
Oxidative stress (OS) is an important contributor to
the damage induced by acute ischemic stroke and is a potent
etiologic factor for chronic neurodegenerative disorders including
Alzheimer's disease (AD), Parkinson's disease (PD), multiple
sclerosis and amyotrophic lateral sclerosis (1,2). The
implication of OS in neurological diseases is associated with its
effect on neurocytes; the structural and functional characteristics
present in neurocytes include easily peroxidizable unsaturated
fatty acids, high oxygen consumption rate and relative paucity of
antioxidant enzymes, resulting in the vulnerability of neurocytes
to OS (2). Furthermore, the
incidence of OS and the opportunity of neurocytes exposed to OS are
increased by exposure to environmental toxins (3), deficient antioxidants present in the
diet source (4) and pathological
conditions such as diabetes (5). An
increase in OS is caused by excessive production of oxidative
radical species (ROS), leading to the endogenous antioxidant
defenses being overwhelmed (6). ROS
exert a strong oxidative property and may result in cell membrane
injury, mitochondrial dysfunction, protein misfolding and DNA
destruction, finally leading neurocyte apoptosis (7).
OS-induced neurocyte apoptosis is a primary
contributor to neurological disease with OS in the
reoxygenation/reperfusion setting of stroke constituting a major
driving force for neurocyte apoptosis (8–10). The
immediate supplement of exogenous antioxidants has been
demonstrated to decrease neuronal mortality and ease the burden of
stroke in vivo and in vitro (8–10). By
contrast, OS derived from microglial activation in PD induces
neurocyte apoptosis and consequently assists the degeneration of
dopaminergic neurons that is a marked pathological feature of PD
(11,12). In AD, the incidence of neuronal
apoptosis is notably elevated, which is associated with the
induction of OS production through the disruption of ceramide
metabolism and accumulation of amyloid β-peptides (13). Neurocytes are unable to be
regenerated following apoptosis and the deficit of neurocytes may
trigger typical characteristics of neurological diseases including
memory loss, cognitive impairment and motor disturbance. Therefore,
counteracting the detrimental effects of ROS, especially
ROS-induced neuronal apoptosis, is an important approach in
treating neurological diseases (10–13).
A number of botanical extracts, specifically those
rich in flavonoids, confer neuroprotective effects via the
inhibition of OS generation and inhibiting OS-mediated deleterious
actions (14). Lin et al
(15) demonstrated that the
Wedelia chinensis extract effectively alleviates tertbutyl
hydroperoxide-induced oxidative damage in neuronal PC12 cells and
D-galactose-induced lipid peroxidation in the cerebral cortex of
mice. Ratanasampil contains numerous Chinese herbs that have
been demonstrated to have neuroprotective effects on OS-induced
neuronal SH-SY5Y cell damage (16).
In the present study, Naphthoflavone, a synthetic derivative of
naturally occurring flavonoids, observed in Passiflora incarnata
Linn (17), was assessed.
Naphthoflavone has the potential to be a readily available and
cheap therapeutic agent, if validated to be effective against
neurological diseases. Naphthoflavone only contains two structural
isomers, α-Naphthoflavone and β-Naphthoflavone, which is convenient
for the investigation of their biological functions. By contrast,
many botanical extracts, despite their neuroprotective effects have
been identified to contain a number of different types of bioactive
components (18). Therefore, the
specific components of botanical extracts that are neuroprotective
and their underlying mechanisms are typically poorly understood,
resulting in restricted application of many botanical extracts in
clinical settings. It has been demonstrated that Naphthoflavone
upregulates the expression or activity of antioxidant-associated
enzymes, including glutathione peroxidase (GPx), quinone
oxidoreductase-1, glutathione transferase and heme oxygenase-1
(19–22), and represses ROS-producing enzymes
such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
(23). This suggests a potential
effect exerted by Naphthoflavone in antioxidation. Currently,
limited studies have assessed the role of Naphthoflavone in neurons
that suffer from OS. Therefore, the present study aimed to
administer α- and β-Naphthoflavone individually and combined to
determine their capacity to counteract the detrimental effects of
OS on neurons in vitro.
Materials and methods
Agents and cell culture
α- and β-Naphthoflavone were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The human neuron
SH-SY5Y cells obtained from the American Type Culture Collection
(Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium (DMEM; Hyclone, Logan, UT, USA) containing 10% fetal bovine
serum (FBS; Hyclone) and 100 U/ml streptomycin and penicillin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in a 5%
CO2 incubator at 37°C.
Cell viability assay
The cell viability was evaluated using a cell
counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) using the following method. SH-SY5Y cells were
seeded in 96-well plates at 1×104 cells/well and
cultured in DMEM without FBS at 37°C for 48 h. The cells were
subjected to H2O2 at concentration of 0–320
µM for 24 h to evaluate the impairment. In an independent
experiment, the cells underwent 20 µM H2O2
for 24 h followed by treatments with α- and β-Naphthoflavone, alone
or in combination. SH-SY5Y cells that were subjected to 20 µM
H2O2 for 24 h were subsequently treated with
0.5 µM SB203580 (Selleck Chemicals, Houston, Texas, USA), a
p38MAPK-specific inhibitor, for 24 h. Finally, 10 µl CCK-8 solution
was added to each well followed by an additional 5–8 h of
incubation at 37°C. The optical density was then measured using a
microplate reader (ELx800; BioTek Instruments, Inc., Winooski, VT,
USA) at a wavelength of 450 nm.
Apoptosis rate analysis
The apoptosis rate was evaluated using the
Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit
I (cat. no. 550475; BD Biosciences, San Jose, CA, USA). A total of
5 µl Annexin V-FITC and 5 µl propidium iodide were added to SH-SY5Y
cells (~2×105) that were treated with Naphthoflavone and
incubated at room temperature for 15 min in the dark. The extent of
apoptosis was analyzed with a dual laser FACSCalibur flow cytometer
(BD Biosciences) and estimated using the ModFit LT™ software
version 2.0 (Verity Software House, Topsham, ME, USA).
Total antioxidant capacity (TAC) and
malondialdehyde (MDA) measurement
The supernatant of the cells was lysed using a
radioimmunoprecipitation assay (RIPA) buffer (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) in order to measure the TAC
using the azino-diethyl-benzthiazoline sulfate (ABTS) method
(24). Total antioxidant capacity
detection kit was purchased from Nanjing KeyGen Biotech Co., Ltd.
Incubation of ABTS with H2O2 and a peroxidase
(metmyoglobin) produces a blue-green radical cation,
ABTS+. Antioxidants in the sample suppress this color
production proportionally to their concentration. Trolox (Nanjing
KeyGen Biotech Co., Ltd.), a water-soluble vitamin E analogue was
used to standardize the system. The results were expressed as µmol
Trolox equivalent/protein concentration of plasma supernatant of
lysed cells. In the MDA assay, 2 ml 0.6% thiobarbituric acid
(Nanjing KeyGen Biotech Co., Ltd.) was added to 2 ml supernatant in
a 10 ml tube. This tube was incubated in boiling water for 15 min
and placed on ice to cool down prior to the optical density being
measured at 532 nm via a microplate reader (ELx800; BioTek
Instruments, Inc.). The results were expressed in nmol MDA/g
protein.
Antioxidant enzyme activity assay
Catalase (CAT), superoxide dismutase (SOD) and GPx
activity detection kits were all obtained from Beyotime Institute
of Biotechnology (Haimen, China). In regard to CAT, cell
homogenates were placed in a cuvette that had contained 250 mM
hydrogen peroxide (H2O2) for 1–5 min. The
remaining hydrogen peroxide was coupled with a chromogenic
substrate (Beyotime Institute of Biotechnology) to generate a red
product,
N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinonemonoimine, which
has a maximal absorption at 520 nm. Testing the remaining hydrogen
peroxide enabled the quantity of H2O2 that
reacted with CAT to be calculated and in turn determine the CAT
activity. In the present study, SOD was present in the samples;
this inhibited the process of superoxide transforming WST-8, a
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
monosodium salt (Nanjing KeyGen Biotech Co., Ltd.), to a stable
water-soluble WST-8 formazan. The latter may be evaluated by
testing the optical density at 450 nm in order to determine SOD
activity. The determination of GPx activity was based on the
principle that NADPH continually diminishes in the cycle of GPx
(6), transforming reduced
glutathione to oxidized glutathione, which is reversed by
glutathione reductase. Detecting reduced NADPH in absorbance at 340
nm may indirectly estimate the activity of GPx.
Western blot analysis
The cells were washed in PBS and lysed with RIPA
buffer (50 mM Tris-buffer, 170 µg/ml leupeptin, 150 mM sodium
chloride, 5 mM EDTA, 1 mM sodium orthovanadate, 1% NP-40, 81 µg/ml
aprotinin, 0.5% deoxycholic acid, and 100 µg/ml
phenylmethylsulfonyl fluoride, all these agents were purchased from
Sigma-Aldrich; Merck Millipore, containing phosphatase inhibitors
(okadaic acid; cat. no. ab120375; Abcam, Cambridge, UK). Protein
concentration was measured using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). A 10% SDS-PAGE gel
loaded with ~20 µg protein from the cell lysis buffer was used to
separate the proteins; these were then transferred onto
polyvinylidene fluoride membranes (Merck Millipore) that were
initially soaked in absolute methanol. The membranes were blocked
with 5% non-fat milk in Tris-buffered saline containing Tween for 1
h. Proteins were probed with the following primary antibodies:
anti-p38 mitogen-activated protein kinase (p38MAPK; 1:1,000,
ab27986), anti-p38MAPK (phospho T180 + Y182; 1:800, ab195049),
anti-cytochrome c (Cyt C; 1:200, ab53056), anti-caspase-3
(1:500, ab47131) purchased from Abcam. Anti-p38MAPK (phospho T322;
1:800, AP50174; Abgent, Inc., San Diego, CA, USA), anti-Bax (1:300,
sc-493; Santa Cruz Biotechnology, Inc., La Jolla, CA, USA), and
anti-GAPDH (1:5,000, ab16884; Abcam) at 37°C for 2 h. Following
three washes, the membranes were incubated with anti-rabbit
IgG-horseradish peroxidase-conjugated secondary antibodies
(1:20,000; cat. no. 611-903-002, Rockland Immunochemicals, Inc.,
Plottstown, PA, USA) at room temperature for 1 h. The blots on the
membranes were scanned to quantify the optical density (Odyssey
Image-forming System; LI-COR Biotechnology, Lincoln, NE, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean following three independent experiments. Data from these
experiments were analyzed using SPSS software, version 12.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance with post hoc
testing was used for multiple comparisons between each group.
Significant differences were determined as P<0.05.
Results
Naphthoflavone attenuates
H2O2-induced cell viability reduction and
apoptosis elevation
H2O2 is recognized as a
powerful oxidant and is commonly used to establish the OS model in
a number of cell types. In the present study, the alteration of
SH-SY5Y cell viability following cell exposure to
H2O2 at concentration from 0–320 µM for 24 h
was investigated. The cell viability was decreased by
H2O2 in a dose-dependent manner, with the
half cell viability loss at 20 µM H2O2
(Fig. 1A). Following the exposure to
20 µM H2O2 for 24 h,
H2O2 was removed and cells were cultivated
for an additional 24 h in the presence or absence of
Naphthoflavone. The dose-response curves for the
H2O2-pretreated cells cultivated with
different doses of α- and β-Naphthoflavone are presented in
Fig. 1B and C, respectively. The
H2O2-inhibited cell viability increased in a
dose-dependent manner by α-Naphthoflavone prior to the cell
viability reaching a plateau at the dose of 20 µM α-Naphthoflavone
(Fig. 1C). By contrast, the cell
viability was increased with β-Naphthoflavone concentration raised
from 0–10 µM, followed by a slight drop (Fig. 1C). Therefore, 20 µM α-Naphthoflavone
and 10 µM β-Naphthoflavone, caused the maximal effect on cell
viability recovery and were used individually and in combination in
subsequent experiments of the present study to counteract 20 µM
H2O2-induced detrimental effects. Results
presented in Fig. 1D indicate that
20 µM H2O2 administered to the positive
control (PC) cells induced a significant reduction of the cell
viability compared to negative control (NC) cells without any
treatment (P<0.05). This reduced cell viability was
significantly increased by 10 µM β-Naphthoflavone (P<0.05 vs.
PC), but not 20 µM α-Naphthoflavone (P=0.064; Fig. 1D). Furthermore, a significant
increase in cell viability was observed following co-treatment with
20 µM α-Naphthoflavone and 10 µM β-Naphthoflavone compared with PC
group (P<0.05). The co-treatment recovered the cell viability to
a level close to that of the NC group.
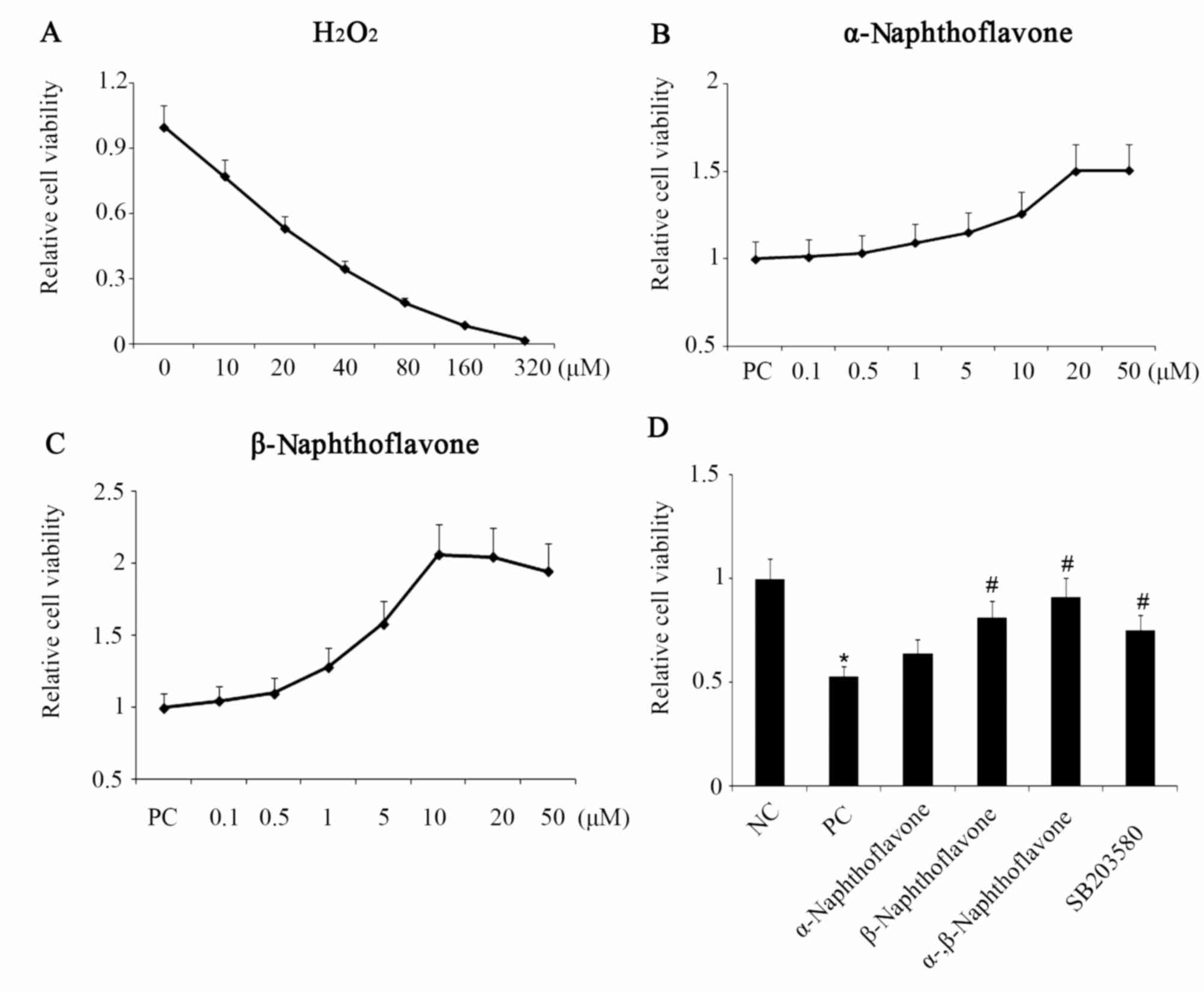 | Figure 1.Naphthoflavone reverses
H2O2-inhibited neuron SH-SY5Y cell viability.
(A) Neuron SH-SY5Y cell viability decreased by
H2O2 in a dose-dependent manner, with half
the cell viability loss at 20 µM H2O2. (B)
H2O2-inhibited neuron SH-SY5Y cell viability
increased in a dose-dependent manner with α-Naphthoflavone, until
the viability reached the plateau at a dose of 20 µM
α-Naphthoflavone. (C) H2O2-inhibited neuron
SH-SY5Y cell viability increased with β-Naphthoflavone
concentration, 0–10 µM, followed by a slight decrease. (D) Neuron
SH-SY5Y cell viability following different treatments. α- and
β-Naphthoflavone at 20 and 10 µM, respectively, were used
individually and in combination following exposure of neuron
SH-SY5Y cells to 20 µM H2O2. *P<0.05 vs.
NC; #P<0.05 vs. PC (n=8). NC, negative control, cells
without any treatment; PC, positive control, cells treated with 20
µM H2O2 only; α-, β-Naphthoflavone,
co-treatment with 20 µM α-Naphthoflavone and 10 µM
β-Naphthoflavone; SB203580, p38MAPK inhibitor;
H2O2, hydrogen peroxide. |
As indicated by the apoptosis rate assay,
H2O2 administration increased the apoptosis
rate of SH-SY5Y cells in the PC group to 24.1%, ~5-fold increase
compared with the NC group (P<0.01). Following this treatment,
the addition of α- and β-Naphthoflavone, respectively,
significantly decreased the apoptosis rate in comparison to the PC
cells (P<0.05; Fig. 2A and B).
Notably, co-treatment with α-Naphthoflavone and β-Naphthoflavone
significantly reduced the apoptosis rate compared with the PC
group, to 7.2% (P<0.01).
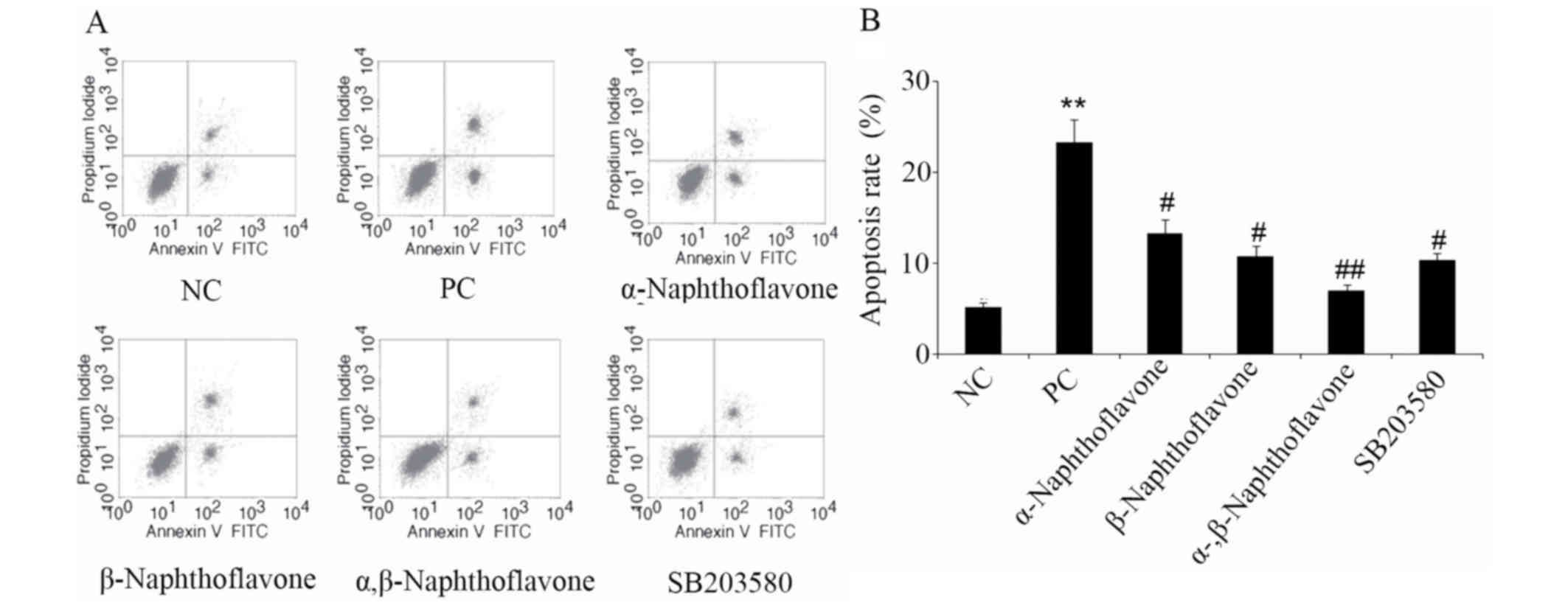 | Figure 2.Naphthoflavone decreases elevation of
apoptosis in H2O2-induced elevation in neuron
SH-SY5Y cells. α- and β-Naphthoflavone at a dose of 20 and 10 µM,
respectively, were used individually and in combination following
exposure of neuron SH-SY5Y cells to 20 µM
H2O2. Apoptosis rate was (A) detected by flow
cytometry and (B) quantified. *P<0.05, **P<0.05 vs. NC;
#P<0.05, ##P<0.01 vs. PC (n=4). NC,
negative control, cells without any treatment; PC, positive
control, cells treated with 20 µM H2O2 only;
α-, β-Naphthoflavone, co-treatment with 20 µM α-Naphthoflavone and
10 µM β-Naphthoflavone; SB203580, p38MAPK inhibitor;
H2O2, hydrogen peroxide. |
Naphthoflavone reverses
H2O2-induced TAC decrease and MDA
increase
To understand the H2O2-induced
oxidative effect and potential antioxidant function of
Naphthoflavone, the oxidative/antioxidative status of SH-SY5Y cells
following different treatments was assessed via TAC and MDA assays.
The TAC of SH-SY5Y cells (PC group) was significantly decreased
following exposure to 20 µM H2O2 (P<0.01,
Fig. 3A). Both α- and
β-Naphthoflavone significantly reversed the
H2O2-induced reduction in TAC compared with
the PC group (P<0.05). Combined treatment with α- and
β-Naphthoflavone had the most marked effect in on TAC, which
significantly increased TAC in comparison to the PC group
(P<0.05). MDA is regarded as a typical and sensitive biochemical
marker following cell exposure to ROS and its accumulation reflects
the extent of OS and indirectly that of cellular antioxidant
capacity (25). In the current
study, MDA in cells subjected to H2O2 (PC
group) increased to ~2.5-fold higher compared with NC cells,
reaching 13.24 µmol/mg protein (P<0.05, Fig. 3B). Post-treatment with α- and
β-Naphthoflavone decreased MDA to 10.24 and 9.01 µmol/mg protein,
respectively, which was a significant reduction compared with the
PC group (P<0.05). Co-treatment with α- and β-Naphthoflavone
reduced MDA to 6.74 µmol/mg, which was a significantly reduction
compared with the PC (P<0.05).
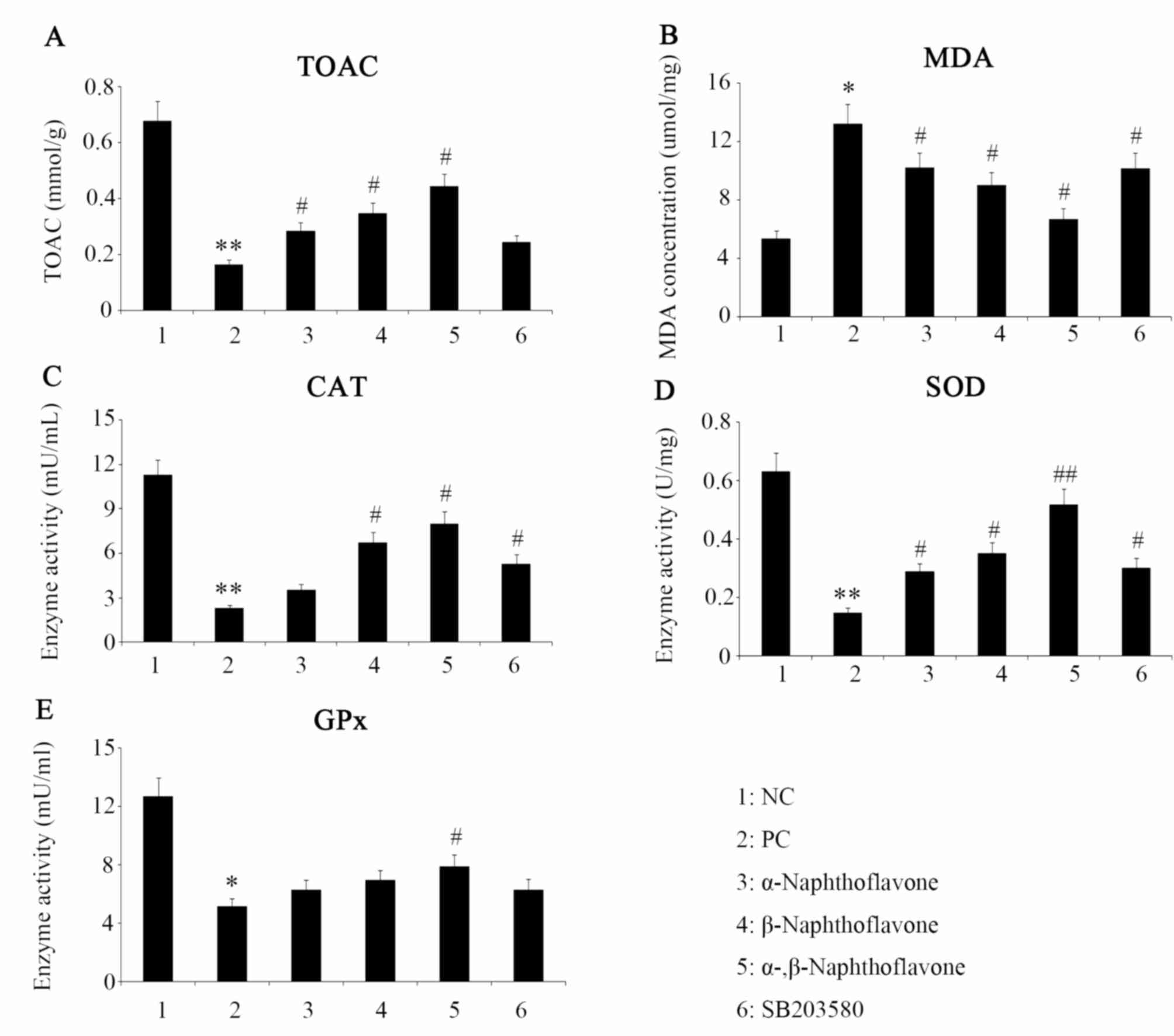 | Figure 3.Oxidative or antioxidative status of
neuron SH-SY5Y cells following different treatments. α- and
β-Naphthoflavone at a dose of 20 and 10 µM, respectively, were used
individually and in combination following exposure of neuron
SH-SY5Y cells to 20 µM H2O2. The oxidative or
antioxidative status of neuron SH-SY5Y cells following treatment
with (A) TAC, (B) MDA, (C) CAT, (D) SOD and (E) GPx. *P<0.05,
**P<0.05 vs. NC; #P<0.05, ##P<0.01
vs. PC (n=4). NC, negative control, cells without any treatment;
PC, positive control, cells treated with 20 µM
H2O2 only; α-, β-Naphthoflavone, co-treatment
with 20 µM α-Naphthoflavone and 10 µM β-Naphthoflavone; SB203580,
p38MAPK inhibitor; TAC, total antioxidant capacity; MDA,
malondialdehyde; CAT, catalase; SOD, superoxide dismutase; GPx,
glutathione peroxidase. |
Antioxidant enzyme activities are
upregulated by Naphthoflavone
As presented in Fig.
3C-E, exposure to H2O2 decreased the
activities of CAT, SOD and GPx by 82, 79 and 63%, compared with
their respective NC values (P<0.01, P<0.01 and P<0.05,
respectively). Post-treatment with α-naphthoflavone significantly
increased SOD activity compared with the PC group (P<0.05;
Fig. 3D), but only marginally
restored the enzyme activities of CAT and GPx (Fig. 3C and E). Post-treatment with
β-Naphthoflavone significantly increased CAT and SOD enzyme
activities compared with the PC group (Fig. 3C and D; P<0.05). Combined
treatment with α- and β-Naphthoflavone produced the most marked
effect, which predominantly elevated SOD enzyme activity compared
to PC (P<0.01; Fig. 1D), as well
as increased CAT and GPx activities significantly compared with the
PC group (Fig. 3C and E;
P<0.05).
Naphthoflavone attenuates
H2O2-induced damage via p38MAPK
inhibition
Previous studies have demonstrated that
H2O2 activates p38MAPK-mediated signaling to
induce neuronal apoptosis (26). In
the present study, SH-SY5Y cells exposed to
H2O2 exhibited a 2.2-fold increase in p38MAPK
phosphorylation at the sites of Thr180 and Tyr182 (P<0.05,
Fig. 4A), although
non-phosphorylated p38MAPK and phosphorylated p38MAPK at site of
Thr322 were almost unaffected, as assessed by western blot analysis
(Fig. 4A). By contrast,
H2O2 administration markedly increased the
expression of apoptosis-associated factors including Bax
(P<0.01, Fig. 4B), Cyt C
(P<0.01, Fig. 4C) and the ratio
of cleaved to non-cleaved caspase-3 (P<0.01, Fig. 4D). Subsequent addition of a
p38MAPK-specific inhibitor (SB203580) effectively reversed the
elevation of p38MAPK phosphorylation on Thr180 and Tyr182
(P<0.05 vs. PC, Fig. 4A), and is
associated with the reduction in the rate of apoptosis identified
in Bax and Cyt C expression (P<0.05 vs. PC, Fig. 4B and C), the cleaved ratio of
caspase-3 (P<0.01 vs. PC, Fig.
4D) and SH-SY5Y cells (P<0.05 vs. PC; Fig. 2). The data from the present study
suggest that H2O2-induced p38MAPK activation
facilitates neuron SH-SY5Y cell apoptosis, as previously considered
(26). There is a limited data
regarding the antioxidant role of SB203580; however, in the present
study the inhibitor exhibited an antioxidant capacity by
effectively reversing the H2O2-mediated
increase of MDA (P<0.05; Fig. 3B)
while H2O2-decreased CAT (P<0.05; Fig. 3C) and SOD activities (P<0.05;
Fig. 3D).
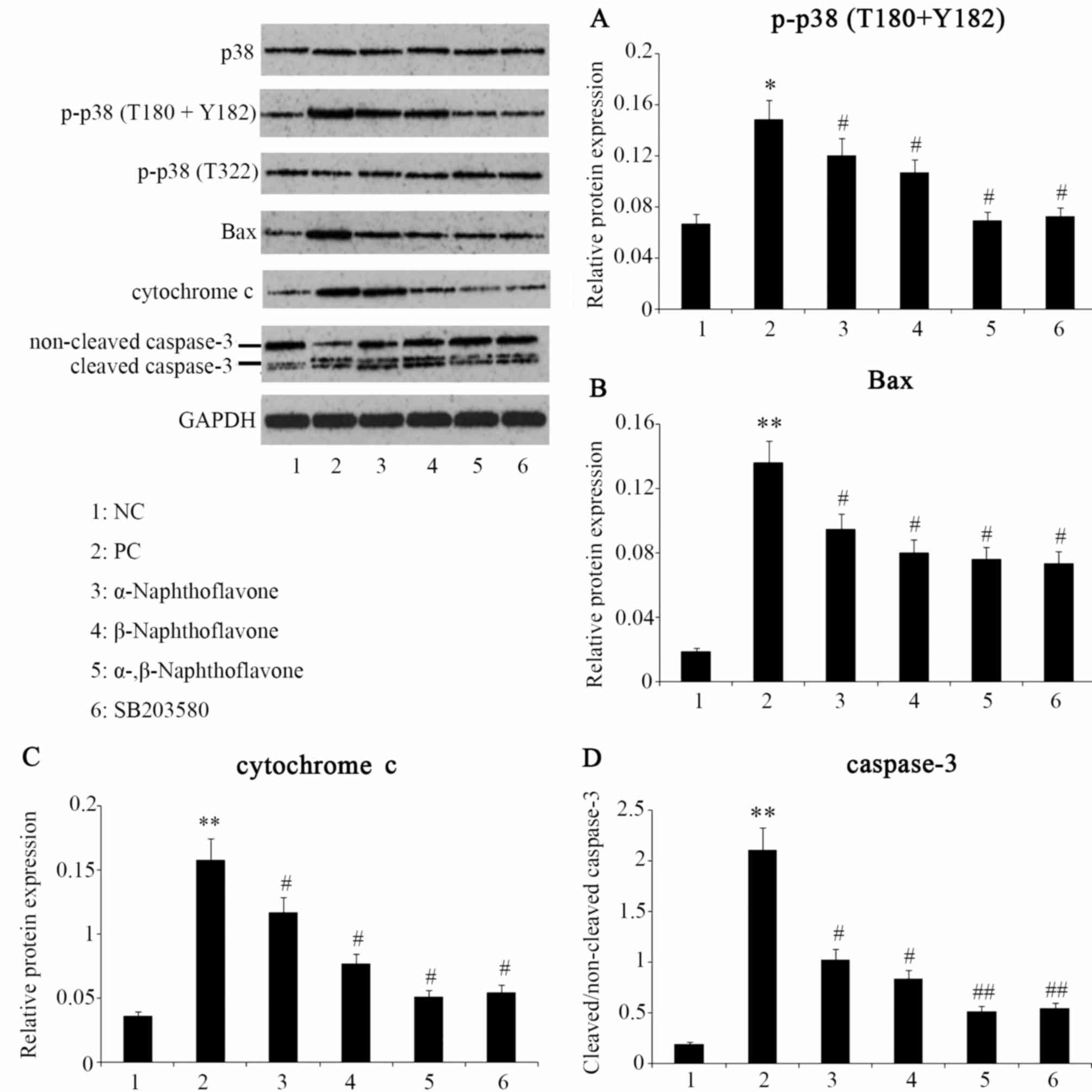 | Figure 4.Expression levels of proteins
following different treatments. α- and β-Naphthoflavone at a dose
of 20 and 10 µM, respectively, were used individually and in
combination following exposure of neuron SH-SY5Y cells to 20 µM
H2O2. (A) Western blot analysis indicates
p38MAPK phosphorylation at Thr180 and Tyr182. The protein
expression of (B) Bax (C) cytochrome c and (D) caspase-3 following
different treatments. *P<0.05, **P<0.05 vs. NC;
#P<0.05, ##P<0.01 vs. PC (n=4,). NC,
negative control, cells without any treatment; PC, positive
control, cells treated with 20 µM H2O2 only;
α-, β-Naphthoflavone, co-treatment with 20 µM α-Naphthoflavone and
10 µM β-Naphthoflavone; SB203580, p38MAPK inhibitor; p-p38,
phosphorylated p38; H2O2, hydrogen
peroxide. |
Post-treatment with Naphthoflavones also repressed
the p38MAPK phosphorylation at the Thr180 and Tyr182 sites and this
inhibitory effect, in order of least to most marked, was exerted by
α-Naphthoflavone (P<0.05 vs. PC), β-Naphthoflavone (P<0.05
vs. PC) and combined α- and β-Naphthoflavone (P<0.05 vs. PC;
Fig. 4A). Additionally,
α-Naphthoflavone decreased the expression of Bax and Cyt C and
cleaved ratio of caspase-3 (P<0.05 vs. PC). These effects were
also exhibited by β-Naphthoflavone, which was more effective than
α-Naphthoflavone, specifically in the inhibition of Cyt C
expression. By contrast, combined utilization of α- and
β-Naphthoflavone minimized Bax and Cyt C expression and the ratio
of cleaved caspase-3 (P<0.05, P<0.05 and P<0.01 vs. PC,
respectively).
Discussion
Naphthoflavone has been documented to alleviate and
confer protection against deleterious effects induced by OS.
Previous studies have indicated that α-Napthoflavone inhibits OS
and serves potential roles is estrogen-induced breast
carcinogenesis and ultraviolet-led human skin aging (27,28).
β-Naphthoflavone has been demonstrated to protect mice against
aristolochic acid-I-induced acute kidney injury, and to attenuate
hyperoxic lung injury in premature infants primarily via mitigating
OS (29,30). In the present study, α- and
β-Naphthoflavone were identified to effectively antagonize the
apoptosis-promoting effect of H2O2 on
neuronal SH-SY5Y cells. β-Naphthoflavone significantly reversed
H2O2-inhibited the cell viability
(P<0.05). Naphthoflavone was represented as a toxic substance in
a previous study (31); however, the
harmful effect of Naphthoflavone was not observed in the
concentration range of α- o β-Naphthoflavone in the present study.
Co-treatment with α- and β-Naphthoflavone abrogated the elevation
of H2O2-induced apoptosis rate and cell
viability reduction, which suggests a synergetic effect between α-
and β-Naphthoflavone in these respects. This advocates the combined
application of α- and β-Naphthoflavone in the clinical settings of
neurological disease treatment.
OS causes structural and functional damage to
neurons, H2O2-induced apoptosis mortality. As
a frequent event in nervous system, it is therefore associated with
a wide range of neurological diseases (1,2). OS is
an important therapeutic target, and the supplement of exogenous
antioxidants has been validated to be an effective approach to
prevent and manage neurological diseases clinically (13–16). In
the present study, neuronal SH-SY5Y cells underwent OS following
exposure to H2O2, as indicated by TAC and MDA
assays, and post-treatment with α-Naphthoflavone and/or
β-Naphthoflavone effectively alleviated
H2O2-induced OS.
A notable property of Naphthoflavone is an ablity to
modulate antioxidant enzyme activity. It has been suggested that
the antioxidant function exerted by natural flavones is completely
dependent on their reducibility, derived from specific phenolic
hydroxyl groups in their molecular structure (32). Evidence from Yang et al
(6) indicates that natural flavones
from grape seed extract elevate the activities of CAT, SOD and GPx,
and mitigate the H2O2 insult to muscle cells,
further suggesting that flavones serve an antioxidant role. It has
been demonstrated previously that β-Naphthoflavone serves as a
modulator of antioxidant enzyme activity (19). However, a previous study has
indicated that antioxidant enzyme activity is unaffected following
treatment with certain flavones (33). Thus, the present results indicate
that the ability of antioxidant enzyme activity regulation is
restricted in a number of flavones. In the current study,
H2O2-inhibited CAT and SOD activities were
promoted by β-Naphthoflavone. Furthermore, α-Naphthoflavone induced
a compensatory increase in SOD activity. The activity of GPx was
not significantly recovered by α- or β-Naphthoflavone, whereas it
was effectively reversed by co-treatment with α- and
β-Naphthoflavone. It has been demonstrated that antioxidant enzyme
activity regulated by certain flavones potentially results in
flavones stimulating or inhibiting aryl hydrocarbon receptors and
subsequently regulating signaling mediated by nuclear factor
erythroid 2-related factor, MAPK and peroxisome
proliferator-activated receptors (6). This was partly supported by the results
of the present study as SB203580 interfered with p38MAPK
activation, resulting in the alteration of CAT and SOD enzyme
activity, which may further explain why the addition of SB203580
may increase TAC and decrease MDA.
It has been indicated that OS-led neuronal apoptosis
represents a major mechanism for the development and progression of
neurological diseases (11–13) and OS-led neuronal apoptosis is at
least in part dependent upon p38MAPK-mediated signaling. Park et
al (26) demonstrated that the
phosphorylation level of p38MAPK is markedly increased in SH-SY5Y
cells following H2O2 stimulation, but those
of extracellular signal regulated kinase and c-Jun N-terminal were
not affected. Furthermore, increased p38MAPK phosphorylation is
associated with an increase in the rate of SH-SY5Y cell apoptosis,
which was eradicated following treatment with SB203580, a p38MAPK
specific inhibitor (26). A similar
outcome was observed in the present study and it was observed that
OS-led neuronal apoptosis relies on p38MAPK phosphorylation at two
sites (Thr180 and Tyr182), rather than at Thr322. α- and
β-Naphthoflavone repressed p38MAPK phosphorylation at Thr180 and
Tyr182, suggesting that α- and β-Naphthoflavone inhibition of
H2O2-led SH-SY5Y cell apoptosis is partly
dependent on p38MAPK signaling. However, it is unclear whether
Naphthoflavone is inhibiting p38MAPK activation completely depends
on repressing OS and its action on p38MAPK phosphorylation, or only
partly depends. The latter suggests that Naphthoflavone may
potentially inhibit p38MAPK activation through direct interference
of its upstream signaling. To understand the mechanisms involved,
future experiments to determine how dependent cell apoptosis in
SH-SY5Y cells is on p38MAPK signaling are required.
Under OS conditions, numerous apoptosis-associated
factors were upregulated or activated. Upregulated Bax and the
released Cyt C from the mitochondria are tightly associated to
mitochondrial dysfunction-mediated apoptosis (34). Conventional western blot assays are
unable to examine proteins present in the mitochondria as the
organelle is not lysed in this setting, and lysing mitochondria
requires more specific conditions. However, a clear upregulation of
Cyt C observed in the western blot analysis in the present study
indicates that Cyt C was released from the mitochondria in large
quantities. Caspase-3 activation is dependent on the cleavage of
its inactive form, and is widely regarded as a key step in
apoptosis execution (34). Notably,
α- and β-Naphthoflavone individually and synergistically suppressed
the expression of Bax and Cyt C and Caspase-3 activation, further
confirming that Naphthoflavone may inhibit OS-led neuronal
apoptosis.
The present results indicate that β-Naphthoflavone
is more effective against OS-led neuron damage compared with
α-Naphthoflavone. α-Naphthoflavone only induced a minor change to
H2O2-inhibited SH-SY5Y cell viability, but it
was markedly reversed by β-Naphthoflavone. Furthermore, the
post-treatment with β-Naphthoflavone led to a reduced apoptosis
rate compared with α-Naphthoflavone post-treatment.
β-Naphthoflavone is more efficient at upregulating the activity of
CAT, as well as inhibiting Cyt C released from the mitochondria
compared with α-Naphthoflavone. Further differences between the
mechanisms underlying α- and β-Naphthoflavone against OS-led neuron
damage may potentially exist, as combined α- and β-Naphthoflavone
had a more marked effect in this regard compared with α- or
β-Naphthoflavone individually. Moreover, increasing doses did not
continue to promote cell viability once the doses of α- and
β-Naphthoflavone reached 20 and 10 µM, respectively. An improved
understanding of the mechanisms by which α- and β-Naphthoflavone
counteract OS-led neuron damage is necessary in future study.
Furthermore, co-treatment with α- and β-Naphthoflavone abrogated
H2O2-induced apoptosis rate elevation and
cell viability reduction. This suggests a synergetic effect between
α- and β-Naphthoflavone. Further analysis demonstrated that the
activities of antioxidant enzymes including CAT, SOD and GPx, were
inhibited by increased H2O2, but enhanced by
α- and/or β-Naphthoflavone. H2O2-stimulated
p38MAPK activation was repressed by α- and/or β-Naphthoflavone,
along with a decreased expression of the apoptosis-related factors
and inhibited caspase-3 activation.
In conclusion, co-treatment with α- and
β-Naphthoflavone minimized H2O2-induced
neuron damage compared with treatment with α- or β-Naphthoflavone
individually. Therefore, it is advocated that utilizing α- and
β-Naphthoflavone together in the clinical setting of neurological
disease treatment may be beneficial.
Acknowledgements
The present study was supported by the Research
Project of Health and Family Planning Commission of Hunan Province
(grant no. B2016196).
References
|
1
|
Weaver J and Liu KJ: Does normobaric
hyperoxia increase oxidative stress in acute ischemic stroke? A
critical review of the literature. Med Gas Res. 5:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amara F, Berbenni M, Fragni M, Leoni G,
Viggiani S, Ippolito VM, Larocca M, Rossano R, Alberghina L, Riccio
P and Colangelo AM: Neuroprotection by cocktails of dietary
antioxidants under conditions of nerve growth factor deprivation.
Oxid Med Cell Longev. 2015:2172582015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choong CJ and Say YH: Neuroprotection of
α-synuclein under acute and chronic rotenone and maneb treatment is
abolished by its familial Parkinson's disease mutations A30P, A53T
and E46K. Neurotoxicology. 32:857–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tchantchou F, Graves M, Ortiz D, Rogers E
and Shea TB: Dietary supplementation with apple juice concentrate
alleviates the compensatory increase in glutathione synthase
transcription and activity that accompanies dietary- and
genetically-induced oxidative stress. J Nutr Health Aging.
8:492–496. 2004.PubMed/NCBI
|
|
5
|
Villegas-Rivera G, Román-Pintos LM,
Cardona-Muñoz EG, Arias-Carvajal O, Rodríguez-Carrizalez AD,
Troyo-Sanromán R, Pacheco-Moisés FP, Moreno-Ulloa A and
Miranda-Díaz AG: Effects of ezetimibe/simvastatin and rosuvastatin
on oxidative stress in diabetic neuropathy: A randomized,
double-blind, placebo-controlled clinical trial. Oxid Med Cell
Longev. 2015:7562942015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang T, Li X, Zhu W, Chen C, Sun Z, Tan Z
and Kang J: Alteration of antioxidant enzymes and associated genes
induced by grape seed extracts in the primary muscle cells of goats
in vitro. PLoS One. 9:e1076702014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soundararajan R, Wishart AD, Rupasinghe
HP, Arcellana-Panlilio M, Nelson CM, Mayne M and Robertson GS:
Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in
vitro against oxidative damage by inducing sterol regulatory
element-binding protein-2-mediated cholesterol biosynthesis. J Biol
Chem. 283:2231–2245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu XH, Li GL, Wang BA, Qin Y, Bai SR, Rong
J, Deng T and Li Q: Diallyl trisufide protects against oxygen
glucose deprivation-induced apoptosis by scavenging free radicals
via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway in B35 neural
cells. Brain Res. 1614:38–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z
and Chen G: Tert-butylhydroquinone alleviates early brain injury
and cognitive dysfunction after experimental subarachnoid
hemorrhage: Role of Keap1/Nrf2/ARE pathway. PLoS One. 9:e976852014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh N, Agrawal M and Doré S:
Neuroprotective properties and mechanisms of resveratrol in vitro
and in vivo experimental cerebral stroke models. ACS Chem Neurosci.
4:1151–1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bournival J, Plouffe M, Renaud J,
Provencher C and Martinoli MG: Quercetin and sesamin protect
dopaminergic cells from MPP+-induced neuroinflammation in a
microglial (N9)-neuronal (PC12) coculture system. Oxid Med Cell
Longev. 2012:9219412012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mena MA, Casarejos MJ, Solano R,
Rodríguez-Navarro JA, Gómez A, Rodal I, Medina M and de Yebenes JG:
NP7 protects from cell death induced by oxidative stress in
neuronal and glial midbrain cultures from parkin null mice. FEBS
Lett. 583:168–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jazvinšćak Jembrek M, Hof PR and Šimić G:
Ceramides in Alzheimer's disease: Key mediators of neuronal
apoptosis induced by oxidative stress and Aβ accumulation. Oxid Med
Cell Longev. 2015:3467832015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonardo CC and Doré S: Dietary flavonoids
are neuroprotective through Nrf2-coordinated induction of
endogenous cytoprotective proteins. Nutr Neurosci. 14:226–236.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin WL, Wang SM, Ho YJ, Kuo HC, Lee YJ and
Tseng TH: Ethyl acetate extract of Wedelia chinensis inhibits
tert-butyl hydroperoxide-induced damage in PC12 cells and
D-galactose-induced neuronal cell loss in mice. BMC Complement
Altern Med. 14:4912014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu A, Wu Z, Meng J, McGeer PL, Zhu Y,
Nakanishi H and Wu S: The neuroprotective effects of ratanasampil
on oxidative stress-mediated neuronal damage in human neuronal
SH-SY5Y cells. Oxid Med Cell Longev. 2015:7923422015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhawan K: Drug/substance reversal effects
of a novel tri-substituted benzoflavone moiety (BZF) isolated from
Passiflora incarnata Linn.-a brief perspective. Addict Biol.
8:379–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Q, Yu J, Yang W, Kimatu BM, Fang Y, Ma
N and Pei F: Identification of flavonoids from Flammulina velutipes
and its neuroprotective effect on pheochromocytoma-12 cells. Food
Chem. 204:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nannelli A, Rossignolo F, Tolando R,
Rossato P, Longo V and Gervasi PG: Effect of beta-naphthoflavone on
AhR-regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and
antioxidant enzymes in various brain regions of pig. Toxicology.
265:69–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrissy S, Strom J, Purdom-Dickinson S
and Chen QM: NAD(P)H: Quinone oxidoreductase 1 is induced by
progesterone in cardiomyocytes. Cardiovasc Toxicol. 12:108–114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins LG and Hayes JD: Mechanisms of
induction of cytosolic and microsomal glutathione transferase (GST)
genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev.
43:92–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reed JR, Cawley GF and Backes WL:
Inhibition of cytochrome P450 1A2-mediated metabolism and
production of reactive oxygen species by heme oxygenase-1 in rat
liver microsomes. Drug Metab Lett. 5:6–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu SY, Liou JW, Cheng TL, Peng SY, Lin
CC, Chu YY, Luo WC, Huang ZK and Jiang SJ: Beta-Naphthoflavone
protects from peritonitis by reducing TNF-alpha-induced endothelial
cell activation. Pharmacol Res. 102:192–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milardovic S, Kereković I, Derrico R and
Rumenjak V: A novel method for flow injection analysis of total
antioxidant capacity using enzymatically produced ABTS*+ and
biamperometric detector containing interdigitated electrode.
Talanta. 71:213–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem. Oct
24–2016.(Epub ahead of print). View Article : Google Scholar
|
|
26
|
Park HR, Lee H, Park H, Jeon JW, Cho WK
and Ma JY: Neuroprotective effects of Liriope platyphylla extract
against hydrogen peroxide-induced cytotoxicity in human
neuroblastoma SH-SY5Y cells. BMC Complement Altern Med. 15:1712015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao PL, Li CH, Chang CY, Lu SR, Lin CH,
Tse LS and Cheng YW: Anti-ageing effects of alpha-naphthoflavone on
normal and UVB-irradiated human skin fibroblasts. Exp Dermatol.
21:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mense SM, Singh B, Remotti F, Liu X and
Bhat HK: Vitamin C and alpha-naphthoflavone prevent
estrogen-induced mammary tumors and decrease oxidative stress in
female ACI rats. Carcinogenesis. 30:1202–1208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao Y, Xue X, Wu YF, Xin GZ, Qian Y, Xie
TP, Gong LK and Ren J: Beta-Naphthoflavone protects mice from
aristolochic acid-I-induced acute kidney injury in a CYP1A
dependent mechanism. Acta Pharmacol Sin. 30:1559–1565. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sinha A, Muthiah K, Jiang W, Couroucli X,
Barrios R and Moorthy B: Attenuation of hyperoxic lung injury by
the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 87:204–212.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kober SL, Meyer-Alert H, Grienitz D,
Hollert H and Frohme M: Intact cell mass spectrometry as a rapid
and specific tool for the differentiation of toxic effects in
cell-based ecotoxicological test systems. Anal Bioanal Chem.
407:7721–7731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Csepregi K, Neugart S, Schreiner M and
Hideg É: Comparative evaluation of total antioxidant capacities of
plant polyphenols. Molecules. 21:pii.E208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orozco TJ, Wang JF and Keen CL: Chronic
consumption of a flavanol- and procyanindin-rich diet is associated
with reduced levels of 8-hydroxy-2′-deoxyguanosine in rat testes. J
Nutr Biochem. 14:104–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burri SH, Kim CN, Fang G, Chang BS,
Perkins C, Harris W, Davis LW, Thompson CB and Bhalla KN: ‘Loop’
domain deletional mutant of Bcl-xL is as effective as p29Bcl-xL in
inhibiting radiation-induced cytosolic accumulation of cytochrome c
(cyt c), caspase-3 activity and apoptosis. Int J Radiat Oncol Biol
Phys. 43:423–430. 1999. View Article : Google Scholar : PubMed/NCBI
|


















