Introduction
Graves' disease (GD) is an organ-specific autoimmune
thyroid disease. The development of GD is influenced by various
genetic and environmental factors and the interactions between them
(1). GD pathogenesis is generally
characterized by abnormalities in T-cell subsets (2,3), innate
immune cells (4), and various
cytokines, which ultimately decreases immune tolerance and
excessively activates B cells, promoting the production of thyroid
stimulating hormone (TSH) receptor antibody (TRAb). The biased
activation of helper T (Th) cells has an important role in GD
development (5,6) and the imbalance of Th1/Th2 cell
immunity may result in a shift toward the Th2-type immune response
(7).
At present, iodine-131 (131I) therapy is
the recommended treatment option for GD; however, prognosis
estimation remains a critical issue (8). It has been previously reported that the
presence of thyroid autoantibodies in patients with GD prior to
131I therapy is an independent predictor of the
occurrence of hypothyroidism (9).
Furthermore, high titers of TRAb in patients with GD prior to
131I therapy are associated with treatment failure,
whereas elevated TRAb following 131I therapy predicts
the occurrence of hypothyroidism (10). However, previous studies have
indicated that the incidence of hypothyroidism following
131I therapy is not affected by the changes in thyroid
autoantibody levels prior to treatment (11,12).
Therefore, the association between the level of thyroid
autoantibodies and the prognosis of GD following 131I
therapy remains controversial. Previous studies on GD have
predominantly focused on the abnormalities of immune cells and
cytokines. Particularly, as markers of immune responses, cytokines
have been intensively investigated (13,14).
However, few studies have been conducted to assess the cytokine
levels in patients with GD following 131I therapy
(15,16).
In the present study, 30 patients with GD who were
currently receiving 131I therapy were followed-up for 18
months. Serum cytokine levels were detected in these patients,
including interleukin (IL)-6, CXC chemokine ligand (CXCL)-10 and
intercellular adhesion molecule (ICAM)-1. Furthermore, the
associations of these cytokine levels with the serum levels of free
triiodothyronine (FT3), free thyroxine index (FT4) and
thyroid-stimulating hormone (TSH) were also evaluated.
Materials and methods
Study subjects
A total of 30 patients with GD (the patient group),
who were admitted to the Linyi People's Hospital (Shandong, China)
between August 2010 and August 2012, participated in the present
study. These patients had been diagnosed with GD according to the
diagnostic criteria from the Guidelines for Diagnosis and Treatment
of Thyroid Diseases in China (2007) (17). Patients with other
autoimmune diseases, hepatic or renal insufficiency, acute or
chronic infection, or thyroid-related eye diseases were excluded.
No patients had been, or were currently being treated with
glucocorticoids or other immunosuppressants. The 131I
treatment dosage was calculated individually for each patient,
according to the Marinelli formula: Treatment dose = [thyroid mass
(g) × 0.1 (mCi / g)] / [radioactive iodine uptake U24
(131I)]. A further 30 gender- and age-matched subjects
were used as the control group. Prior written and informed consent
were obtained from every patient and the methodology was approved
by the Ethics Review Board of the Linyi People's Hospital.
Laboratory measurements
Fasting venous blood (10 µl) was collected into
sodium citrate tubes from each control subject and patient with GD
prior to 131I therapy, after 1 week, and 1, 2, 3, 6, 12
and 18 months following treatment. Blood samples were centrifuged
at 2,500 × g for 15 min. Serum was separated and stored at −80°C
prior to laboratory tests. Serum concentrations of FT3, FT4, TSH
and thyroid peroxidase antibodies (TPOAb) were measured via
chemiluminescence assay (Unicel Dxi800) and serum TRAb
concentration was measured via radioreceptor assay using commercial
kits (all Beckman Coulter, Inc., Brea, CA, USA), according to the
manufacturer's protocols. Serum levels of IL-6, CXCL-10 and ICAM-1
were measured using ELISA kits (R&D Systems, Inc., Minneapolis,
MN, USA), according to the manufacturer's protocol.
Statistical analysis
Data were expressed as mean ± standard deviation.
Statistical analysis was performed using the SPSS 19.0 software
package (IBM SPSS, Armonk, NY, USA). The χ2 test was
used for count data, Student's t-test for differences between
dependent variables, and one-way analysis of variance for in-group
comparisons, with Student-Newman-Keuls and Fisher's least
significant difference tests. Pearson's correlation was used for
association analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and clinical features of
patients with GD prior to 131I therapy
Demographic and clinical features of 30 control
subjects and 30 patients with GD prior to 131I therapy
are listed in Table I. No
significant differences in gender and age were found between the
patient group and control subjects (P>0.05). Serum levels of
FT3, FT4, TSH, TPOAb and TRAb in the patient group prior to
131I treatment were significantly higher than those in
the control group (P<0.01). Serum levels of IL-6, CXCL-10 and
ICAM-1 in the patient group prior to 131I therapy were
also significantly higher than those in the control group
(P<0.01). These results demonstrated the significant
immune/inflammatory responses in patients with GD prior to
treatment.
 | Table I.Demographic and clinical
characteristics of patients with GD prior to iodine-131 therapy and
control subjects. |
Table I.
Demographic and clinical
characteristics of patients with GD prior to iodine-131 therapy and
control subjects.
| Characteristic | Controls n=30 | Patients with GD
n=30 | P-value |
|---|
| Gender | 4 males/26
females | 5 males/25
females | 0.74 |
| Age (years) | 35.72±3.16 | 36.12±2.34 | 0.33 |
| FT3 (mmol/l) | 4.82±1.28 | 20.75±6.79 |
<0.01 |
| FT4 (mmol/l) | 16.72±3.49 | 42.01±8.92 |
<0.01 |
| TSH (uIU/ml) | 2.34±1.62 | 0.01±0.01 |
<0.01 |
| TPOAb (IU/ml) | 32.17±16.94 | 479.83±36.72 |
<0.01 |
| TRAb (IU/l) | 0.85±0.36 | 11.73±3.95 |
<0.01 |
| IL-6 (pg/ml) | 43.70±5.05 | 114.93±12.35 |
<0.01 |
| CXCL-10
(pg/ml) | 78.07±8.83 | 183.67±18.52 |
<0.01 |
| ICAM-1 (pg/ml) | 161.45±7.64 | 345.69±16.83 |
<0.01 |
Changes in serum levels of IL-6,
CXCL-10, and ICAM-1 in patients with GD following 131I
therapy
Serum levels of IL-6, CXCL-10, and ICAM-1 were
determined in the patient group at the indicated time points
following 131I therapy and compared with the before
treatment level and corresponding control group. As presented in
Fig. 1, in the patient group, the
serum IL-6 level increased rapidly following 131I
therapy, peaking at 1 week post-treatment (P<0.01) and
subsequently declining. Serum levels of IL-6 in the patient group
at 12 and 18 months following 131I therapy were
significantly lower than the before-treatment value (P<0.05),
although they remained significantly higher than those in the
control group (P<0.01). Furthermore, as presented in Fig. 2, the serum level of CXCL-10 was also
significantly elevated in the patient group at 1 week following
131I therapy (P<0.01). In the subsequent six months,
serum CXCL-10 levels in the patient group gradually declined, to a
level comparable with the before-treatment value. At 12 and 18
months post-treatment, serum CXCL-10 levels had declined further
and were significantly lower than the before-treatment value
(P<0.05); however, they remained significantly higher than those
in the control group (P<0.01). Conversely, the serum ICAM-1
level exhibited a less-marked increase in the patient group in the
first week following 131I therapy, which continuously
increased over the next 12 months (P<0.05) and a relatively
stable level was achieved thereafter (Fig. 3). These results suggest that the
inflammatory response is maintained in the patients with GD
following 131I therapy.
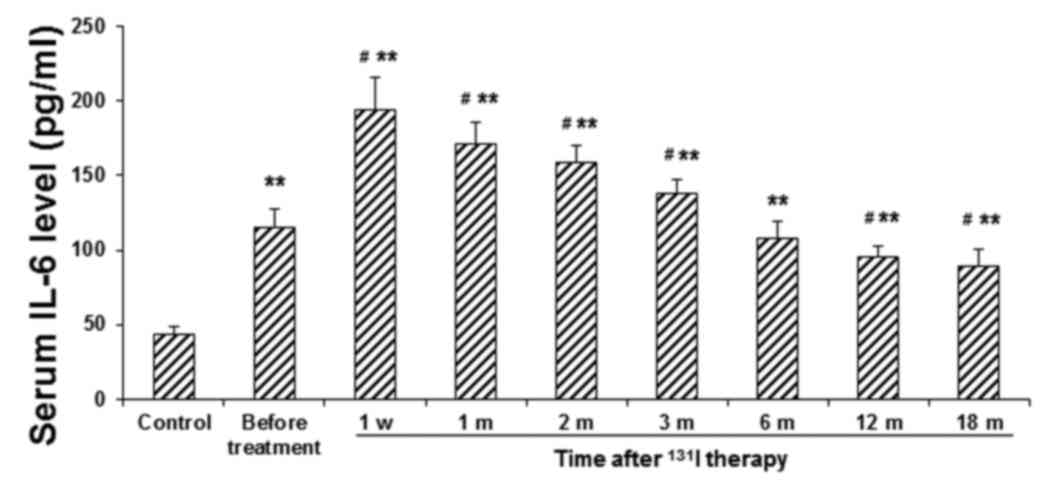 | Figure 1.Serum levels of IL-6 in patients with
GD before and after 131I therapy. Serum levels of IL-6
in patients with GD were determined using ELISA, prior to
131I therapy, at 1 w, and 1, 2, 3, 6, 12 and 18 m
post-treatment. **P<0.01 vs. the corresponding control group;
#P<0.05 vs. the before-treatment level in patients
with GD. IL, interleukin; GD, Graves' disease; 131I,
iodine-131; w, week; m, month. |
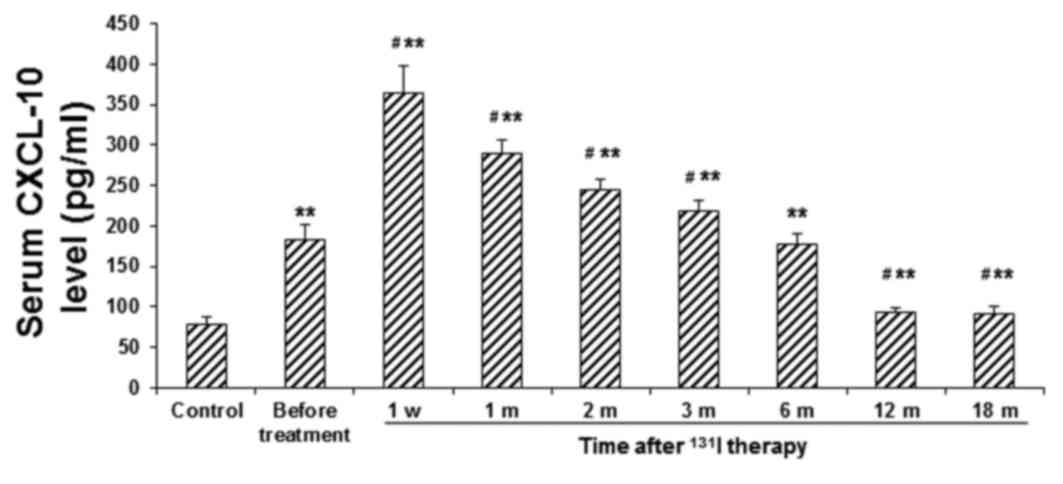 | Figure 2.Serum levels of CXCL-10 in patients
with GD before and after 131I therapy. Serum levels of
CXCL-10 in patients with GD were determined using ELISA, prior to
131I therapy, at 1 w, and 1, 2, 3, 6, 12 and 18 m
post-treatment. **P<0.01 vs. the corresponding control group;
#P<0.05 vs. the before-treatment level in patients
with GD. CXCL, CXC chemokine ligand; GD, Graves' disease;
131I, iodine-131; w, week; m, month. |
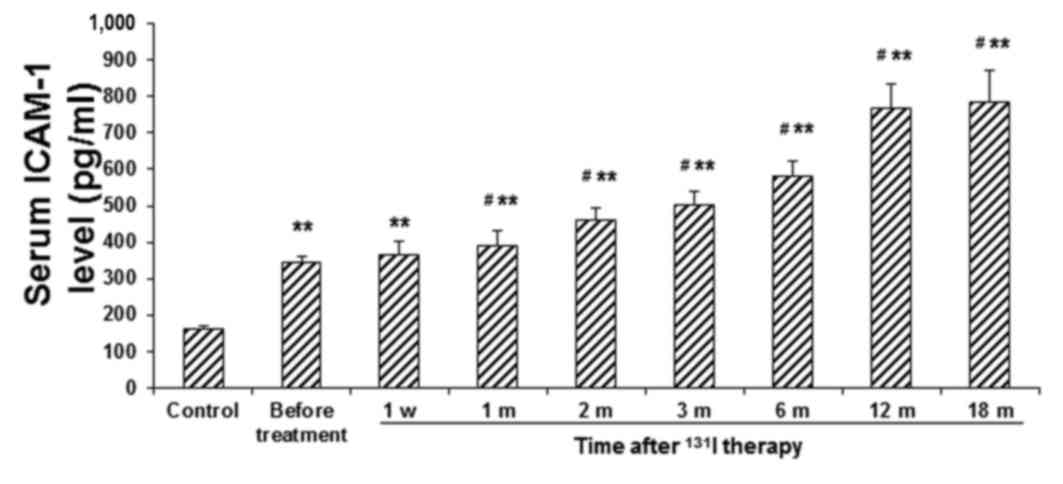 | Figure 3.Serum levels of ICAM-1 in patients
with GD before and after 131I therapy. Serum levels of
ICAM-1 in patients with GD were determined using ELISA, prior to
131I therapy, at 1 w, and 1, 2, 3, 6, 12 and 18 m
post-treatment. **P<0.01 vs. the corresponding control group;
#P<0.05 vs. the before-treatment level in patients
with GD. ICAM, intracellular adhesion molecule; GD, Graves'
disease; 131I, iodine-131; w, week; m, month. |
The present results from the Pearson's correlation
analysis demonstrated that serum levels of IL-6, CXCL-10, and
ICAM-1 were not associated with FT3, FT4 and TSH in the patient
group (Table II). These results
suggest that changes in the serum levels of IL-6, CXCL-10 and
ICAM-1 in patients with GD following 131I therapy may be
associated with changes in immune/inflammatory responses, rather
than alterations in thyroid function.
 | Table II.Correlation analysis of serum levels
of IL-6, CXCL-10, and ICAM-1 with FT3, FT4, and TSH in patients
with GD and control subjects. |
Table II.
Correlation analysis of serum levels
of IL-6, CXCL-10, and ICAM-1 with FT3, FT4, and TSH in patients
with GD and control subjects.
| Cytokine | Statistical
value | FT3 | FT4 | TSH |
|---|
| IL-6 | R | 0.037 | 0.046 | −0.018 |
|
| P-value | 0.61 | 0.54 | 0.61 |
| CXCL-10 | R | 0.072 | 0.063 | −0.014 |
|
| P-value | 0.24 | 0.29 | 0.61 |
| ICAM-1 | R | 0.047 | 0.053 | −0.061 |
|
| P-value | 0.36 | 0.31 | 0.23 |
Discussion
GD is a common organ-specific autoimmune disease
(1). Abnormalities in T-cell subsets
(2) and other innate immune cells
(4), and the production of TRAb due
to excessively activated B cells (18), are important pathogenic events in GD.
As one of the most commonly used treatments for GD, 131I
therapy is able to induce cellular apoptosis, which may induce the
rupture of thyroid follicular cells and induce the release of
cellular contents into the bloodstream. During this process,
antibody production may be enhanced by antigens released by the
rupture of thyroid follicular cells. Furthermore, the immune
response and inflammatory reactions in the thyroid may be altered
by the ionizing radiation.
Various cytokines have been determined to be
associated with the pathology of GD, including ILs, tumor necrosis
factor-α, interferon-γ and RANTES, which are markers for immune and
inflammatory responses (13,19,20).
Despite intensive investigation, few cytokines have been confirmed
to be altered in patients with GD following 131I therapy
(15,16). It has been demonstrated previously
that IL-6 (21), CXCL-10 (22) and ICAM-1 (23) levels are significantly higher in
patients with GD than in healthy subjects, which may be associated
with immune/inflammatory responses in the thyroid. However, it has
not yet been fully elucidated whether this occurs in patients with
GD following 131I therapy.
IL-6, as a Th2 cytokine, predominantly regulates B-
and T-lymphocyte functions (24). In
patients with GD, IL-6 is produced by infiltrating immune cells and
thyrocytes (25). Serum IL-6 levels
in patients with GD have been reported to be significantly higher
than those in healthy controls (21,26).
However, the clinical significance of serum IL-6 levels remains
controversial. Wahrenberg et al (21) have determined that the serum level of
IL-6 indicates the tendency of inflammatory response. Furthermore,
Heuer et al (26) claim that
the elevated IL-6 concentration may reflect disease severity. In
the present study, it was demonstrated that serum IL-6 levels in
patients with GD were significantly higher than those in the
control group. The serum IL-6 level increased and peaked rapidly
following 131I therapy, and subsequently returned to the
before-treatment level within six months, prior to declining
further by 18 months post-treatment. Pearson's correlation analysis
demonstrated that changes in serum IL-6 levels in the patient group
following 131I therapy were not correlated with thyroid
function, suggesting that the changes in serum IL-6 may be
associated with changes in immune/inflammatory responses.
Conversely, the present results indicated that the serum level of
IL-6 in the patient group at 18 months following 131I
therapy was significantly higher than in the control group,
suggesting a marked inflammatory response in the thyroid following
131I therapy. Further studies are required to clarify
whether this is related to the thyroid dysfunction following
131I therapy.
CXCL-10, also known as interferon-γ-inducible
protein 10 (IP-10), is associated with the infiltration,
localization and activation of lymphocytes in the thyroid (27). In addition to endothelial and T
cells, CXCL-10 is predominately secreted by the thyroid cells in
inflammatory responses, which contributes to the pathogenesis of GD
(22,28). The present results demonstrated that
serum CXCL-10 levels in the patient group were significantly higher
than those in the control group, which is in accordance with
previous findings (22,28). Furthermore, the serum level of
CXCL-10 in the patient group rapidly increased following
131I therapy and subsequently declined to the
before-treatment level within six months, with a significantly
lower level at 18 months post-treatment, while remaining
significantly higher that of the control group. These results were
consistent with those observed for the serum IL-6 levels. CXCL-10
and IL-6 mediate the Th1/Th2 responses; therefore, these findings
suggest that 131I therapy for GD may significantly
enhance cellular and humoral immune/inflammatory responses.
ICAM-1, which is a member of the adhesion molecule
immunoglobulin superfamily, has an important role in lymphocyte
adhesion, T-cell activation and the antigen-presenting process,
which indicates non-specific inflammation (29). In accordance with a previous study
(23), the present results
demonstrated that the serum ICAM-1 level in the patient group was
significantly higher than that of the control group. Serum ICAM-1
levels have been observed to be altered by treatment with
anti-thyroid drugs (30), suggesting
that the inflammatory responses in patients with GD may be
alleviated by these drugs. Furthermore, the present results
demonstrated that the serum levels of ICAM-1 in patients with GD
were gradually increased during the 18 months following
131I therapy, suggesting inflammatory activity. This
phenomenon is markedly different from the changes observed in the
serum levels of IL-6 and CXCL-10. As ICAM-1 is a marker for the
activities of endothelial cells and fibroblasts (31), the continuously elevated ICAM-1 level
following 131I therapy may be associated with the
involvement of endothelial cells and fibroblasts in the repair of
the thyroid.
In conclusion, the present results demonstrated that
serum levels of IL-6, CXCL-10 and ICAM-1 were significantly
elevated in patients with GD prior to treatment, and that
131I therapy was able to alter the serum cytokine levels
in these patients. However, the changes in serum levels of IL-6,
CXCL-10 and ICAM-1 were not associated with thyroid function.
Furthermore, no data regarding the thyroid autoantibodies prior to
and following the 131I therapy in these patients was
obtained in the present study, and the relationship between thyroid
autoantibody changes and cytokine alterations cannot be evaluated.
Therefore, these findings suggest that these cytokines cannot be
used as prognostic markers for the 131I therapy of
GD.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grants nos. 30971595 and 30771017),
Natural Science Foundation of Shandong Province, China (grant no.
ZH2011HM067), and the Medical and Health Science Technology
Development Program of Shandong Institute (grant no.
2011HW023).
References
|
1
|
Prummel MF, Strieder T and Wiersinga WM:
The environment and autoimmune thyroid diseases. Eur J Endocrinol.
150:605–618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saitoh O, Abiru N, Nakahara M and Nagayama
Y: CD8+CD122+ T cells, a newly identified
regulatory T subset, negatively regulate Graves' hyperthyroidism in
a murine model. Endocrinology. 148:6040–6046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammerstad SS, Jahnsen FL, Tauriainen S,
Hyöty H, Paulsen T, Norheim I and Dahl-Jørgensen K: Immunological
changes and increased expression of myxovirus resistance protein a
in thyroid tissue of patients with recent onset and untreated
Graves' disease. Thyroid. 24:537–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawashima A, Tanigawa K, Akama T,
Yoshihara A, Ishii N and Suzuki K: Innate immune activation and
thyroid autoimmunity. J Clin Endocrinol Metab. 96:3661–3671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations and implications for health and disease. Altern Med
Rev. 8:223–246. 2003.PubMed/NCBI
|
|
6
|
Rapoport B and McLachlan SM: Graves'
hyperthyroidism is antibody-mediated but is predominantly a
Th1-type cytokine disease. J Clin Endocrinol Metab. 99:4060–4061.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morshed SA, Latif R and Davies TF:
Delineating the autoimmune mechanisms in Graves' disease. Immunol
Res. 54:191–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chair RS Bahn, Burch HB, Cooper DS, Garber
JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM,
Rivkees SA, et al: Hyperthyroidism and other causes of
thyrotoxicosis: Management guidelines of the American Thyroid
Association and American Association of Clinical Endocrinologists.
Thyroid. 21:593–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmad AM, Ahmad M and Young ET: Objective
estimates of the probability of developing hypothyroidism following
radioactive iodine treatment of thyrotoxicosis. Eur J Endocrinol.
146:767–775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiovato L, Fiore E, Vitti P, Rocchi R,
Rago T, Dokic D, Latrofa F, Mammoli C, Lippi F, Ceccarelli C and
Pinchera A: Outcome of thyroid function in Graves' patients treated
with radioiodine: Role of thyroid-stimulating and
thyrotropin-blocking antibodies and of radioiodine-induced thyroid
damage. J Clin Endocrinol Metab. 83:40–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andrade VA, Gross JL and Maia AL: The
effect of methimazole pretreatment on the efficacy of radioactive
iodine therapy in Graves' hyperthyroidism: One-year follow-up of a
prospective, randomized study. J Clin Endocrinol Metab.
86:3488–3493. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gómez-Arnaiz N, Andía E, Gumà A, Abós R,
Soler J and Gómez JM: Ultrasonographic thyroid volume as a reliable
prognostic index of radioiodine-131 treatment outcome in Graves'
disease hyperthyroidism. Horm Metab Res. 35:492–497. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikos H, Mikos M, Obara-Moszynska M and
Niedziela M: The role of the immune system and cytokines involved
in the pathogenesis of autoimmune thyroid disease (AITD).
Endokrynologia Polska. 65:150–155. 2014.PubMed/NCBI
|
|
14
|
Khan FA, Al-Jameil N, Khan MF, Al-Rashid M
and Tabassum H: Thyroid dysfunction: An autoimmune aspect. Int J
Clin Exp Med. 8:6677–6681. 2015.PubMed/NCBI
|
|
15
|
Dong QY, Li SJ, Gao GQ, Liu XM, Li WX,
Liang CG, Du WH and Wsng YL: Short-term effect of radioactive
iodine therapy on CXCL-10 production in Graves' disease. Clin
Invest Med. 34:E2622011.PubMed/NCBI
|
|
16
|
Jurgilewicz DH, Rogowski F, Łebkowska U,
Citko A, Jaroszewicz E and Parfieńczyk A: E-selectin, L-selectin,
ICAM-1 and IL-6 concentrations changes in the serum of patients
with hyperthyroidism in the early period of radioiodine I-131
therapy. Nucl Med Rev Cent East Eur. 5:39–42. 2002.PubMed/NCBI
|
|
17
|
Chinese Society of Endocrinology, .
Medical guidelines for the management of Thyroid disease in China.
Clin J Intern Med. 46(10)2007.
|
|
18
|
Zeki K, Tanaka Y, Fujihira T, Watanabe K,
Suzuki H, Yamashita U and Eto S: Immunological abnormality of
peripheral blood B cells in patients with autoimmune thyroid
disease. Endocrinol Jpn. 36:335–342. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mysliwiec J, Palyga I, Nikolajuk A,
Kowalska A and Gorska M: Serum interleukin-16 and RANTES during
treatment of Graves' orbitopathy with corticosteroids and
teleradiotherapy. Endokrynol Pol. 63:92–96. 2012.PubMed/NCBI
|
|
20
|
Niyazoglu M, Baykara O, Koc A, Aydoğdu P,
Onaran I, Dellal FD, Tasan E and Sultuybek GK: Association of
PARP-1, NF-κB, NF-κBIA and IL-6, IL-1β and TNF-α with Graves
Disease and Graves Ophthalmopathy. Gene. 547:226–232. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wahrenberg H, Wennlund A and Hoffstedt J:
Increased adipose tissue secretion of interleukin-6, but not of
leptin, plasminogen activator inhibitor-1 or tumour necrosis factor
alpha, in Graves' hyperthyroidism. Eur J Endocrinol. 146:607–611.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonelli A, Rotondi M, Fallahi P,
Romagnani P, Ferrari SM, Buonamano A, Ferrannini E and Serio M:
High levels of circulating CXCL chemokine ligand 10 are associated
with chronic autoimmune thyroiditis and hypothyroidism. J Clin
Endocrinol Metab. 89:5496–5499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arao T, Morimoto I, Kakinuma A, Ishida O,
Zeki K, Tanaka Y, Ishikawa N, Ito K, Ito K and Eto S: Thyrocyte
proliferation by cellular adhesion to infiltrating lymphocytes
through the intercellular adhesion molecule-1/lymphocyte
function-associated antigen-1 pathway in Graves' disease. J Clin
Endocrinol Metab. 85:382–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvi M, Girasole G, Pedrazzoni M, Passeri
M, Giuliani N, Minelli R, Braverman LE and Roti E: Increased serum
concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in
patients with Graves' disease. J Clin Endocrinol Metab.
81:2976–2979. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heuer M, Aust G, Ode-Hakim S and Scherbaum
WA: Different cytokine mRNA profiles in Graves' disease,
Hashimoto's thyroiditis and nonautoimmune thyroid disorders
determined by quantitative reverse transcriptase polymerase chain
reaction (RT-PCR). Thyroid. 6:97–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antonelli A, Rotondi M, Ferrari SM,
Fallahi P, Romagnani P, Franceschini SS, Serio M and Ferrannini E:
Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in
Graves' ophthalmopathy: Modulation by peroxisome
proliferator-activated receptor-gamma agonists. J Clin Endocrinol
Metab. 91:614–620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antonelli A, Ferrari SM, Corrado A, Di
Domenicantonio A and Fallahi P: Autoimmune thyroid disorders.
Autoimmun Rev. 14:174–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Makgoba MW, Sanders ME, Luce GE Ginther,
Dustin ML, Springer TA, Clark EA, Mannoni P and Shaw S: ICAM-1 a
ligand for LFA-1-dependent adhesion of B, T and myeloid cells.
Nature. 331:86–88. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wenisch C, Myskiw D, Parschalk B, Hartmann
T, Dam K and Graninger W: Soluble endothelium-associated adhesion
molecules in patients with Graves' disease. Clin Exp Immunol.
98:240–244. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Crescioli C, Cosmi L, Borgogni E,
Santarlasci V, Gelmini S, Sottili M, Sarchielli E, Mazzinghi B,
Francalanci M, Pezzatini A, et al: Methimazole inhibits CXC
chemokine ligand 10 secretion in human thyrocytes. J Endocrinol.
195:145–155. 2007. View Article : Google Scholar : PubMed/NCBI
|

















