Introduction
Spinal cord injury (SCI) is the primary cause of
paraplegia. Nerve regeneration and restoration is considered
extremely difficult and is a major focus of current neuroscience
research. The secondary mechanisms that occur following SCI include
enclosed blood circulation obstacles, changes in the levels of
biologically active substances and the development of energy
metabolic disorders (1). However,
one popular hypothesis is that the primary problem resulting from
secondary spinal injury is chemical hypoxia (2), and further damage following SCI is
caused by decreased local blood flow and hypoxia. It has been
demonstrated that hypoxia inducible factor 1 (HIF-1) is specific
and sensitive to the hypoxia signaling response (3).
As well as the characteristics of its existence and
the regulation of target genes, HIF-1 is considered to be the low
oxygen related regulatory gene in the core of the most important
hypoxia-related transcription factors (4). When tissues or cells are in a hypoxic
environment, HIF-1 is one of the most important transcription
factors regulating oxygen metabolism (5). HIF-1 expression is increased following
hypoxia and may specifically bind to the hypoxia response element
of the vascular endothelial growth factor (VEGF) promoter (6), strengthening the stability of the
biological functions of VEGF and promoting VEGF expression,
transcription and translation. The primary function of VEGF in the
nervous system is associated with angiogenesis, and by promoting
angiogenesis and improving local microcirculation, VEGF may
indirectly protect the nerve from ischemic and anoxic injury
(7).
At present, research into the role that HIF-1/VEGF
signaling pathways serve in the response to SCI is very limited.
ML228 was selected for further characterization as it exhibited the
most improved potency within the chemotype and was considered to be
an interesting tool compound. ML228 represents a novel chemotype
available to the research community for the study of HIF activation
and its therapeutic potential. Not only is the compound
substantially different in structure from other known HIF
activators, ML228 also lacks the acidic functional group almost
universally present in prolyl hydroxylase inhibitors, which may be
important for certain disease applications. ML228 was demonstrated
to potently activate HIF in vitro as well as its downstream
target, VEGF (8). Therefore, the
present study evaluated the effect of ML228, which has been shown
to activate HIF as well as its downstream target VEGF (9), on the HIF-1/VEGF signaling pathway
following the induction of SCI in a rat model. In addition, the
effect of the activation of this signalling pathway on the response
to SCI was investigated. Therefore, the effect of ML228 on recovery
from SCI was evaluated in the current study.
Materials and methods
Animals and reagents
A total of 90 Sprague Dawley rats (female, 12 weeks
old; weight, 220–255 g) were purchased from the SCXK Experimental
Animal Center of Henan Province (Zhengzhou, China). Antibodies for
HIF-1α (BA0912-2) and VEGF (PB0084), and goat anti-mouse secondary
antibody (BA1051), were purchased from Wuhan Boster Biotechnology,
Ltd. (Wuhan, China). Hematoxylin and eosin (H&E) dye was
purchased from Nanjing Jiancheng Technology, Co., Ltd. (Nanjing,
China). The specific activator of HIF-1/VEGF signaling pathways
ML228 (10) was purchased from
MedChemExpress China (Shanghai, China).
Animal model
Sprague Dawley rats were randomly divided into the
following three groups (n=30 each): Sham group; the control group
(SCI without ML228 treatment); and treatment group (SCI rats that
received 1 µg/kg ML228 treatment). All rats were anaesthetized by
intraperitoneal injection of cocktail (2 ml/kg) of xylazine (1.3
mg/ml; X1126; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany),
ketamine (25 mg/ml; 16519-5; Cayman Chemical Company, Ann Arbor,
MI, USA) and acepromazine (0.25 mg/ml; A7111; Sigma-Aldrich; Merck
Millipore). Following back shaving and sterilization, an incision
was made on the back posterior to the lower thoracic region. When
the back muscles had been infiltrated, laminectomy at the T10 level
exposed the dorsal surface of the spinal cord and the lower
thoracic cord was subsequently transected using fine scissors.
Finally, the surgical wound was closed in two layers. Sham rats
underwent a similar operation to rats in the SCI groups, with the
exception that the lower thoracic cord was exposed but not
transected. Daily assistance in bladder emptying was given to rats
that had undergone SCI until spontaneous miction recovered. All
rats had ad libitum access to food and water, and were fed
with commercial rat chow comprising 0.95% calcium and 0.67%
phosphate. Rats were housed in a controlled environment at 22°C and
50% humidity with a 12 h light/dark cycle. To prevent infection,
all rats received an intramuscular injection of 80,000 units
penicillin every day for 7 days. One rat succumbed to mortality on
the first day; therefore, a new rat was added to maintain the total
number of 90. All experiments were completed according to the
protocols of the University Animal Welfare and Ethical Review
Committees of Zheng Gu hospital.
Behavioral testing
Basso, Beattie and Bresnahan (BBB) scores were
assigned on the day prior to operation, and 1, 3 and 7 days
following operation (10). All rats
were randomly assigned to groups, ensuring that initial locomotor
scores were equalized among the groups. Severe compression injury
produces spontaneous recovery to a BBB score of 4 (11). Behavioral testing was performed daily
by two individuals blinded to the treatment groups, and functional
recovery was assessed using the BBB locomotor rating scale. To
determine whether these functional recoveries were due to axonal
regeneration or activated local reflex of the segment below the
injured site, transections on all rats were performed one segment
distal (T10) from the previously injured spinal level. An
anesthesia cocktail (2 ml/kg) of ketamine, xylazine and
acepromazine was administered, and the transection was performed as
described previously (12). Samples
were frozen at −80°C prior to further experiments. Rats were
sacrificed using an overdose of pentobarbital sodium (100 mg/kg via
intraperitoneal injection; P3761; Sigma-Aldrich; Merck Millipore)
followed by transcardial perfusion.
H&E staining
H&E staining was performed as follows: Frozen
sections of spinal cord (thickness, 2 µg) were dried in air
following polysine coating, washed in distilled water for 1–2 min,
immersed in hematoxylin solution for 1 min and washed again.
Following treatment with 1% hydrochloric acid for 3 sec, sections
were washed and treated with saturated lithium carbonate solution
for 1 min, followed by washing. Then, sections were dehydrated in
80% alcohol for 1–2 min, stained in eosin for 1–2 min and
subsequently washed. Following dehydration in graded alcohol and
transparentization in xylene, sections were mounted with neutral
gum. Sections were observed using light microscopy (DMLP-MP30;
Leica Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
Transverse sections of spinal cords were lysed using
radioimmunoprecipitation assay buffer (AR0105-10; Wuhan Boster
Biotechnology, Ltd.) following treatment. Lysates (20 µl/lane) were
separated by 10% SDS-PAGE and transferred to PVDF membranes.
Membranes were probed with primary anti-HIF-1α and anti-VEGF
antibodies (both 1:500), and goat anti-mouse secondary antibody
(1:1,000) and treated with chemiluminescence detection kit
(A3417_5000-1; AppliChem GmbH, Darmstadt, Germany) according to the
manufacturer's protocol. Band intensities were quantified using
Image J (http://imagej.net/). β-actin (1:500;
BM0627; Wuhan Boster Biotechnology, Ltd.) was used as a
reference.
Immunohistochemistry
On 1, 3, 7 day following SCI or sham operation, 10
rats per group were sacrificed using an overdose of pentobarbital
sodium (100 mg/kg via intraperitoneal injection), followed by
transcardial perfusion of 0.9% saline for 5 min and then 4%
paraformaldehyde (Sigma-Aldrich; Merck Millipore) in 0.1 M
phosphate-buffered saline (PBS), pH 7.4, for 15 min at room
temperature. Two 3-mm spinal cord segments, 1.5 mm rostral and 1.5
mm caudal to the lesion epicenter, were removed from each rat and
post-fixed in the same fixative for 2 h at 4°C. Subsequently,
segments were cryopreserved in 30% sucrose solution in PBS for 2–3
days at 4°C. Spinal cords were frozen in Tissue-Tek®
optimum cutting temperature compound (Sakura Finetek USA, Inc.,
Torrance, CA, USA) and 15 µm transverse sections were cut and
mounted on gelatin-coated glass slides. Sections were pre-incubated
in a blocking solution containing 8% normal donkey serum
(SP-072-VX10, ImmunoReagents, Inc., Raleigh, NC, USA) in diluent
solution (1% bovine serum albumin (45496; EMD Millipore, Billerica,
MA, USA) and 0.3% Triton X-100 in 0.01 M PBS, pH 7.5) for 1 h at
room temperature. Sections were subsequently incubated overnight at
4°C with the anti-HIF-1α and anti-VEGF antibodies (both 1:500 in
the diluent solution. Sections were washed three times in 0.01 M
PBS for 10 min each and then incubated with the goat anti-mouse
antibodies (1:1,000) diluted in 0.01 M PBS for 1 h at room
temperature in the dark. Sections were counterstained with
toluidine blue (C0053; Shanghai Baoman Biotechnology Co., Ltd.,
Shanghai, China). Negative controls were treated with the secondary
antibody alone. All samples were examined under a phase contrast
microscope (08/357317, Olympus Corp., Tokyo, Japan.) and images
were acquired using a digital camera and image-capturing software
(both from Diagnostic Instruments, Inc., Sterling Heights, MI,
USA).
Statistical analysis
The data were analyzed by one-way analysis of
variance followed by Student-Newman-Keuls tests of multiple
comparisons to determine whether there were significant differences
between individual groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
BBB score measurement following
operation
To verify whether SCI induction was successful, the
BBB scores in the three groups were examined (Table I). There were no differences in the
BBB scores among the three groups prior to operation. Immediately
following the operation, the BBB scores in the three groups
decreased to different extents. BBB scores in the SCI groups (both
control and treatment groups) were significantly lower than in the
sham group, suggesting that the SCI was successful (P<0.05).
Following 1, 3 and 7 days, BBB scores in the three groups had
recovered to different extents and were significantly higher in the
treatment group than in the control group (P<0.05). This
suggests that administration of ML228 may alleviate SCI of the
central nervous system and relieve associated symptoms.
 | Table I.Basso, Beattie, and Bresnahan scores
at different time points (n=90). |
Table I.
Basso, Beattie, and Bresnahan scores
at different time points (n=90).
| Group | Prior to
operation | 1 day after
operation | 3 days after
operation | 7 days after
operation |
|---|
| Sham group
(n=30) | 21.00±0.00 | 18.34±0.42 | 18.96±0.33 | 19.34±0.41 |
| Treatment group
(n=30) | 21.00±0.00 |
1.51±0.55a,b |
4.37±0.51a,b |
8.49±0.43a,b |
| Control group
(n=30) | 21.00±0.00 |
1.84±0.47a |
2.09±0.44a |
4.56±0.39a |
H&E staining following
operation
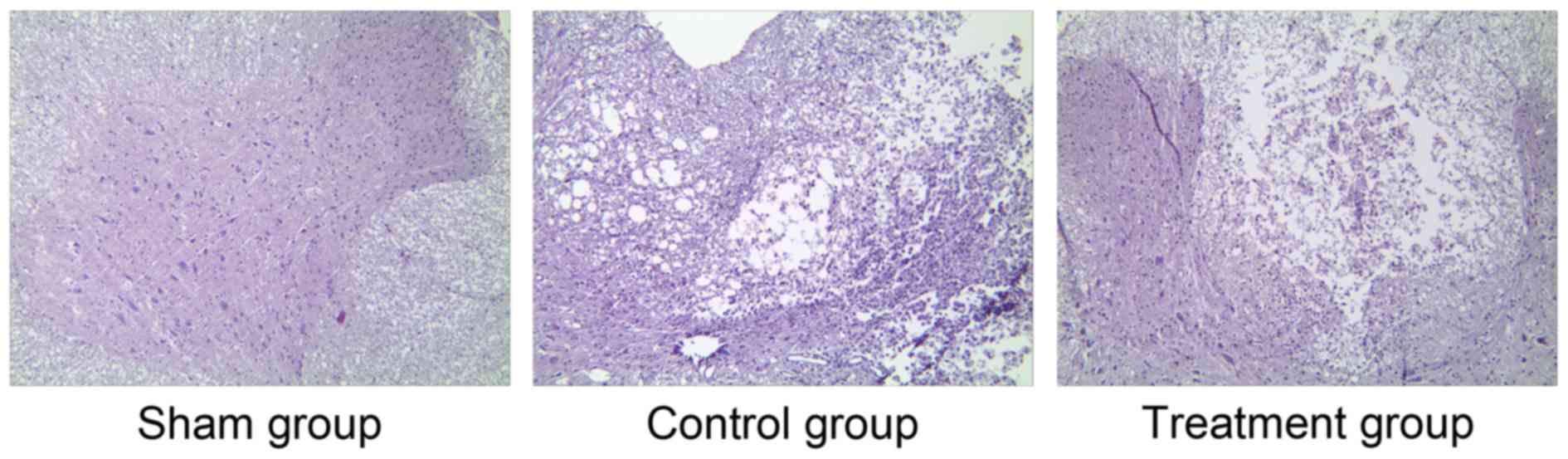
To further verify the success of SCI induction,
H&E staining of the spinal cord sections was performed. Under a
high magnification (×200), the organizational structure of the
spinal cord in the sham group was clear and there was no obvious
cell damage in any of the groups (Fig.
1). However, there were obvious disorders and a loose
arrangement of structure in the control and treatment groups.
Moreover, clear blank areas were visible, suggesting the formation
of glial scars (Fig. 1). Cell
structures in the SCI groups were incomplete and the cells
exhibited demyelinating changes, neuron vacuole degeneration and
nuclear pyknosis. Furthermore, there were a large number of
polymorphonuclear cells and extensive macrophage infiltration, and
the control group exhibited edema and massive bleeding. However,
compared with the control group, the structure in the treatment
group was more ordered and clear, and fewer necrosis areas and
glial scars were observed (Fig. 1).
These results further verified the success of SCI induction and the
effect of ML228 in relieving SCI.
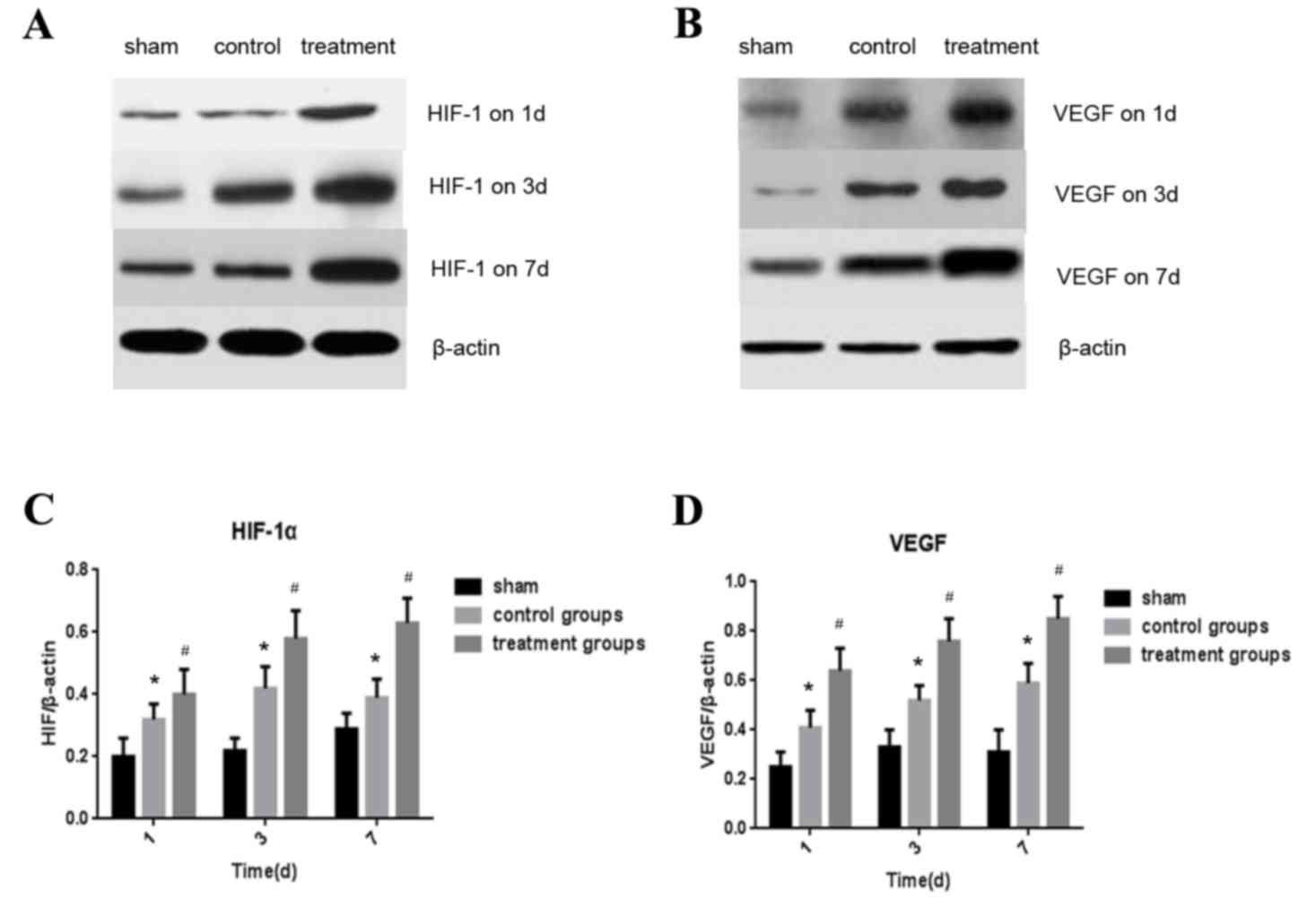
Expression of HIF-1α and VEGF in the
spinal cord detected by western blotting
The expression of HIF-1α and VEGF proteins in the
spinal cord were measured by western blotting 1, 3 and 7 days
following the operation. (Fig. 2A and
B) Levels of HIF-1α expression were significantly higher in the
control and treatment groups compared with the sham group
(P<0.05; Fig. 2C). Furthermore,
the expression of HIF-1α in the treatment group was significantly
higher than in the control group (P<0.05; Fig. 2C). Similar results were observed
regarding the expression of VEGF. In the control group, VEGF
expression of control group was significantly higher than in the
sham group (P<0.05) and expression of VEGF was significantly
higher in the treatment group compared with the control group
(P<0.05; Fig. 2D).
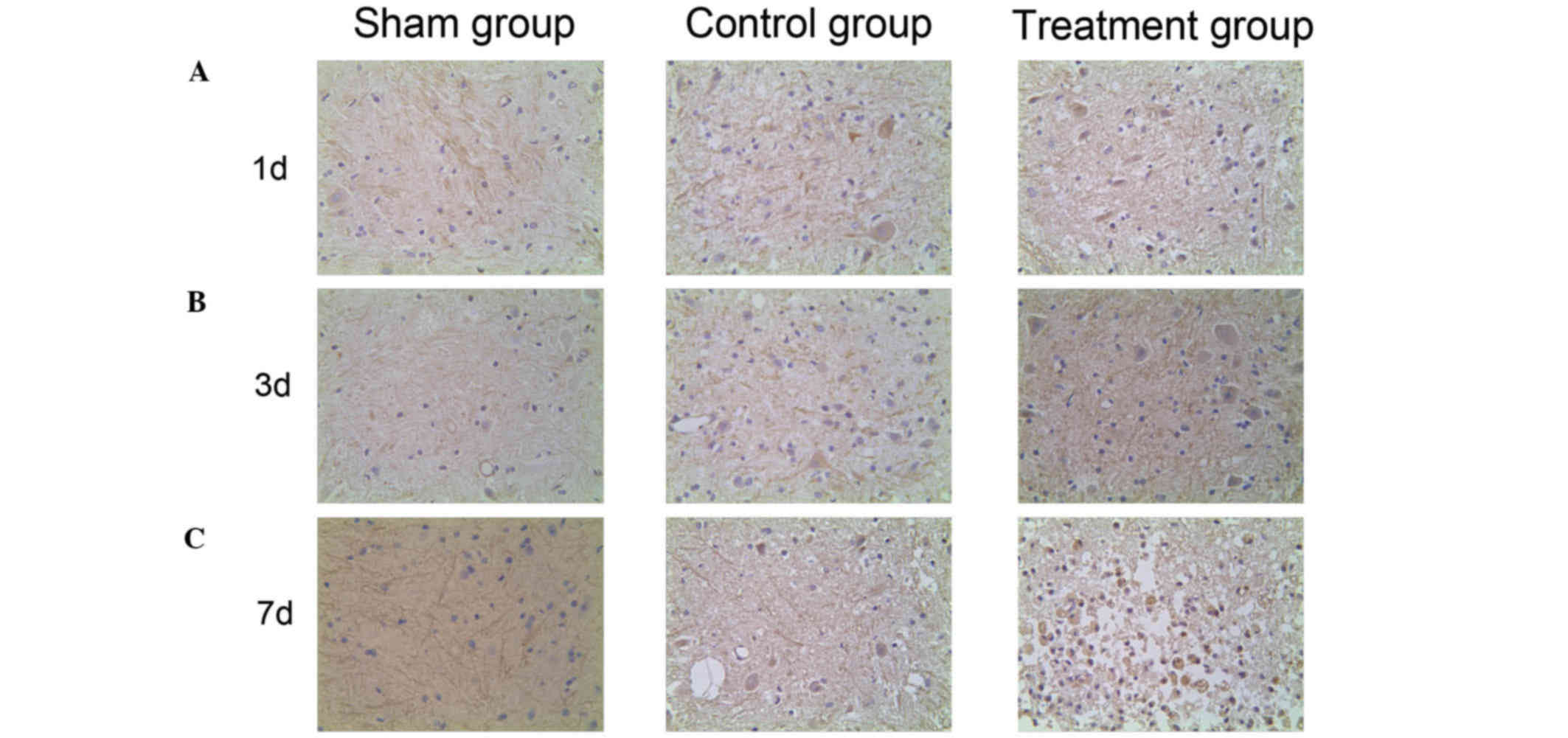
Expression of HIF-1α and VEGF in the
spinal cord detected by immunohistochemistry
To investigate the HIF-1α/VEGF signaling pathway
expression profiling of SCI in vivo, the distribution of
HIF-1α and VEGF expression was detected using immunohistochemistry.
HIF-1α-positive cells were dyed lightest in color and were most
highly dispersed in the sham group. Stronger dyeing of HIF-1α was
observed in the control group and the number of HIF-1α-positive
cells was higher than in the sham group. The strongest and most
prevalent HIF-1α staining was observed in the treatment group
(Fig. 3). This indicates that HIF-1α
expression increases following SCI, and that HIF-1α expression
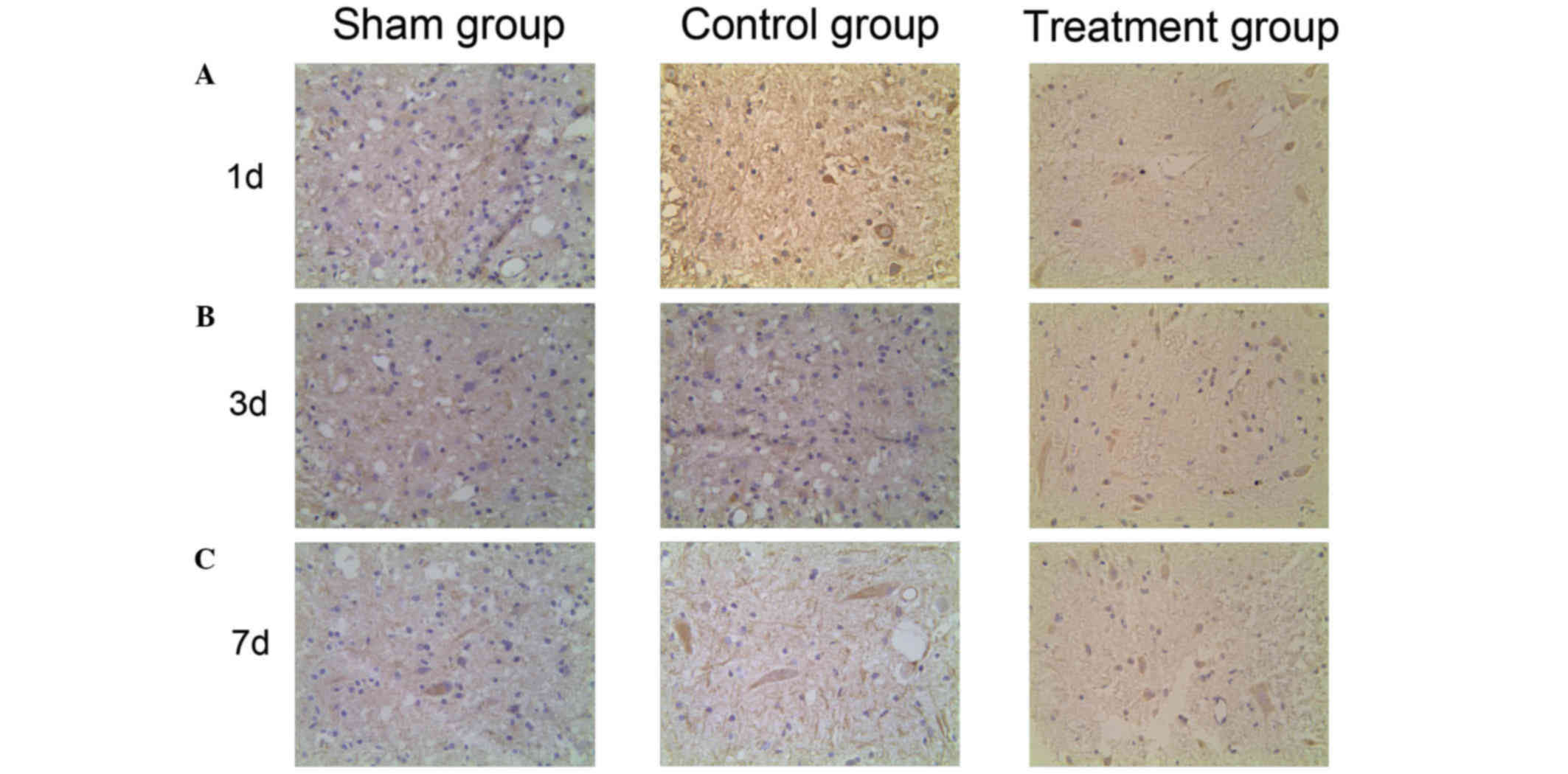
levels are further increased by ML228 treatment. VEGF positive
cells were dyed lightest in color and were highly dispersed in the
sham group. Stronger dyeing of VEGF was observed in the control
group and the number of VEGF positive cells was higher than in the
sham group. The strongest and most prevalent VEGF staining was seen
in the treatment group (Fig. 4).
This indicates that VEGF expression increases following SCI and
that levels of VEGF expression are further increased by ML228
treatment.
Discussion
At present, SCI is a medical problem worldwide;
figures indicate that the morbidity of SCI is 20–40 per million
(13). Due to improvements in
treatment, the mortality rate from SCI has decreased from ~50% in
the early 20th century to 6% at present; however, the recovery from
nerve damage remains poor.
HIF-1 is a transcriptional activator consisting of
the oxygen regulation subunit HIF-lα and the structural subunit
HIF-lβ, and regulates the expression of genes following changes in
oxygen concentrations within cells (14). HIF-lα serves a crucial role in the
regulation of multiple equilibrium in hypoxia. It is a
transcription factor that improves the adaptation of cells and
tissues to ischemic and hypoxic environments (15) and activates the transcription of a
number of factors, including erythropoietin and VEGF (16). Recently, it has been demonstrated
that HIF-1 is widely involved in the adaption to hypoxia in
mammalian cells. It is an important transcriptional regulator of
the cellular adaptation to hypoxia and is associated with a number
of diseases (17). VEGF was
initially identified as an endothelial specific growth factor,
however it has been since been demonstrated that VEGF has a variety
of different functions (18,19). VEGF is the most powerful angiogenesis
promoting factor, which can directly act on vascular endothelial
cells, stimulate the proliferation and migration of vascular
endothelial cells, and promote angiogenesis (20). Studies have suggested that VEGF is a
type of nerve protection factor that acts by a separate mechanism
to protect nerve cells and promote the survival of endothelial
cells in an anti-apoptotic way, not only in angiogenesis (21,22).
Furthermore, studies have demonstrated that, as well as having a
nervous protective effect, VEGF has the ability to promote nerve
regeneration and stimulate the growth and survival of neurons,
glial cells and axons (23–25). HIF-1 is the upstream regulatory
protein of VEGF transcription, which is itself upregulated by
ischemia or hypoxia. The role of VEGF in various tumors and hypoxic
ischemic heart disease is clear, however only a limited number of
studies into the role of VEGF in spinal cord injury have been
performed. Therefore, the present study investigated ML228, a known
HIF-1/VEGF activator, and tested whether the activation of the
HIF-1/VEGF signaling pathway was able to improve recovery from
SCI.
The results demonstrated that the functional
recovery, measured using the BBB scoring system, was improved by
treatment with ML228. In addition, very low expression of HIF-1α
and VEGF was detected in the spinal cord of the sham group.
However, expression of these proteins significantly increased
following SCI, indicating that a large number of HIF-1 and VEGF
proteins are produced in order to adapt to the hypoxic ischemia
induced by SCI in rats. Subsequently, as expected, following
administration of the HIF-1/VEGF signaling pathway activator to the
treatment group, HIF-1α and VEGF expression increased compared with
the control group, suggesting that HIF-1/VEGF activation
contributed to recovery from SCI.
The reduction of blood flow in the spinal cord is
the primary cause of necrosis and nervous dysfunction (26). Regardless of the degree of trauma,
ischemia and infarction appear in the gray matter 1–2 h after SCI,
while the extent of trauma in the white matter is related to
changes in blood flow (27). The
severity of nerve dysfunction is determined by the ischemia extent
of the white matter, and ischemia is one of the primary causes of
secondary spinal cord injury (28).
Mahon et al (29)
demonstrated that ischemia and hypoxia of the spinal cord occur
immediately following SCI, and determined that microcirculation
changes following injury are a key mechanism of secondary damage.
Therefore, other scientists have concluded that hypoxia may be a
useful technique for tissue specific gene therapy, as it has
reduced side effects and increased efficacy (30). Long et al (31) determined that in hypoxic conditions
following SCI, the upregulation of VEGF is consistent with
increasing HIF-1α in acute periods, in accordance with the results
of the current study. Furthermore, there is a correlation between
VEGF expression and angiogenesis (protecting vascular endothelial
cells, increasing blood vessel density and improving regional blood
flow), neurogenesis (antiapoptosis, neurotrophy and attenuating
axonal degradation), and locomotor ability improvement (32,33).
In conclusion, intervening to increase the activity
of the HIF-1/VEGF signaling pathway by administrating ML228
following SCI may improve the local hypoxic ischemia environment,
reduce SCI secondary injury and promote the recovery of
neurological function. Further studies on the mechanism of SCI are
required and may provide novel therapeutic strategies for future
SCI treatment.
References
|
1
|
Anwar MA, Al Shehabi TS and Eid AH:
Inflammogenesis of secondary spinal cord Injury. Front Cell
Neurosci. 10:982016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwab JM, Zhang Y, Kopp MA, Brommer B and
Popovich PG: The paradox of chronic neuroinflammation, systemic
immune suppression, autoimmunity after traumatic chronicspinal cord
injury. Exp Neurol. 258:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong W, Bazer FW, Song G and Kim J:
Expression of hypoxia-inducible factor-1 by trophectoderm cells in
response to hypoxia and epidermal growth factor. Biochem Biophys
Res Commun. 469:176–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stampas A and Tansey KE: Spinal cord
injury medicine and rehabilitation. Semin Neurol. 34:524–533. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gui L, Liu B and Lv G: Hypoxia induces
autophagy in cardiomyocytes via a hypoxia-inducible factor
1-dependent mechanism. Exp Ther Med. 11:2233–2239. 2016.PubMed/NCBI
|
|
6
|
Kimura H, Weisz A, Ogura T, Hitomi Y,
Kurashima Y, Hashimoto K, D'Acquisto F, Makuuchi M and Esumi H:
Identification of hypoxia-inducible factor1 ancillary sequence and
its function in vascular endothelial growth factor gene induction
by hypoxia and nitric oxide. J Biol Chem. 276:2292–2298. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Savas S, Savas C, Altuntas I and Adiloglu
A: The correlation between nitric oxide and vascular endothelial
growth factor in spinal cord injury. Spinal Cord. 46:113–117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing J and Lu J: HIF-1α activation
attenuates IL-6 and TNF-α pathways in hippocampus of rats following
transient global ischemia. Cell Physiol Biochem. 39:511–520. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Theriault JR, Felts AS, Bates BS, Perez
JR, Palmer M, Gilbert SR, Dawson ES, Engers JL, Lindsley CW and
Emmitte KA: Discovery of a new molecular probe ML228: An activator
of the hypoxia inducible factor (HIF) pathway. Bioorg Med Chem
Lett. 22:76–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open filed
testing in rats. Neurotrauma. 12:1–21. 1995. View Article : Google Scholar
|
|
11
|
Scheff SW, Saucier DA and Cain ME: A
statistical method for analyzing rating scale data: The BBB
locomotor score. J Neurotrauma. 19:1251–1260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu P, Graham L, Wang Y, Wu D and Tuszynski
M: Promotion of survival and differentiation of neural stem cells
with fibrin and growth factor cocktails after severe spinal cord
injury. J Vis Exp. e506412014.PubMed/NCBI
|
|
13
|
Chavez JC and LaManna JC: Activation of
hypoxia-inducibled factor-1 in the rat cerebral cortex after
transient global ischemia: Potential role of insulin-like growth
faetor-l. J Neurosci. 22:8922–8931. 2002.PubMed/NCBI
|
|
14
|
Linke S, Stojkoski C, Kewley RJ, Booker
GW, Whitelaw ML and Peet DJ: Substrate reqnirements of the
oxygen-sensing asparaginyl hydroxylase factor inhibiting
hypoxia-inducible factor. J Bid Chem. 279:14391–14397. 2004.
View Article : Google Scholar
|
|
15
|
Jantsch J and Schödel J: Hypoxia and
hypoxia-inducible factors in myeloid cell-driven host defense and
tissue homeostasis. Immunobiology. 220:305–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L,
Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional
regulation of vascular endothelial cell responses to hypoxia by
HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zimna A and Kurpisz M: Hypoxia-Inducible
Factor-1 in physiological and pathophysiological Angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basagiannis D, Zografou S, Murphy C,
Fotsis T, Morbidelli L, Ziche M, Bleck C, Mercer J and
Christoforidis S: VEGF induces signalling and angiogenesis by
directing VEGFR2 internalisation via macropinocytosis. J Cell Sci.
pii(jcs): 1882192016.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Vasta S, Di Martino A, Zampogna B, Torre
G, Papalia R and Denaro V: Role of VEGF, nitric oxide, and
sympathetic neurotransmitters in the pathogenesis of tendinopathy:
A review of the current evidences. Front Aging Neurosci. 8:1862016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Alessio A, Proietti G, Lama G, Biamonte
F, Lauriola L, Moscato U, Vescovi A, Mangiola A, Angelucci C and
Sica G: Analysis of angiogenesis related factors in glioblastoma,
peritumoral tissue and their derived cancer stem cells. Oncotarget.
2016.(Epub ahead of print). View Article : Google Scholar
|
|
21
|
Ortuzar N, Argandoña EG, Bengoetxea H and
Lafuente JV: Combination of intracortically administered VEGF and
environmental enrichment enhances brain protection in developing
rats. J Neural Transm (Vienna). 118:135–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tovar-Y-Romo LB and Tapia R: VEGF protects
spinal motor neurons against chronic excitotoxic degeneration in
vivo by activation of PI3-K pathway and inhibition of p38MAPK. J
Neurochem. 115:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song S, Park JT, Na JY, Park MS, Lee JK,
Lee MC and Kim HS: Early expressions of hypoxia-inducible factor
1alpha and vascular endothelial growth factor increase the neuronal
plasticity of activated endogenous neural stem cells after focal
cerebral ischemia. Neural Regen Res. 9:912–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding XM, Mao BY, Jiang S, Li SF and Deng
YL: Neuroprotective effect of exogenous vascular endothelial growth
factor on rat spinal cord neurons on vitro hypoxia. Chin Med
J(Engl). 118:1644–1650. 2005.PubMed/NCBI
|
|
25
|
Jin K, Mao XO and Greenberg DA: Vascular
endothelial growth factor stimulates neurite outgrowth from
cerebral cortical neurons via Rho kinase signaling. J Neurobiol.
66:236–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu K, Liang CL, Chen HJ, Chen SD, Hsu HC,
Liliang PC, Lin TK and Cho CL: Injury severity and cell death
mechanisms: Effects of concomitant hypovolemic hypotension on
spinal cord ischemia-reperfusion in rats. Exp Neurol. 185:120–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Natarajan R, Salloum FN, Fisher BJ,
Kukreja RC and Fowler AA III: Hypoxia inducible factor-1
upregulates adiponectin in diabetic mouse hearts and attenuates
post-ischemic injury. J Cardiovasc Pharmacol. 51:178–187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamosaityte S, Galli R, Uckermann O,
Sitoci-Ficici KH, Later R, Beiermeister R, Doberenz F, Gelinsky M,
Leipnitz E, Schackert G, et al: Biochemical monitoring of spinal
cord injury by FT-IR spectroscopy-effects of therapeutic alginate
implant in rat models. PLoS One. 10:e01426602015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahon PC, Hirota K and Semenza GL: FIH·1:
A novel protein that interacts with HIF-lalpha transcriptional
activivity. Genes Dev. 15:2675–2686. 2005. View Article : Google Scholar
|
|
30
|
Rhim T, Lee DY and Lee M: Hypoxia as a
target for tissue specific gene therapy. J Control Release.
172:484–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long HQ, Li GS, Hu Y, Wen CY and Xie WH:
HIF-1α/VEGF signaling pathway may play a dual role in secondary
pathogenesis of cervical myelopathy. Med Hypotheses. 79:82–84.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herrera JJ, Nesic O and Narayana PA:
Reduced vascular endothelial growth factor expression in contusive
spinal cord injury. J Neurotrauma. 26:995–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Figley S, Spratt SK, Lee G, Ando D,
Surosky R and Fehlings MG: An engineered transcription factor which
activates VEGF-A enhances recovery after spinal cord injury.
Neurobiol Dis. 37:384–393. 2010. View Article : Google Scholar : PubMed/NCBI
|