Introduction
Benign prostatic hyperplasia (BPH) is one of the
most common chronic diseases in men (1). A previous epidemiological study has
determined that >50% of men over the age of 50 years exhibit
symptoms of BPH, such as urinary urgency and retention, and the
incidence rate gradually increases with age (2). Aging and androgens are known to be the
two main factors associated with the development of BPH, and it has
previously been reported that inflammation may be another key
factor in prostatic enlargement (3,4). In
addition, repeated tissue damage due to chronic inflammation may
provoke compensatory cellular proliferation, which increases the
risk of hyperplastic growth (3,5). Chronic
inflammation is able to induce proliferation in prostate tissue by
affecting apoptotic protein expression. Cyclooxygenase (COX)-2
inhibition is able to significantly increase apoptotic activity in
prostate cells via directly upregulating B-cell lymphoma (Bcl)-2
expression (6). Cell growth in the
normal prostate is regulated via a delicate balance of cell death
and proliferation; therefore, disruption of the molecular
mechanisms that regulate these processes may lead to a state of
epithelial and stromal hyperplasia (7,8).
At present, therapy for BPH is largely based on the
use of α1-andrenergic receptor blockers and 5α-reductase
inhibitors, which relax prostatic smooth muscle and reduce
prostatic volume, respectively (9,10). Data
from a previous systematic review indicates improved urine flow,
nocturia and quality of life resulting from these treatments when
used in combination or as monotherapies (11). Recently, the use of phosphodiesterase
type 5 (PDE5) inhibitors was also recognized as an effective
treatment for BPH (12). These
pharmacological agents are effective; however, they induce
undesirable adverse effects, including blood loss, urinary
incontinence, infection, sexual dysfunction and morphological
changes in the prostate (13,14).
Therefore, it is necessary to identify novel effective herbal
products for the treatment of BPH that are also safe for long-term
use.
Ga-Gam-Nai-Go-Hyan (GGN) is an oriental herbal blend
that has been used for a long time in Korea (15). It is composed of nine herbs:
Morinda officinalis Haw, Cistanche salsa, Cornus
officinalis, Cuscuta japonica Chois, Psoralea
corylifolia L, Dendrobium nobile Lindley, Trigonella
foenumgraecum L, Foeniculum vulgare Mill and Aconitum
carmichaeli Debeaux (Table I).
It has previously been reported that GGN is effective in the
treatment of pallor, dizziness, chronic prostatitis, impotence and
BPH (15). In addition, GGN has been
used to treat patients with genital herpes, which may support its
use in the treatment of GGN, as Trichomonas vaginalis has
been detected in the urine of patients with BPH, which may indicate
an association between infections of the genital system and BPH
(16). A Korean medicine book
entitled The Treasured Mirror of Eastern Medicine reported that the
primary therapeutic facets of herbs used in GGN are similar to
those of therapeutic agents used in the treatment of BPH (17). Certain major active components of
GGN, including quercetin, kaempferol, coumarins and lignin
glycosides, have been previously reported to exhibit
anti-inflammatory and anti-oxidative qualities (18–21).
Furthermore, a recent study by our group revealed that Cistanche
salsa extract elicits an anti-proliferative effect on the
prostate tissue of rats with BPH (22). Although studies on the physiological
functions of the major active components of GGN have been
performed, the molecular mechanism(s) underlying the effect of GGN
on BPH have not yet been investigated; therefore, the aim of the
present study was to assess the anti-proliferative effects of GGN
in a testosterone-induced rat model of BPH, and to demonstrate that
it functions through regulation of the inflammatory response and
apoptotic protein expression.
 | Table I.Recipe of Ga-Gam-Nai-Go-Hyan
formulation used. |
Table I.
Recipe of Ga-Gam-Nai-Go-Hyan
formulation used.
| Species | Parts used | Weight (g) |
|---|
| Morinda
officinalis Haw | Roots | 120 |
| Cistanche
salsa | Context stem | 120 |
| Cornu
sofficinalis | Fruit | 120 |
| Cuscuta
japonica Chois | Seed | 120 |
| Psoralea
corylifolia L | Seed | 100 |
| Dendrobium
nobile Lindley | Above ground | 80 |
| Trigonella
foenumgraecum L. | Seed | 80 |
| Foeniculum
vulgare Mill. | Fruit | 40 |
| Aconitum
carmichaeli Debeaux | Roots | 20 |
Materials and methods
Materials and reagents
All herbs used to prepare GGN were purchased from
Omniherb (Dong Woo Dang Pharmacy Co. Ltd., Yeongcheon, Korea).
Finasteride was obtained from Merck & Co., Inc. (Whitehouse
Station, NJ, USA). Antibodies against inducible nitric oxide
synthase (iNOS; M-19; sc-650), COX-2 (C-20; sc-1745), procaspase-3
(E-8; sc-7272), procaspase-8 (C-20; sc-6136), B-cell lymphoma-2
(Bcl-2; C-2; sc-7382), Bcl-extra large (Bcl-xL; H-5; sc-8392),
Bcl-2-associated X protein (Bax; B-9; sc-7480), p53 (FL-393;
sc-6243), Fas (A-20; sc-1023), Fas ligand (Fas-L; C-178; sc-6237)
and β-actin (ACTBD11B7; sc-81178) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). An antibody against
Fas-associated protein with death domain (FADD; ab24533) was
purchased from Abcam (Cambridge, UK). The horeseadish
peroxidase-conjugated secondary antibodies (goat anti-rabbit,
111-035-003; rabbit anti-mouse, 315-035-003; and donkey anti-goat,
705-035-003) were purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). All other reagents were
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany).
Preparation of GGN
GGN consists of nine different herbs; Morinda
officinalis How (120 g), Cistanche deserticola Y. C. Ma.
(120 g), Cornus officinalis Sieb. et. Zucc. (120 g),
Cuscuta chinensis Lamark (120 g), Psoralea
corylifolia L. (100 g), Dendrobium nobile Lindl. (80 g),
Trigonella foenum-graecum L. (80 g), Foeniculum
vulgare Mill. (40 g) and Aconitum carmichaeli Debx. (20
g). The herbs had a moisture content of <13% by weight and were
air-dried. The combination of herbs was extracted with 50% (v/v)
ethanol-water at 60°C for 8 h. The extracts were then filtered
through 15-µm cartridge paper and ethanol was removed via vacuum
rotary evaporation (Eyela; Tokyo Rikakikai Co., Ltd., Tokyo Japan).
The concentrates were freeze-dried and the yield was 12%. Powders
were dissolved in distilled water prior to experiments and residual
powders were stored at −20°C.
Animals
Ten-week-old male Wistar rats (n=24; weight, 200±20
g) were purchased from Daehan Biolink Co., Ltd., (Republic of
Korea). Rats were housed under constant conditions (temperature,
22±2°C; humidity, 55±9%; 12 h light/dark cycle; ad libitum
access to food and water) in accordance with the Guide for the Care
and Use of Laboratory Animals. All procedures were approved by the
Ethics Committee for Animal Care and the Use of Laboratory Animals
at Sang-ji University (Wonju, Korea; approval documents, 2013-03;)
and conducted in accordance with University guidelines. Rats were
randomly distributed into four groups (n=6 in each); the
sham-operated group (Con; administered with 200 µl distilled water
orally), the BPH model group (BPH), the BPH-induced group
administrated with finasteride (Fina; 5 mg/kg/day; p.o. Merck &
Co., Inc.) and the BPH-induced group administrated with GGN (GGN;
100 mg/kg/day; p.o.). BPH was induced in rats via castration and
subsequent subcutaneous injections of 100 µl testosterone
propionate (Waco Pure Chemical Industries, Ltd., Osaka, Japan; 10
mg/kg/day), as previously reported (23). At the end of the four-week period,
body weight was recorded and rats were fasted for 12 h. The
following day, all rats were sacrificed by cervical dislocation
following anesthetization with Zoletil 50 (20 mg/kg
intraperitoneally; Virbac, Carros, France), and blood samples were
obtained via cardiac puncture. Prostatic tissue was excised,
rinsed, weighed and stored at −70°C until use.
Prostate weight to body weight
ratio
Prostatic tissues were harvested, rinsed, and
weighed immediately following sacrifice. The prostate weight to
body weight (PW/BW) ratio was calculated. The relative prostate
weight ratio was calculated as follows: Relative prostate weight
ratio=prostate weight of rats in the experimental groups (BPH,
Fina, GGN)/prostate weight of rats in the Con group.
Serum analysis
Serum concentrations of testosterone were determined
using a testosterone enzyme immunoassay kit (582701; Cayman
Chemical Co., Ann Arbor, MI, USA). Serum concentrations of
dihydrotestosterone (DHT) were determined via enzymatic methods
using a commercially available assay kit (11-DHTHU-E01; ALPCO,
Salem, NH, USA) according to the manufacturer's protocol.
Histological analysis
The prostatic tissue was fixed in 4% buffered
formalin and embedded in paraffin, and 4-µm thick sections were
cut. The sections were stained with hematoxylin and eosin for
histological examination, and images were acquired using an SZX10
microscope (Olympus Corp., Tokyo, Japan). The thickness of the
epithelium in the prostate tissue (TETP) was measured using the
Leica Application Suite software (LAS ver. 3.3.0; Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
Western blot analysis
The prostatic tissue from each rat was homogenized
in PRO-PREP lysis buffer (Intron Biotechnology Inc., Seongnam,
Korea). Tissue extracts were centrifuged at 16,000 × g (4°C)
for 20 min, and the resulting supernatant was transferred to a
clean tube. The protein concentration was determined using a
Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) according to the manufacturer's protocol. Aliquots of each
protein sample (30 µg) were separated by 10–12% SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane (Immobilion-P
transfer membrane; Merck Millipore). The membranes were blocked
with 2.5% skimmed milk at 4°C for 30 min and incubated overnight
with 1:1,000 dilutions of the primary antibodies (anti-iNOS,
anti-COX-2, anti-caspase-3, anti-caspase-8, anti-Bcl-xL, anti-Bax,
anti-P-53, anti-Fas, anti-Fas-L, anti-FADD and anti-β-actin). The
blots were washed three times with Tween-20/TBS and then incubated
with 1:2,000 dillutions of corresponding secondary antibodies
(Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. Blots
were again washed three times with Tween-20/TBS and the
immunoreactive protein bands were visualized via enhanced
chemiluminescence, and the developed blots were exposed to X-ray
film (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Each blot
was performed in triplicate and densitometric analysis was
performed using Bio-rad Quantity One Software (version 4.6.3;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean for six rats. Data were analyzed using one-way analysis of
variance with Dunnett's test and P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS statistical analysis software
(version 19.0; International Business Machines, Armonk, NY,
USA).
Results
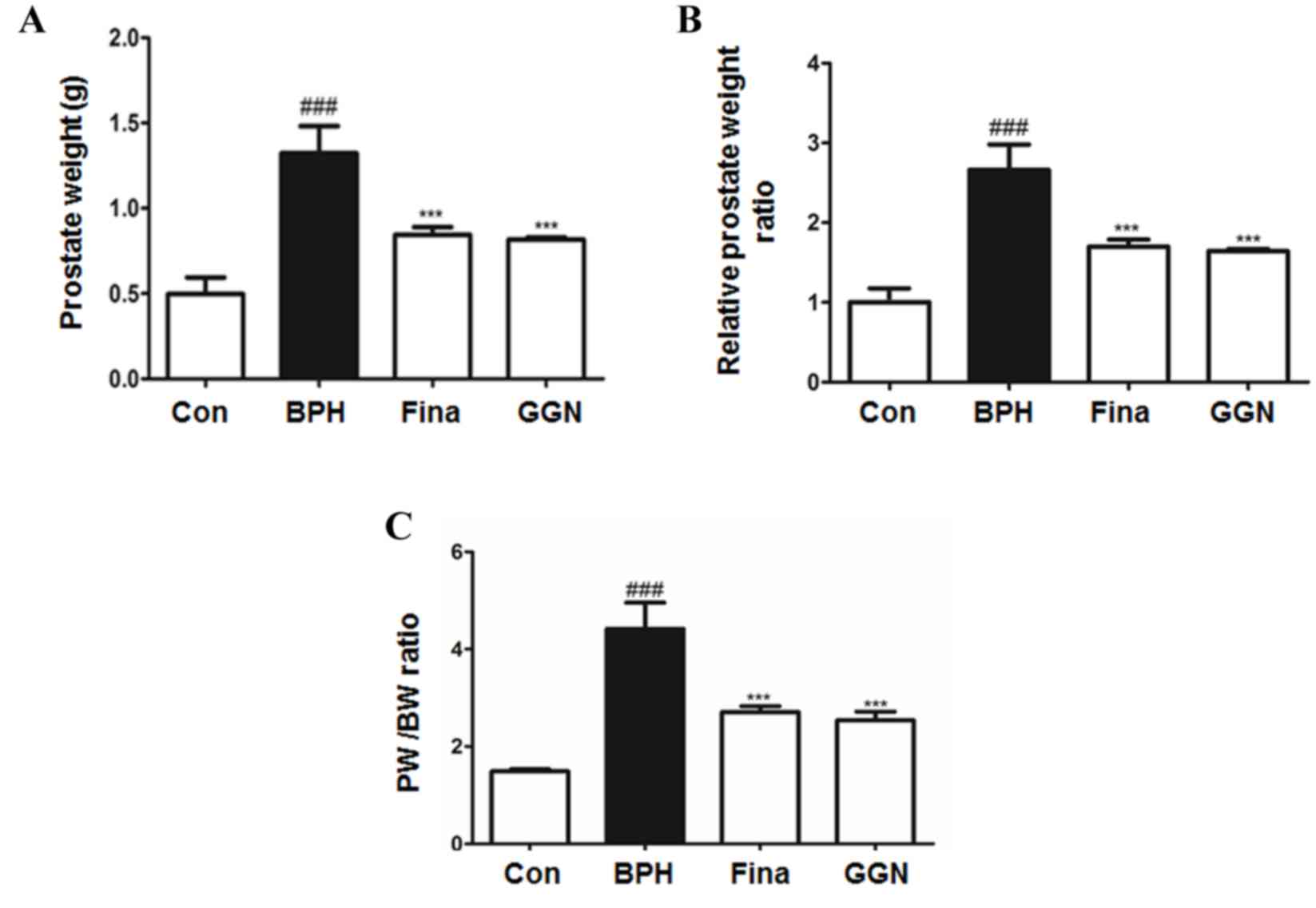
GGN decreases prostate weight in a rat
model of BPH
The mean prostate weight of rats in the BPH group
was significantly higher than that of rats in all other groups
(P<0.001). Compared with BPH rats, the prostate weight of rats
in the Fina and GGN groups were significantly decreased
(P<0.001; Fig. 1A). The relative
prostate weight ratio in the BPH group was 2.66 times that in the
Con group, which was a significant increase (P<0.001), whereas
in the GGN group, prostate weight was only 1.64 times that in the
Con group (Fig. 1B). In addition,
the PW/BW ratio in the BPH group was also significantly higher than
that in the other groups (P<0.001). Compared with the BPH group,
the PW/BW ratio was significantly lower in the Fina and GGN groups
(P<0.001), whereas no significant difference was observed
between the PW/BW ratios of the GGN group (2.54) and the Fina group
(2.71).
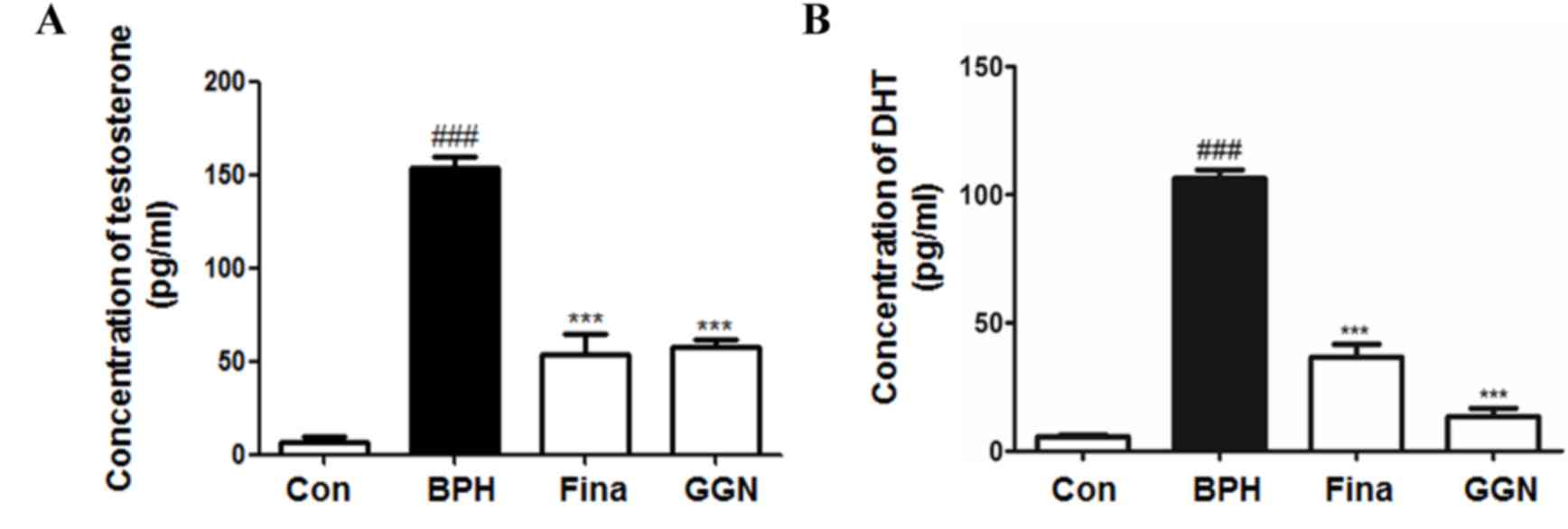
GGN decreases serum testosterone and
DHT levels in a rat model of BPH
The serum testosterone and DHT levels of the rats in
each group are displayed in Fig. 2.
The concentrations of the testosterone and DHT in the BPH group
were significantly higher than those in all other groups
(P<0.001). In the Fina and GGN groups, serum testosterone and
DHT levels were significantly lower than those in the BHP group
(P<0.001). Of note, DHT levels in the GGN group were markedly
lower than those in the Fina group.
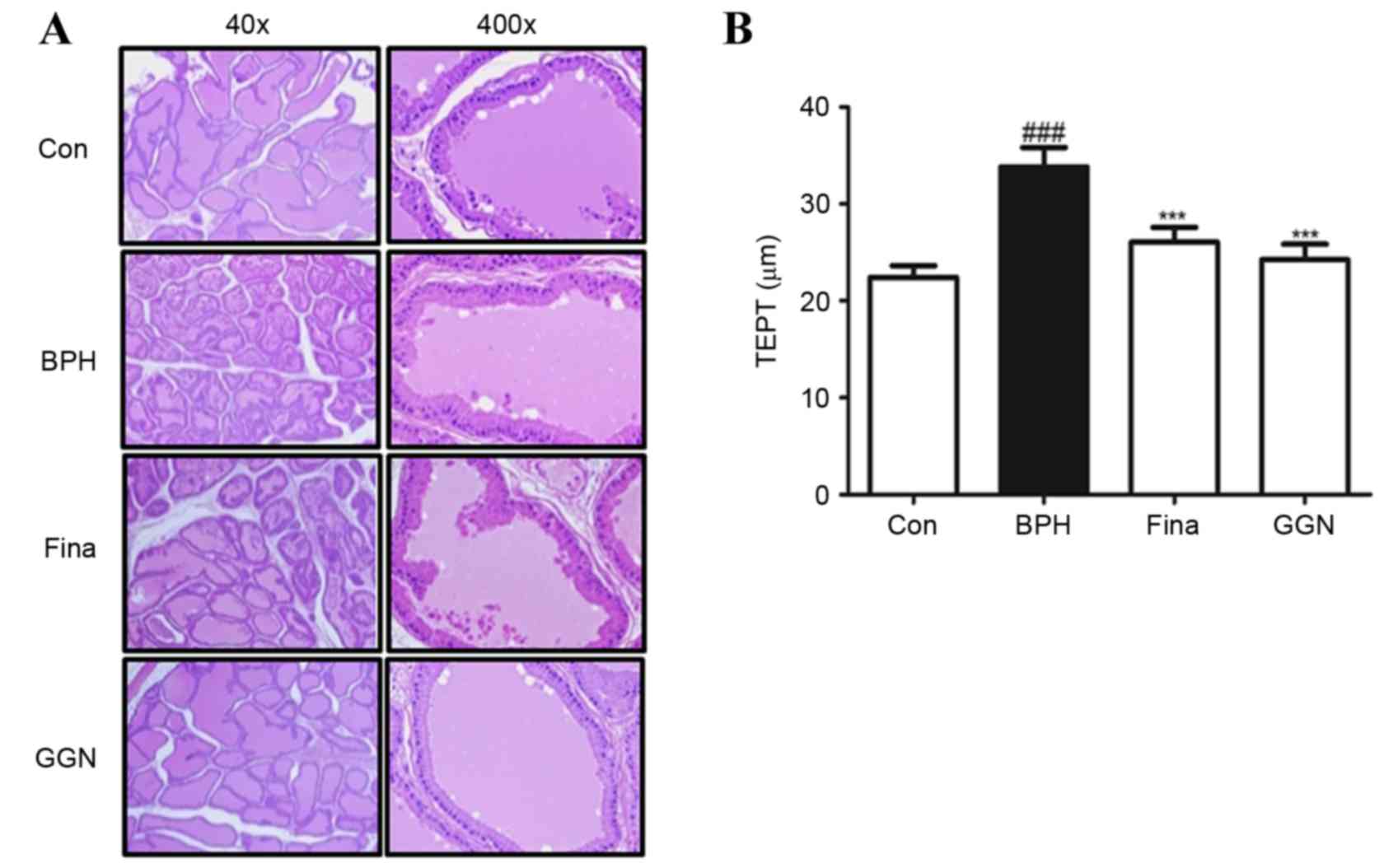
GGN attenuates testosterone-induced
morphological changes of the prostate gland
The effect of GGN on prostate gland morphology was
investigated by histological analysis (Fig. 3). Rats in the BPH group exhibited
histological changes typical of prostatic hyperplasia: Thickened
glandular epithelium, vacuolated cytoplasm pointing into the
glandular lumen and a decreased glandular luminal area (Fig. 3A). Administration of GGN for four
weeks suppressed these typical histological patterns. The TETP was
significantly higher in the BPH-induced group than in the other
groups (P<0.001; Fig. 3B). In
brief, histologic examination revealed that administration of
finasteride or GGN ameliorated the major features of prostatic
hyperplasia observed in the BPH group, although certain minor
features of prostatic hyperplasia remained.
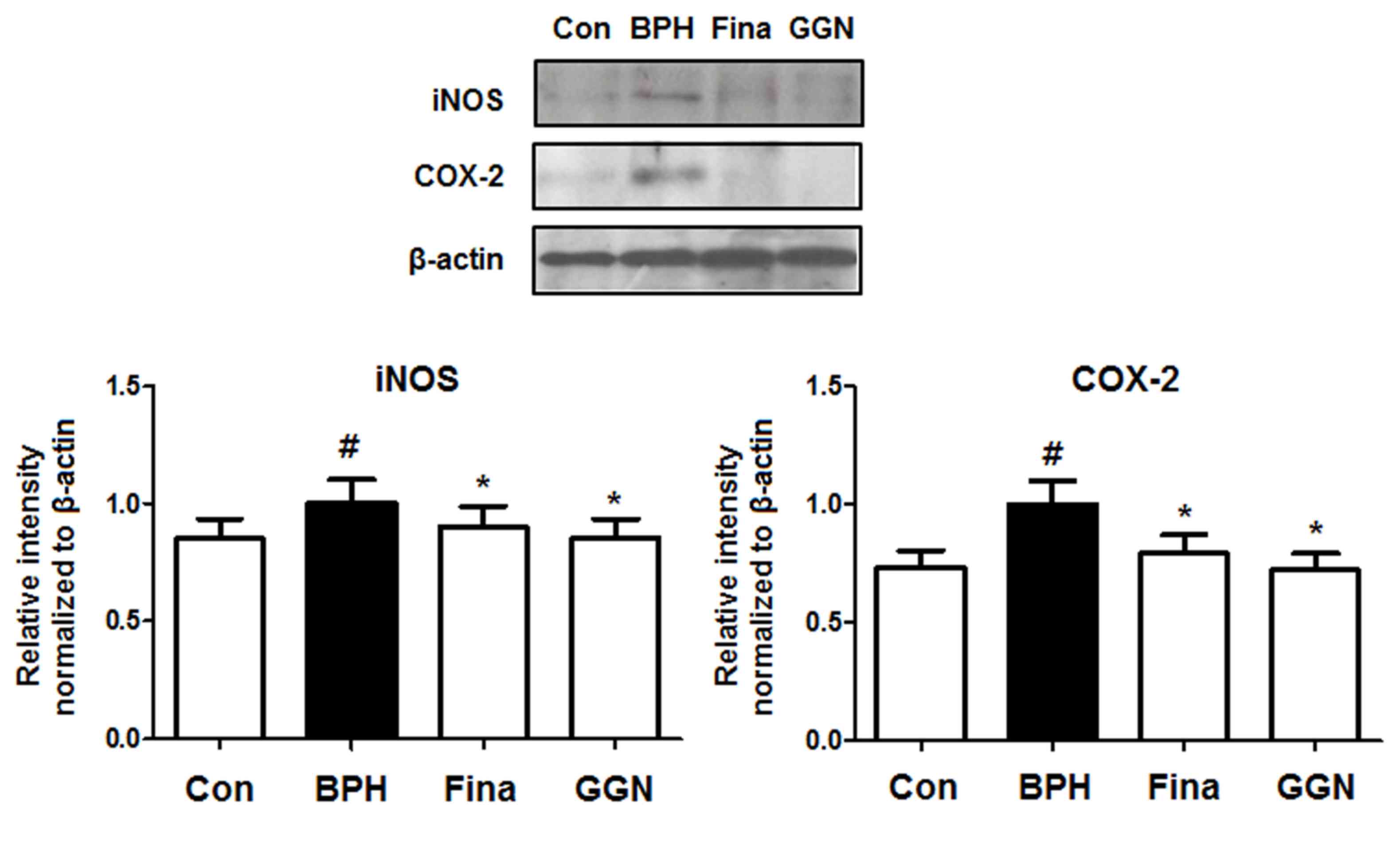
GGN decreases inflammatory proteins in
prostatic tissue of rats with BPH
Inflammation is associated with the increased
proliferation of prostatic epithelial cells (24). In patients with BPH, iNOS, which
provides a sustained release of reactive nitrogen species that may
induce cell damage, is typically present (25). In addition, COX-2, which generates
pro-inflammatory prostaglandins, is detected in inflammatory cells
(4). In the present study, the
levels of iNOS and COX-2 in the prostatic tissue of BPH-induced
rats were analyzed by western blotting to investigate the effects
of GGN on inflammation. Compared with the Con group, treatment with
testosterone increased the levels of iNOS and COX-2 in the
BPH-induced group. By contrast, the Fina and GGN groups showed
markedly reduced levels of these inflammatory proteins compared
with the BPH group (Fig. 4).
GGN enhances apoptotic signaling in
prostatic tissue of rats with BPH
BPH arises from an imbalance between cell
proliferation and apoptosis (3). The
two main apoptotic pathways are the intrinsic and extrinsic
pathways. In the extrinsic pathway, apoptosis is induced by death
activators binding to receptors at the cell surface and
accumulation of the adaptor molecule FADD, which leads to the
activation of initiator caspase (26). In the present study, the expression
levels of proteins in the extrinsic pathway were assessed (Fig. 5A), and the Fina and GGN-treated group
exhibited higher protein levels of Fas-L, Fas, FADD and p53, and
lower protein levels of procaspase-8 and procaspase-3 compared with
the levels in the BPH group.
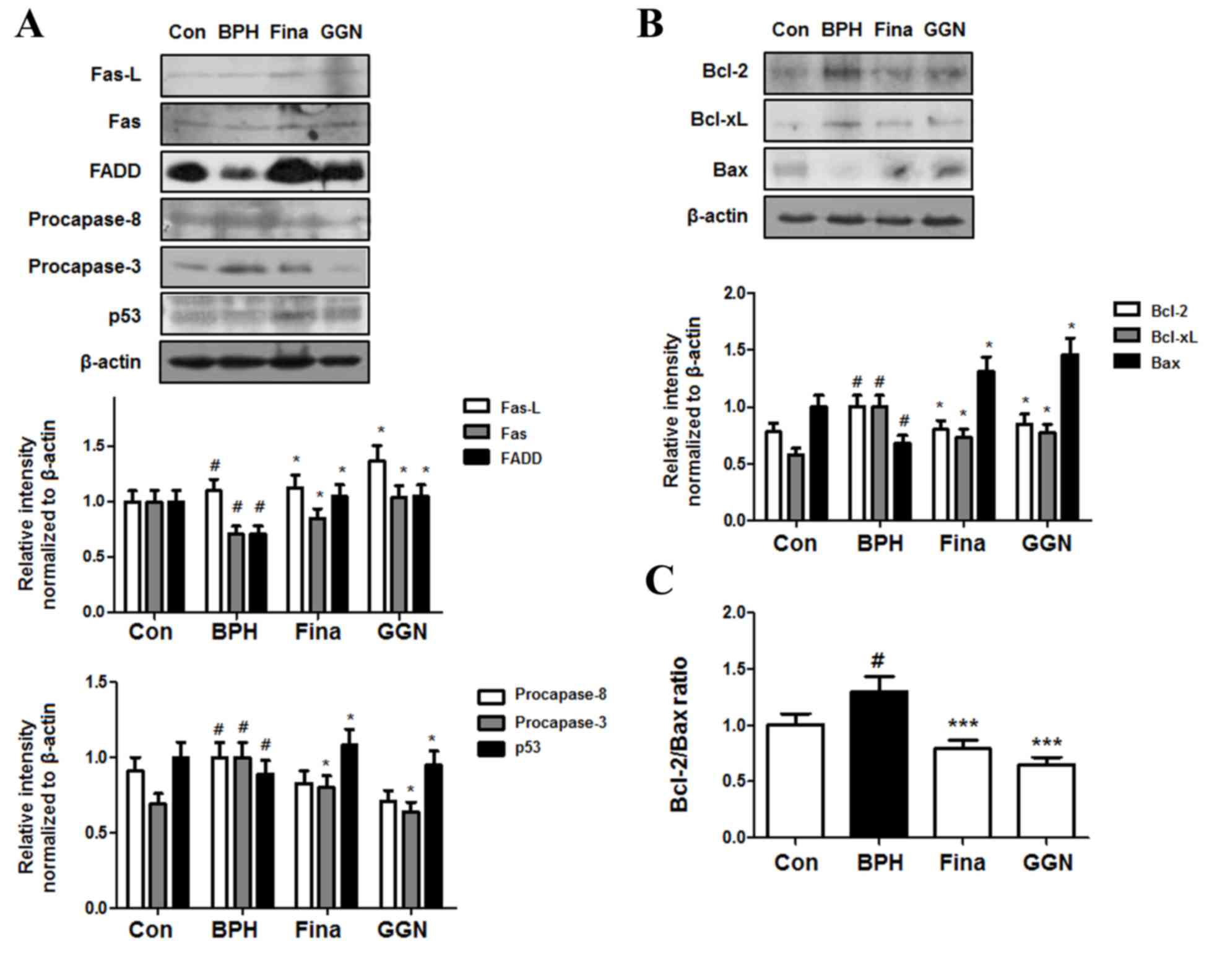 | Figure 5.Effect of GGN administration on the
expression of apoptotic proteins. (A) The expression levels of
P-53, death receptor proteins and procaspase-3 were determined via
western blotting using specific antibodies. (B) The expression
levels of Bcl-2 family proteins were determined by western blotting
using specific antibodies. β-actin was used as internal controls.
(C) Densitometric analysis of Bcl-2 and Bax bands was performed,
and the data (relative density normalized to β-actin) were plotted
as the Bcl-2/Bax ratio. The data shown represents mean ± standard
error of six rats per group. #P<0.05 vs. Con group;
*P<0.05, ***P<0.001 vs. BPH group. GGN, Ga-Gam-Nai-Go-Hyan;
Bcl, B-cell lymphoma; Bax, bcl-2-associated X protein; BPH, benign
prostatic hyperplasia; fina, finasteride-administered group; FADD,
Fas-associated protein with death domain; Fas-L, Fas ligand. |
In the intrinsic pathway, apoptosis, including
disruption of the mitochondrial membrane potential, is critically
regulated by Bcl-2 family proteins (27). As demonstrated in Fig. 5B, the Fina and GGN group exhibited
lower levels of the anti-apoptotic proteins Bcl-2 and Bcl-xL, and
higher levels of the pro-apoptotic protein Bax than the BPH group.
Therefore, although the ratio of Bcl-2 to Bax was significantly
increased in the BPH group compared with the control group
(P<0.05), the ratio decreased significantly following treatment
with Fina and GGN (P<0.001), suggesting that GGN-induced
apoptosis is regulated by the Bcl-2 family of proteins, which are
important mediators of apoptosis (Fig.
5C).
Discussion
BPH is the most prevalent urologic health concern in
the male population, with a histological prevalence at autopsy of
50% in men aged 50–60 years and 90% in men aged >80 years
(28). It is characterized by the
non-malignant overgrowth of prostatic tissue surrounding the
urethra, and is usually present with one or more co-morbidities,
including bladder dysfunction and hypertrophy, which may lead to
acute urinary retention (2).
Previously, androgens and age have been considered as the main
determinants of prostate enlargement; however, the potentially
important role of chronic inflammation in the pathogenesis of BPH
has recently been identified (29).
Hormonal imbalance has an important role in the
pathogenesis of BPH via induction of inflammatory responses and
reduction of apoptosis, and testosterone has been shown to be
associated with BPH (4). In the
present study, rats were administered testosterone for four weeks
to induce BPH. Prostate weight ratios and DHT levels in the BPH
group were higher than those in the Con group, indicating that
testosterone administration successfully induced BPH. However, four
weeks of GGN treatment effectively inhibited BPH, and these effects
were comparable to those observed in the Fina group.
In the inflammatory cells of the prostate, iNOS is
the factor that predominantly activates reactive nitrogen, which is
able to damage cells (30).
Hyperplastic prostate tissue is characterized by increased NOS
expression in the epithelial cells compared with normal tissue
(31). In addition, nitric oxide may
also be converted by COX enzymes to proinflammatory prostaglandins
(32), and COX-2 has been detected
in the epithelium and interstitial spaces of inflammatory cells
(30). In the present study, the
protein expression levels of COX-2 and iNOS were analyzed in a rat
model of BPH using western blotting. The levels of COX-2 and iNOS
proteins in the BPH group were higher than those in the Con group.
By contrast, COX-2 and iNOS levels in the GGN group were lower than
those in the BPH group. These results indicated that GGN may
suppress the growth of prostatic cells through its
anti-inflammatory effects.
In mammalian cells, apoptosis proceeds via the
extrinsic and intrinsic pathways. The extrinsic pathway is
triggered by the binding of extracellular signaling factors to
death receptors at the plasma membrane. Fas is a cell-surface
receptor protein belonging to the tumor necrosis factor receptor
superfamily, and its physiological ligand, Fas-L, is a member of
the corresponding tumor necrosis factor cytokine family (33). Fas-L-Fas signaling triggers apoptosis
through FADD, and activation of the aspartate-specific cysteine
protease, caspase-8, initiating a cascade of caspase activation
leading to phagocytosis of the cell (34). The intrinsic pathway is activated by
intracellular damage, such as oxidative stress, and is controlled
by the mitochondria, which are regulated by the Bcl-2 protein
family. Bcl-2 proteins are categorized based on their structure and
function; this family includes anti-apoptotic proteins, such as
Bcl-2 and Bcl-xL, and proapoptotic proteins, such as Bax (27). In various systems, the Bcl-2 family
modulates apoptosis, with the Bcl-2/Bax ratio serving as a rheostat
to determine a cell's susceptibility to apoptosis (35). p53 induces the release of various
proapoptotic factors from the intermembrane space of the
mitochondria (36). p53 interacts
with Bax by binding to its Bcl-2 homolog 3 domains, leading to
cytochrome C release by mitochondria into the cytoplasm
(37). Cytochrome C activates
caspase-9, which in turn activates a series of caspases, including
caspase-3 (38). In the present
study, it was demonstrated that administration of GGN increased the
levels of Fas, Fas-L, FADD and p53, and decreased the levels of
procaspase-3 and procaspase-8. In addition, GGN reduced the levels
of Bcl-2 and Bcl-xL and increased the levels of Bax protein. These
results indicated that GGN-induced apoptosis in a rat model of BPH
is most likely due to stimulation of death receptors and the
mitochondrial pathway.
In conclusion, the findings of the present study
suggested that GGN is able to prevent BPH by regulating
inflammatory responses and apoptosis. Based on this hypothesis, GGN
may have applications as a therapeutic agent for the treatment of
patients with BPH.
Acknowledgements
The present study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry of
Health & Welfare, Republic of Korea (grant no. HI16C0477).
References
|
1
|
Boyle P, Maisonneuve P and Napalkov P:
Incidence of prostate cancer will double by the year 2030: The
argument for. Eur Urol. 29:(Suppl 2). S3–S9. 1996.
|
|
2
|
McVary KT: BPH: Epidemiology and
comorbidities. Am J Manag Care. 12:(Suppl 5). S122–S128.
2006.PubMed/NCBI
|
|
3
|
Sciarra A, Di Silverio F, Salciccia S,
Gomez AM Autran, Gentilucci A and Gentile V: Inflammation and
chronic prostatic diseases: Evidence for a link? Eur Urol.
52:964–972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sciarra A, Mariotti G, Salciccia S, Gomez
A Autran, Monti S, Toscano V and Di Silverio F: Prostate growth and
inflammation. J Steroid Biochem Mol Biol. 108:254–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robert G, Descazeaud A, Nicolaïew N, Terry
S, Sirab N, Vacherot F, Maillé P, Allory Y and de la Taille A:
Inflammation in benign prostatic hyperplasia: A 282 patients'
immunohistochemical analysis. Prostate. 69:1774–1780. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Silverio F, Bosman C, Salvatori M,
Albanesi L, Pannunzi L Proietti, Ciccariello M, Cardi A, Salvatori
G and Sciarra A: Combination therapy with rofecoxib and finasteride
in the treatment of men with lower urinary tract symptoms (LUTS)
and benign prostatic hyperplasia (BPH). Eur Urol. 47:72–78;
discussion 78–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholson TM and Ricke WA: Androgens and
estrogens in benign prostatic hyperplasia: Past, present and
future. Differentiation. 82:184–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyprianou N, Tu H and Jacobs SC: Apoptotic
versus proliferative activities in human benign prostatic
hyperplasia. Hum Pathol. 27:668–675. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rittmaster RS: 5alpha-reductase inhibitors
in benign prostatic hyperplasia and prostate cancer risk reduction.
Best Pract Res Clin Endocrinol Metab. 22:389–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartsch G, Rittmaster RS and Klocker H:
Dihydrotestosterone and the concept of 5alpha-reductase inhibition
in human benign prostatic hyperplasia. Eur Urol. 37:367–380. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tacklind J, Fink HA, Macdonald R, Rutks I
and Wilt TJ: Finasteride for benign prostatic hyperplasia. Cochrane
Database Syst Rev. CD0060152010.PubMed/NCBI
|
|
12
|
Oelke M, Bachmann A, Descazeaud A,
Emberton M, Gravas S, Michel MC, N'dow J, Nordling J and de la
Rosette JJ: European Association of Urology: EAU guidelines on the
treatment and follow-up of non-neurogenic male lower urinary tract
symptoms including benign prostatic obstruction. Eur Urol.
64:118–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kyprianou N: Doxazosin and terazosin
suppress prostate growth by inducing apoptosis: Clinical
significance. J Urol. 169:1520–1525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morelli A, Filippi S, Comeglio P,
Sarchielli E, Chavalmane AK, Vignozzi L, Fibbi B, Silvestrini E,
Sandner P, Gacci M, et al: Acute vardenafil administration improves
bladder oxygenation in spontaneously hypertensive rats. J Sex Med.
7:107–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JKSB, Lee EJ and Kim HK: Study on the
treatment of benign prostatic hyperplasia (BPH) in oriental
medicine. The Journal of Korean Oriental Medical Society.
19:211–227. 1998.
|
|
16
|
Kim JH, Kim SS, Han IH, Sim S, Ahn MH and
Ryu JS: Proliferation of prostate stromal cell induced by benign
prostatic hyperplasia epithelial cell stimulated with Trichomonas
vaginalis via crosstalk with mast cell. Prostate. 76:1431–1444.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heo J: The Treasured Mirror of Eastern
Medicine. 2. 3rd. Yoekang Press; Seoul: 2005
|
|
18
|
Zou W, Liu W, Yang B, Wu L, Yang J, Zou T,
Liu F, Xia L and Zhang D: Quercetin protects against
perfluorooctanoic acid-induced liver injury by attenuating
oxidative stress and inflammatory response in mice. Int
Immunopharmacol. 28:129–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Devi KP, Malar DS, Nabavi SF, Sureda A,
Xiao J, Nabavi SM and Daglia M: Kaempferol and inflammation: From
chemistry to medicine. Pharmacol Res. 99:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng KW, Yu Q, Liao LX, Song FJ, Lv HN,
Jiang Y and Tu PF: Anti-neuroinflammatory effect of MC13, a novel
coumarin compound from Condiment Murraya, through inhibiting
lipopolysaccharide-induced TRAF6-TAK1-NF-kB, P38/ERK MAPKS and
Jak2-Stat1/Stat3 pathways. J Cell Biochem. 116:1286–1299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HS, Kang P, Kim KY and Seol GH:
Foeniculum vulgare Mill. Protects against
lipopolysaccharide-induced acute lung injury in mice through
ERK-dependent NF-kappaB activation. Korean J Physiol Pharmacol.
19:183–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon EJ, Chung KS and An HJ:
Anti-proliferation effects of Cistanches salsa on the progression
of benign prostatic hyperplasia. Can J Physiol Pharmacol.
94:104–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo QL, Ding QL and Wu ZQ: Effect of
baicalein on experimental prostatic hyperplasia in rats and mice.
Biol Pharm Bull. 27:333–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Nunzio C, Kramer G, Marberger M,
Montironi R, Nelson W, Schröder F, Sciarra A and Tubaro A: The
controversial relationship between benign prostatic hyperplasia and
prostate cancer: The role of inflammation. Eur Urol. 60:106–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baltaci S, Orhan D, Gögüs C, Türkölmez K,
Tulunay O and Gögüs O: Inducible nitric oxide synthase expression
in benign prostatic hyperplasia, low- and high-grade prostatic
intraepithelial neoplasia and prostatic carcinoma. BJU Int.
88:100–103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu TH, Lan KY, Yang MD, Lin JJ, Hsia TC,
Wu CT, Yang JS, Chueh FS and Chung JG: Diallyl sulfide promotes
cell-cycle arrest through the p53 expression and triggers induction
of apoptosis via caspase- and mitochondria-dependent signaling
pathways in human cervical cancer Ca Ski cells. Nutr Cancer.
65:505–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petros AM, Olejniczak ET and Fesik SW:
Structural biology of the Bcl-2 family of proteins. Biochim Biophys
Acta. 1644:83–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elkahwaji JE: The role of inflammatory
mediators in the development of prostatic hyperplasia and prostate
cancer. Res Rep Urol. 5:1–10. 2012.PubMed/NCBI
|
|
29
|
Fibbi B, Penna G, Morelli A, Adorini L and
Maggi M: Chronic inflammation in the pathogenesis of benign
prostatic hyperplasia. Int J Androl. 33:475–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chughtai B, Lee R, Te A and Kaplan S: Role
of inflammation in benign prostatic hyperplasia. Rev Urol.
13:147–150. 2011.PubMed/NCBI
|
|
31
|
Wang W, Bergh A and Damber JE: Chronic
inflammation in benign prostate hyperplasia is associated with
focal upregulation of cyclooxygenase-2, Bcl-2, and cell
proliferation in the glandular epithelium. Prostate. 61:60–72.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugar LM: Inflammation and prostate
cancer. Can J Urol. 13:(Suppl 1). 46–47. 2006.PubMed/NCBI
|
|
33
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor signaling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boatright KM, Renatus M, Scott FL,
Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP,
Green DR and Salvesen GS: A unified model for apical caspase
activation. Mol Cell. 11:529–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salakou S, Kardamakis D, Tsamandas AC,
Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos
DS, Papapetropoulos T, Petsas T and Dougenis D: Increased Bax/Bcl-2
ratio up-regulates caspase-3 and increases apoptosis in the thymus
of patients with myasthenia gravis. In Vivo. 21:123–132.
2007.PubMed/NCBI
|
|
36
|
Sun Y, Holley AK and St Clair DK: p53
regulation of energy metabolism and mitochondria regulation of p53
in cancer cells: An insight into the role of manganese superoxide
dismutase. Curr Pharm Biotechnol. 14:261–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galluzzi L, Morselli E, Kepp O, Tajeddine
N and Kroemer G: Targeting p53 to mitochondria for cancer therapy.
Cell Cycle. 7:1949–1955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Teissier S, Ben Khalifa Y, Mori M, Pautier
P, Desaintes C and Thierry F: A new E6/P63 pathway, together with a
strong E7/E2F mitotic pathway, modulates the transcriptome in
cervical cancer cells. J Virol. 81:9368–9376. 2007. View Article : Google Scholar : PubMed/NCBI
|