Introduction
Huperzine A is an acetylcholinesterase inhibitor
isolated from the Chinese herb Huperzia serrata. It easily
penetrates the blood-brain barrier (1) and possesses diverse pharmacological
functions (2,3). Huperzine A protects cortical neurons
from β-amyloid-induced apoptosis in vitro (4), and regulates the expression of nerve
growth factor and its receptors (5).
Importantly, it selectively inhibits acetylcholinesterase (6). Compared with other acetylcholinesterase
inhibitors, including physostigmine, donepezil and rivastigmine,
huperzine A is more effective at increasing the level of cortical
acetylcholine and has a longer lasting effect (6,7).
Currently, huperzine A is being investigated as a potential
treatment for neurodegenerative diseases, such as Alzheimer's
disease (8,9). Additionally, acetylcholinesterase
inhibitors have been recognized as promising therapeutic agents for
drug addiction (10). Therefore,
assessing the effects of huperzine A on drug addiction is
important.
It has been suggested that behavioral sensitization
is linked to drug craving and compulsive drug seeking (11). Behavioral sensitization refers to a
phenomenon characterized by enhanced responsiveness following
repeated, intermittent treatment with psychomotor stimulants
(12,13). Locomotor activity, as a measure of
spontaneous behavior, has been primarily assessed in behavioral
sensitization studies (14,15). Additionally, behavioral sensitization
has been established in rodents that have repeatedly been
administered the same or incremental doses of a drug (16–19). It
has been demonstrated that behavioral sensitization may also be
applied in a model of testing addictive behavior associated with
drug seeking and relapse (12,20).
Relapse is the resumption of drug seeking/drug consumption
following a protracted period abstinence (14). In an animal model of relapse, when
contextual conditioning is repeatedly paired with drug abuse, it
has a distinct ‘incentive salience’, consequently leading to
compulsive drug craving, seeking and relapse (21–23).
Theories of contextual conditioning emphasize that a ‘context’ not
only consists of fixed geometric features of the environment but
also includes multi-modal sensory (visual, tactile and olfactory)
cues and temporal or episodic context (24). A previous study has determined that
the contextual conditioning may modulate behavioral effects of drug
abuse, such as the sensitization to psychomotor stimulant effects
of amphetamine (23).
Carlezon et al (25) and Wise et al (26) proposed the ‘state-dependency’
hypothesis based on studies of bromocriptine sensitization. This
hypothesis suggests that sensitized behavior should only be evident
under the same conditions in which the same type of drugs (an
addictive drug and a non-addictive drug) and dosage were injected
just as sensitized behavior was exhibited in the past. Therefore,
sensitized response exhibited while under the influence of both
drugs may only be recalled when a rat is in the same state. The
rats did not exhibit sensitization when they were challenged with
the addictive drug alone, which was due to the different states in
which the sensitized behavior developed. Therefore, it has been
suggested that state-dependency should be considered in studies
that involve the inhibition of behavioral sensitization (27).
The purpose of the present study was to examine the
effects of huperzine A on behavioral sensitization caused by
morphine and relapse induced by contextual conditioning. It was
also assessed whether the state-dependency hypothesis may explain
the effects of huperzine A on morphine-induced sensitization.
Materials and methods
Animals
A total of 72 Male Wistar rats used in this study
were 8 weeks old and weighted 220–250 g (Academy of Military
Medical Sciences, Beijing, China). They were housed in groups of
three per cage in a 12-h light-dark cycle (lights on at 7:00 a.m.)
at 22±1°C and relative humidity of 50–60% with ad libitum
access to food and water. Prior to the study experiments, animals
were provided adaptive feeding for 7 days. A previous study
suggested that there were individual differences in the initial
locomotor response to a novel environment in rats (28). Therefore, the adaptive feeding may
make rats familiar with the test environment in advance and avoid
the effect of a novel environment on locomotor activity. The
adaptive feeding procedure was as follows: On days 1–2, rats were
housed in groups of three per cage as aforementioned; on days 3–5,
the animals were acclimated to locomotor chambers (Shanghai Mobile
Datum Information Technology Co., Ltd., Shanghai, China) for 1 h;
on days 6 and 7, locomotor activity was recorded for 2 h once a day
between 8:00 and 16:00. An average baseline for locomotor activity
of each rat was established and expressed as locomotor data
obtained from the two tests on days 6 and 7. All experimental
procedures were carried out in accordance with the 1996 National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (29) and all experimental
procedures were approved by the Institutional Animal Care and Use
Committee, Capital Normal University (Beijing, China).
Apparatus
Locomotor activity was measured in a locomotor
activity chamber 40×40×50 cm (length × width × height; Shanghai
Mobile Datum Information Technology Co., Ltd.). The chamber was
placed in a sound attenuating, dimly illuminated container with an
exhaust fan in order to avoid the interference of external
unrelated conditions on rat's locomotor activity during the testing
and to maintain ventilation. Locomotor activity was measured based
on the horizontal distance (mm) traveled and was recorded
automatically with an infrared video-tracking system.
Drugs
The following drugs were used: sterile 0.9% saline
(prepared by our laboratory) and morphine hydrochloride (10 mg/ml,
Shenyang First Pharmaceutical Factory, Shenyang, China), which was
diluted to 5 mg/ml in sterile saline prior to intraperitoneal
injection. Huperzine A (purity >98%, Jiangxi Herbal Tiangong
Technology Co., Ltd., Jiangxi, China), an acetylcholinesterase
inhibitor, was dissolved in phosphate-buffered saline. The doses of
huperzine A were administered at concentrations of 0.2, 0.3 and 0.4
mg/kg, as described previously (30,31). All
drugs were injected intraperitoneally at a volume of 1 m/kg between
8:00 and 16:00.
Procedures
Two experiments were completed to examine locomotor
activity. The first was to assess the effects of huperzine A
administration alone on locomotor activity. It has been
demonstrated that acetylcholinesterase inhibitors decrease
locomotor activity (32); therefore,
a supplementary experiment was set up in the current study to
examine the effects of huperzine A administration alone on
locomotor activity and analyze the side effects of huperzine A on
locomotor activity (32,33). In the current experiment, based on
the baseline data of locomotor activity obtained from the adaptive
feeding phase, these baseline data were ranked from highest to
lowest, and then grouped by S-type. A total of 32 rats were evenly
divided into four groups (n=8 rats per group) and treated with:
Saline (Sal), 0.2 mg/kg huperzine A (0.2 HupA), 0.3 mg/kg huperzine
A (0.3 HupA) or 0.4 mg/kg huperzine A (0.4 HupA). On days 1–5, each
group was administered the corresponding injection alone (saline or
huperzine A) in their cage once daily. Locomotor activity was
recorded for 2 h starting 25 min (30) following each injection.
The second experiment assessed the effects of
huperzine A on morphine-induced behavioral sensitization. To
examine the effects of huperzine A on behavioral sensitization
induced by morphine, 40 rats which were different from the rats
used in the first experiment were assigned to five groups (n=8 rats
per group) and treated with: Saline+saline (Sal+Sal), saline + 5
mg/kg morphine (Sal+Mor), 0.2 mg/kg huperzine A + 5 mg/kg morphine
(0.2 HupA+Mor), 0.3 mg/kg huperzine A + 5 mg/kg morphine (0.3
HupA+Mor) or 0.4 mg/kg huperzine A + 5 mg/kg morphine (0.4
HupA+Mor). This experimental procedure involved three phases
(Table I). The first injection was
administered to all rats between 8:00 and 16:00 and the second
injection was administered 25 min later (30). During the development phase (days
1–5), the rats in the Sal+Sal group received saline injections
twice daily and rats in the Sal+Mor group were administered saline
and morphine every day. The remaining three groups were
administered different doses of huperzine A and morphine every day.
During the withdrawal phase (days 6–12), no treatments were
administered. During the expression phase (day 13), all animals
were administered 1 ml/kg saline to examine the effects of repeated
huperzine A administration during the development phase of morphine
sensitization, to determine whether relapse is induced by
contextual conditioning alone. Conditioned locomotor activity
induced by saline injection may be regarded as a relapse caused by
contextual conditioning when this follows repeated and intermittent
exposure to morphine (34). Bevins
and Bardo also suggested that when a multisensory environment was
reliably paired with morphine in rats, that context, in a drug-free
test, could evoked a hyperactive conditioned locomotor activity
(35). On day 14, all rats were
challenged with 5 mg/kg morphine to determine the effects of
huperzine A on the development of morphine-induced sensitization.
On day 18, the rats in the Sal+Mor group were injected with saline
and morphine (5 mg/kg). The other three groups received
co-administration of huperzine A (0.2, 0.3 or 0.4 mg/kg) and 5
mg/kg morphine. Considering the data regarding development and
morphine challenge, the current study assessed the state-dependency
hypothesis using these challenges on day 18. On day 22, the rats in
the Sal+Mor group were injected with the appropriate dose of
huperzine A and 5 mg/kg morphine to examine the effect of huperzine
A on the expression of morphine-induced sensitization. The
preferred dose of huperzine A (0.4 mg/kg) was selected from 0.2,
0.3 and 0.4 mg/kg based on the results of preliminary experiments
in the present study. On day 26, only the Sal+Mor rats were
injected with saline and 5 mg/kg morphine to investigate whether
sensitization was weakened or disappeared over time. Locomotor
activity was recorded for 2 h immediately following the
administration of the final injection. Particularly, in order to
avoid elimination of any conditioned hyperactivity and to fully
model the relapse induced by contextual conditioning, when all the
groups were challenged with saline on day 13 in this experiment,
their locomotor activities were recorded for 1 h, as described
previously (36–40).
 | Table I.Different treatments in all groups
over the experiments completed. |
Table I.
Different treatments in all groups
over the experiments completed.
|
| Treatments |
|---|
|
|
|
|---|
| Groups | Days 1–5 | Days 6–12 | Day 13 | Day 14 | Day 18 | Day 22 | Day 26 |
|---|
| Sal+Sal | Sal+Sal, LMA test
(2 h) | Withdrawal
period | Sal challenge, LMA
test (1 h) | Mor challenge, LMA
test (2 h) |
|
|
|
| Sal+Mor | Sal+Mor, LMA test
(2 h) | Withdrawal
period | Sal challenge, LMA
test (1 h) | Mor challenge, LMA
test (2 h) | Sal+Mor, LMA test
(2 h) | 0.4 mg/kg
HupA+Mor | Mor challenge, LMA
test (2 h) |
| 0.2 HupA+Mor | 0.2 mg/kg HupA+Mor,
LMA test (2 h) | Withdrawal
period | Sal challenge, LMA
test (1 h) | Mor challenge, LMA
test (2 h) | 0.2 mg/kg HupA+
Mor, LMA test (2 h) |
|
|
| 0.3 HupA+Mor | 0.3 mg/kg HupA+Mor,
LMA test (2 h) | Withdrawal
period | Sal challenge, LMA
test (1 h) | Mor challenge, LMA
test (2 h) | 0.3 mg/kg HupA+Mor,
LMA test (2 h) |
|
|
| 0.4 HupA+Mor | 0.4 mg/kg HupA+Mor,
LMA test (2 h) | Withdrawal
period | Sal challenge, LMA
test (1 h) | Mor challenge, LMA
test (2 h) | 0.4 mg/kg HupA+
Mor, LMA test (2 h) |
|
|
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
mean ± standard error of the mean (mean ± SEM), unless otherwise
indicated. Data obtained from repeated huperzine A administration
alone in experiment one and development of sensitization in
experiment two were analyzed by two-way (groups × time) repeated
measures analysis of variance (ANOVA). Data regarding the drug
challenge on days 13, 14 and 18 in experiment 2 were respectively
analyzed by one-way ANOVA. This was followed by the least
significant difference (LSD) post hoc comparison when there was
significance among groups. One-way repeated measures ANOVA was used
to compare the data of the Sal+Mor group on days 18, 22 and 26 in
experiment 2. P<0.05 was considered to represent a statistically
significant difference.
Results
Administration of huperzine A alone
does not affect locomotor activity
As demonstrated by the two-way (groups × time)
repeated measures ANOVA, repeated huperzine A injection alone had
no significant effect on locomotor activity (Fig. 1). The interaction effects of groups ×
time [F (12,112)=1.126, P=0.346], the main effects of the groups [F
(3,28)=0.849, P=0.479] and time [F
(4,112)=2.370, P=0.057] did not have significant effects on
locomotor activity (P>0.05). According to the average locomotor
activity of days 1–5, there was no significant difference between
the Sal group and all HupA groups (0.2, 0.3 and 0.4 mg/kg) (mean
difference ± SEM: 4,337.631±2,873.362; 2,687.724±2,873.362;
1,232.341±2,873.362; P>0.05). Therefore, repeated huperzine A
injection alone did not alter the motor function of the rats.
Higher doses of huperzine A
immediately inhibit morphine-induced addictive behavior at the
development phase
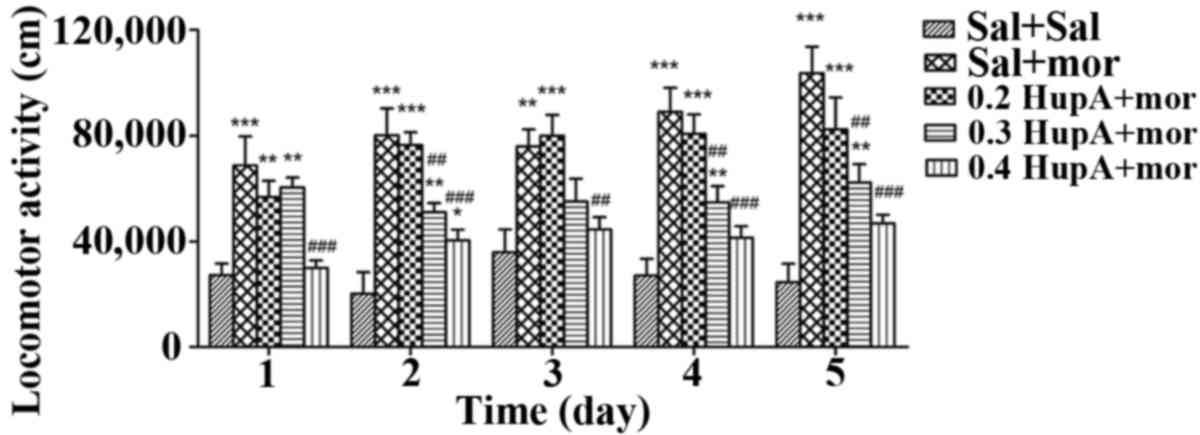
The results of two-way ANOVA indicated that the
interaction effects of groups × time [F (16,140)=2.381, P=0.004]
and the main effects of the groups [F (4,35)=16.729, P<0.001] and time [F
(4,140)=7.131, P<0.001] all had significant effects on locomotor
activity when rats were co-administered huperzine and morphine
(P<0.05). With respect to the daily locomotor activities of each
group, the LSD post hoc comparison indicated that daily locomotor
activities in the Sal+Mor group were significantly greater than
those in the Sal+Sal group (P≤0.001 on days 1–5), which indicated
that repeated morphine administration caused addictive behavior
(Fig. 2). Furthermore, the daily
locomotor activities in the 0.2 HupA+Mor group were significantly
greater than those in the Sal+Sal group (P=0.002 and P<0.001 on
days 1 and 2–5, respectively). However, compared to the Sal+Mor
group, no significant difference in locomotor activity was observed
in the 0.2 HupA+Mor group (P=0.194, P=0.701, P=0.703, P=0.392 and
P=0.083 on days 1–5, respectively), suggesting that 0.2 mg/kg
huperzine A did not prevent the locomotor behavior induced by
morphine. The locomotor activities in the 0.3 HupA+Mor group were
markedly increased compared to those in the Sal+Sal group (P=0.001,
P=0.002, P=0.007 and P=0.003 on days 1, 2, 4 and 5, respectively;
except for P=0.077 on day 3); however, they were significantly less
than those in the Sal+Mor group only on days 2, 4 and 5 (P=0.004,
P=0.001 and P=0.001, respectively). These results suggested that
0.3 mg/kg huperzine A inhibited morphine-induced locomotor
increases. However, the daily locomotor activities in the 0.4
HupA+Mor group were significantly lower than those in the Sal+Mor
group (P<0.001, P<0.001, P=0.005, P<0.001 and P<0.001
on days 1–5, respectively). There were no significant differences
in the locomotor activity between the 0.4 HupA+Mor group and the
Sal+Sal group (P=0.761, P=0.417, P=0.148 and P=0.069 on days 1, 3,
4 and 5, respectively), with the exception of day 2 (P=0.040).
Therefore, 0.4 mg/kg huperzine A rapidly and effectively rescued
the addictive behavior induced by morphine (Fig. 2).
Huperzine A does not inhibit the
relapse induced by contextual conditioning
Saline-challenged locomotor activity was markedly
different among the groups [F (4,35)=7.234,
P<0.001] (Fig. 3). LSD post hoc
comparison analysis indicated a clear increase in locomotor
activity in the Sal+Mor group compared with that in the Sal+Sal
group. (mean difference ± SEM: 25,439.104±6,318.852, P<0.001;
Fig. 3). Similarly, the locomotor
activities in the 0.2 HupA+Mor group, 0.3 HupA+Mor group and 0.4
HupA+Mor group were all significantly greater than those in the
Sal+Sal group (P<0.001, P=0.029 and P=0.006, respectively;
Fig. 3); however, there were no
significant differences in locomotor activity in the HupA treated
groups compared with the Sal+Mor group (P=0.343, P=0.088 and
P=0.280, respectively; Fig. 3).
Therefore, huperzine A did not inhibit the relapse induced by the
saline challenge.
Huperzine A does not inhibit the
development of morphine-induced behavioral sensitization
Considering morphine-challenged locomotor
activities, a one-way ANOVA revealed a significant difference among
the groups [F (4,35)=2.979, P=0.032]. The results of the LSD
post hoc comparison indicated that the locomotor activity in the
Sal+Mor group was significantly greater than that of the Sal+Sal
group (mean difference ± SEM: 30,567.659±12,713.000, P=0.022;
Fig. 4), which indicated that
behavioral sensitization was established. Furthermore, the
locomotor activities in the 0.2 HupA+Mor group, 0.3 HupA+Mor group
and 0.4 HupA+Mor group were all significantly greater than those in
the Sal+Sal group (P=0.047, P=0.024 and P=0.002, respectively;
Fig. 4); however, no differences
were observed when compared with the Sal+Mor group (P=0.732,
P=0.963 and P=0.382, respectively; Fig.
4). Therefore, huperzine A failed to block the development of
morphine-induced behavioral sensitization.
The current study does not support the
state-dependency hypothesis
When the rats in the Sal+Mor group and the three
HupA+Mor groups were challenged with saline and morphine or
different doses of huperzine A and morphine, the difference in
locomotor activity among the groups was significant [F (3,28)=7.900,
P=0.001]. LSD post hoc comparison analysis revealed that the
locomotor activities in the 0.4 HupA+Mor group were significantly
lower compared with those in the Sal+Mor group (mean difference ±
SEM: 49,737.813±10,660.000, P<0.001; Fig. 5). However, the locomotor activities
in the 0.2 HupA+Mor group (mean difference ± SEM:
13,219.521±10,660.000, P=0.225) and 0.3 HupA+Mor group (mean
difference ± SEM: 15,830.700±10,660.000, P=0.149) did not differ
significantly to those in the Sal+Mor group (P>0.05; Fig. 5). Based on the 5-day locomotor
activity data obtained from the development phase and the
morphine-challenged locomotor activity data from the expression
phase, state-dependency was not supported by these results.
A higher dose of huperzine A inhibits
the expression of morphine-induced behavioral sensitization
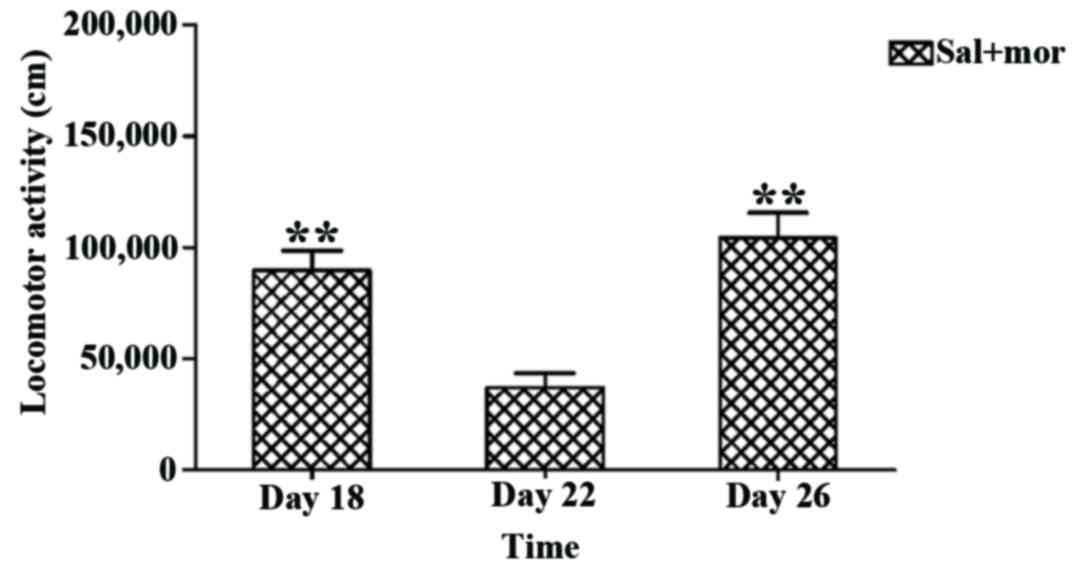
Based on the aforementioned findings, 0.4 mg/kg
huperzine A, as the preferred dose, was used in the following
experiment. A one-way repeated measures ANOVA demonstrated that
time had a significant influence on locomotor activity [F (2,14)=13.367, P=0.001]. With regard to the
Sal+Mor group, the results of LSD post hoc comparison analysis
indicated that the locomotor activity measured following
co-administration of 0.4 mg/kg huperzine A and morphine on day 22
was significantly lower than the group challenged with saline and
morphine on day 18 (mean difference ± SEM: −52,622.189±14,140.000,
P=0.007; Fig. 6) and the group
challenged with saline and morphine on day 26 (mean difference ±
SEM: −67,220.744±12,160.000, P=0.001; Fig. 6). However, no significant difference
was observed between the locomotor activities on days 18 and 26
(mean difference ± SEM: −14,598.555±14,610.000, P=0.351; Fig. 6). Consequently, it was determined
that 0.4 mg/kg huperzine A inhibited the expression of
morphine-induced behavioral sensitization.
Discussion
In the present study, three different doses of
huperzine A, which were co-administered with morphine during the
development of the sensitization phase (days 1–5), failed to
inhibit locomotor activity when morphine challenge was instigated
on day 14. This indicates that huperzine A did not stop the
development of morphine-induced behavioral sensitization. Notably,
during the 5-day development of sensitization, 0.4 mg/kg huperzine
A blocked the increase in locomotor activities caused by morphine.
Additionally, considering the evidence indicating that the three
different doses of huperzine A did not affect the motor function of
the animals, it is likely that the inhibition of 0.4 mg/kg
huperzine A on morphine-induced hyperactivity during the 5-day
developmental phase was immediate and temporary. Locomotor activity
in the 0.4 HupA+Mor group was significantly higher when challenged
with morphine compared with the Sal+Sal group. It was demonstrated
that sensitization developed normally.
Previous studies have demonstrated that
acetylcholinesterase inhibitors may markedly affect cholinergic
interneurons in the striatum (41,42).
Furthermore, the balance between dopamine and acetylcholine in the
striatum may modulate motor function (43). Therefore, the immediate suppression
of locomotor activities was potentially induced by simultaneous
inhibition of dopamine release caused by morphine and acetylcholine
release following huperzine A administration in the striatum.
However, this alternative hypothesis and mechanism of the immediate
inhibition of huperzine A requires additional investigation.
The appearance of addictive behavior during the
development of sensitization was prevented by 0.4 mg/kg huperzine;
however, it remains unknown why the initial inhibition by huperzine
A did not last longer. By contrast, the development of behavioral
sensitization was associated with external factors, including the
frequency, dose, interval and contextual conditioning of injections
(44) in addition to the
insufficient frequency of administration and the short development
period in the present study. This may have resulted in a temporary
inhibitory effect. With regard to the Sal+Mor group, the locomotor
activity induced by co-administration of 0.4 mg/kg huperzine A and
morphine on day 22 was not as marked compared with the group
challenged with saline and morphine on day 18. This indicates that
0.4 mg/kg huperzine A may suppress the expression of behavioral
sensitization caused by morphine, which was in agreement with the
results obtained from previous studies investigating other
acetylcholinesterase inhibitors, such as physostigmine (17).
The locomotor activity in the Sal+Mor group
challenged with saline and morphine on day 26 increased
significantly again, indicating that behavioral sensitization was
not weakened or eliminated with time and confirming the inhibitory
effect of 0.4 mg/kg huperzine A on the expression of
morphine-induced behavioral sensitization. However, huperzine A
failed to inhibit the development of morphine-induced behavioral
sensitization. This phenomenon may be explained by the different
mechanisms of development and expression of morphine-induced
behavioral sensitization (45). The
development of morphine sensitization primarily requires
stimulation of µ-opioid receptors within the ventral tegmental area
but not the dopamine receptor (46,47).
However, its expression requires stimulation of the dopamine
receptor, specifically the dopamine D2 receptor
(47). Furthermore, the nucleus
accumbens has been recognized as the brain region that is most
closely associated with the expression of behavioral sensitization
(48). Repeated morphine
administration may enhance the release of dopamine, which may
stimulate the dopamine D2 receptor (45,47),
leading to an increase in locomotor activities. The increasing
concentration of acetylcholine in the nucleus accumbens may
suppress the addictive behavior caused by morphine (10). It has been demonstrated that
acetylcholinesterase inhibitors administered to the nucleus
accumbens, but not to the ventral tegmental area, suppress the
rewarding response to opioids (10,49).
Thus, huperzine A potentially increased the release of
acetylcholine, which may inhibit the release of dopamine in the
nucleus accumbens, resulting in a blockade of expression for
behavioral sensitization induced by morphine.
The present study demonstrated that three different
doses of huperzine A (0.2, 0.3 and 0.4 mg/kg), which were injected
with morphine during development of the sensitization phase (days
1–5), failed to suppress relapse when challenged with saline alone
on day 13. As huperzine A did not stop the development of morphine
sensitization, the contextual conditioning that is associated with
the drug rewarding may have potentially possessed ‘incentive
salience’ (12,50) and the reward-associated contextual
conditioning may induce relapse (23). Incentive salience, just as a
psychological process, transforms the perception of stimuli,
imbuing them with salience, making them attractive, ‘wanted’,
incentive stimuli (12). By
contrast, huperzine A may be selectively distributed in the
cerebral cortex, hippocampus and other brain regions associated
with learning and memory (2,51). In particular, the hippocampus is
involved in the conditioning of contextual stimuli, contextual
learning and memory consolidation (22,23).
Therefore, huperzine A may enhance the release of acetylcholine in
the central nervous system and improve the normal or impaired
learning and memory functions of animals (31,52–54).
Consequently, contextual conditioning was more closely associated
with relapse.
The state-dependency hypothesis was previously used
to interpret the following phenomenon: Rats repeatedly injected
with MK-801 and bromocriptine exhibited progressive augmentation of
locomotor activities during the development phase (24,27).
However, the rats did not exhibit sensitized behavior when they
were challenged with bromocriptine alone, until they were
challenged with MK-801+bromocriptine (25). Carlezon et al (25) and Wise et al (26) suggested that MK-801 became a
conditioned stimulus to recall the sensitized response only when
co-administered with bromocriptine during a challenge period. The
contextual dependency of sensitization was extended to the drug
state when sensitization was induced (27).
In contrast to the results obtained from the
aforementioned previous studies (25), the present study demonstrated that
locomotor activity in the 0.4 HupA+Mor group was prevented when 0.4
mg/kg huperzine A and morphine were injected in the development and
expression phases, but did not experience behavioral sensitization.
Following withdrawal, 0.4 HupA+Mor rats expressed sensitized
behavior when challenged with morphine alone, but did not exhibit
inhibition of behavior. Therefore, the results of the present study
do not support the state-dependency hypothesis. As discussed, the
inhibition of huperzine A on behavioral sensitization in the
present study was potentially due to the unique pharmacological
effects of huperzine A on the central nervous system or different
mechanisms between the development and expression of
morphine-induced behavioral sensitization.
In conclusion, the present study examined the
effects of huperzine A on behavioral sensitization induced by
morphine and relapse induced by contextual conditioning. The
current study demonstrated that 0.4 mg/kg huperzine A may inhibit
the expression of behavioral sensitization induced by morphine but
not the development of sensitization. Additionally,
co-administration of huperzine A with morphine during the
development period did not inhibit the relapse induced by
contextual conditioning. Therefore, the results of the current
study did not support the state-dependency hypothesis. The
inhibition mechanisms of huperzine A on morphine-induced behavioral
sensitization require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31470989).
References
|
1
|
Wang R, Yan H and Tang XC: Progress in
studies of huperzine A, a natural cholinesterase inhibitor from
Chinese herbal medicine. Acta Pharmacol Sin. 27:1–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma T, Gong K, Yan Y, Zhang L, Tang P,
Zhang X and Gong Y: Huperzine A promotes hippocampal neurogenesis
in vitro and in vivo. Brain Res. 1506:35–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Yang J and Jiang Q: The protective
effect of huperzine A against hepatic ischemia reperfusion injury
in mice. Transplant Proc. 46:1573–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ou LY, Tang XC and Cai JX: Effect of
huperzine A on working memory in reserpine- or yohimbine-treated
monkeys. Eur J Pharmacol. 433:151–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HY, Yan H and Tang XC:
Non-cholinergic effects of huperzine A: Beyond inhibition of
acetylcholinesterase. Cell Mol Neurobiol. 28:173–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang XC: Huperzine A (shuangyiping): A
promising drug for Alzheimer's disease. Zhongguo Yao Li Xue Bao.
17:481–484. 1996.PubMed/NCBI
|
|
7
|
Liang YQ and Tang XC: Comparative effects
of huperzine A, donepezil and rivastigmine on cortical
acetylcholine level and acetylcholinesterase activity in rats.
Neurosci Lett. 361:56–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HY and Tang XC: Neuroprotective
effects of huperzine A: New therapeutic targets for
neurodegenerative disease. Trends Pharmacol Sci. 27:619–625. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma X and Gang DR: In vitro production of
huperzine A, a promising drug candidate for Alzheimer's disease.
Phytochemistry. 69:2022–2028. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hikida T, Kitabatake Y, Pastan I and
Nakanishi S: Acetylcholine enhancement in the nucleus accumbens
prevents addictive behaviors of cocaine and morphine. Proc Natl
Acad Sci USA. 100:6169–6173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Handal M, Ripel A, Skurtveit S and Mørland
J: Behavioural sensitization in mice induced by
morphine-glucuronide metabolites. Pharmacol Biochem Behav.
90:578–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robinson TE and Berridge KC: The neural
basis of drug craving: An incentive-sensitization theory of
addiction. Brain Res Brain Res Rev. 18:247–291. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierce RC and Kalivas PW: Repeated cocaine
modifies the mechanism by which amphetamine releases dopamine. J
Neurosci. 17:3254–3261. 1997.PubMed/NCBI
|
|
14
|
Sanchis-Segura C and Spanagel R:
Behavioural assessment of drug reinforcement and addictive features
in rodents: An overview. Addict Biol. 11:2–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tortorelli LS, Engelke DS, Lunardi P, E
Souza T Mello, Santos-Junior JG and Gonçalves CA: Cocaine
counteracts LPS-induced hypolocomotion and triggers locomotor
sensitization expression. Behav Brain Res. 287:226–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Didone V, Quoilin C, Tirelli E and
Quertemont E: Parametric analysis of the development and expression
of ethanol-induced behavioral sensitization in female Swiss mice:
Effects of dose, injection schedule and test context.
Psychopharmacology (Berl). 201:249–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Li JX, Zhu X, Cui R and Jiao J:
Effects of physostigmine on the conditioned hyperactivity and
locomotor sensitization to morphine in rats. Behav Brain Res.
206:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ball KT, Klein JE, Plocinski JA and Slack
R: Behavioral sensitization to 3,4-methylenedioxymethamphetamine is
long-lasting and modulated by the context of drug administration.
Behav Pharmacol. 22:847–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collins GT, Truong YN, Levant B, Chen J,
Wang S and Woods JH: Behavioral sensitization to cocaine in rats:
Evidence for temporal differences in dopamine D3 and D2 receptor
sensitivity. Psychopharmacology (Berl). 215:609–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Li JX, Zheng JW, Liu RK and Liang
JH: L-type Ca(2+) channel blockers inhibit the development but not
the expression of sensitization to morphine in mice. Eur J
Pharmacol. 467:145–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brody AL, Mandelkern MA, London ED,
Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR Jr,
Madsen D and Jarvik ME: Brain metabolic changes during cigarette
craving. Arch Gen Psychiatry. 59:1162–1172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weiss F: Neurobiology of craving,
conditioned reward and relapse. Curr Opin Pharmacol. 5:9–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zironi I, Burattini C, Aicardi G and Janak
PH: Context is a trigger for relapse to alcohol. Behav Brain Res.
167:150–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulteis G, Liu J, Amitai N and Tzeng S:
Context- and cue-conditioned potentiation of acute morphine
dependence and withdrawal. Pharmacol Biochem Behav. 82:82–89. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carlezon WA Jr, Mendrek A and Wise RA:
MK-801 disrupts the expression but not the development of
bromocriptine sensitization: A state-dependency interpretation.
Synapse. 20:1–9. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wise RA, Mendrek A and Carlezon WA Jr:
MK-801 (dizocilpine): Synergist and conditioned stimulus in
bromocriptine-induced psychomotor sensitization. Synapse.
22:362–368. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stephens DN, Elliman TD and Dunworth SJ:
State-dependent behavioural sensitization: Evidence from a
chlordiazepoxide state. Behav Pharmacol. 11:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gancarz AM, San George MA, Ashrafioun L
and Richards JB: Locomotor activity in a novel environment predicts
both responding for a visual stimulus and self-administration of a
low dose of methamphetamine in rats. Behav Processes. 86:295–304.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Institute of Laboratory Animal Resources;
Commission on Life Sciences; National Research Council, . Guide for
the Care and Use of Laboratory Animals. National Academy Press;
Washington, D.C: 1996
|
|
30
|
Ye JW, Shang YZ, Wang ZM and Tang XC:
Huperzine A ameliorates the impaired memory of aged rat in the
Morris water maze performance. Acta Pharmacol Sin. 21:65–69.
2000.PubMed/NCBI
|
|
31
|
Wang ZF, Tang LL, Yan H, Wang YJ and Tang
XC: Effects of huperzine A on memory deficits and neurotrophic
factors production after transient cerebral ischemia and
reperfusion in mice. Pharmacol Biochem Behav. 83:603–611. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Myhrer T, Enger S and Aas P: Behavioral
side effects in rats treated with acetylcholinesterase inhibitors
suggested used as prophylactics against nerve agents. Pharmacol
Biochem Behav. 95:338–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gawel K, Labuz K, Jenda M, Silberring J
and Kotlinska JH: Influence of cholinesterase inhibitors, donepezil
and rivastigmine on the acquisition, expression and reinstatement
of morphine-induced conditioned place preference in rats. Behav
Brain Res. 268:169–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JL, Milton AL and Everitt BJ:
Cue-induced cocaine seeking and relapse are reduced by disruption
of drug memory reconsolidation. J Neurosci. 26:5881–5887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bevins RA and Bardo MT:
Morphine-conditioned changes in locomotor activity: Role of the
conditioned stimulus. Exp Clin Psychopharm. 6:131–138. 1998.
View Article : Google Scholar
|
|
36
|
Xinwang L, Aihong X, Bin Z, Ping Y and
Chunyan G: Physostigmine blocks behavioral locomotor sensitization
induced by morphine in rats. Acta Psychologica Sinica. 37:362–365.
2005.
|
|
37
|
Xinwang L, Aihong X, Bin Y, Jia W and
Chunyan G: Effects of scopolamine on behavioral sensitization
induced by morphine in rats. Acta Psychologica Sinica. 39:299–305.
2007.
|
|
38
|
Bevins RA, Besheer J and Pickett KS:
Nicotine-conditioned locomotor activity in rats: Dopaminergic and
GABAergic influences on conditioned expression. Pharmacol Biochem
Behav. 68:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reid MS, Ho LB and Berger SP: Behavioral
and neurochemical components of nicotine sensitization following
15-day pretreatment: Studies on contextual conditioning. Behav
Pharmacol. 9:137–148. 1998.PubMed/NCBI
|
|
40
|
Wei S and Li X: Differential effects of
propranolol on conditioned hyperactivity and locomotor
sensitization induced by morphine in rats. Sci Rep.
4:37862014.PubMed/NCBI
|
|
41
|
Kawaguchi Y, Wilson CJ, Augood SJ and
Emson PC: Striatal interneurones: Chemical, physiological and
morphological characterization. Trends Neurosci. 18:527–535. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao Q and Tang XC: Effects of huperzine A
on acetylcholinesterase isoforms in vitro: Comparison with tacrine,
donepezil, rivastigmine and physostigmine. Eur J Pharmacol.
455:101–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakanishi S, Kaneko S, Hikida T, Watanabe
D and Pastan I: Role of synaptic integration of dopaminergic and
cholinergic transmission in basal ganglia function. International
Congress Series. 1250:487–492. 2003. View Article : Google Scholar
|
|
44
|
Powell KR and Holtzman SG: Parametric
evaluation of the development of sensitization to the effects of
morphine on locomotor activity. Drug Alcohol Depend. 62:83–90.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kalivas PW and Stewart J: Dopamine
transmission in the initiation and expression of drug- and
stress-induced sensitization of motor activity. Brain Res Brain Res
Rev. 16:223–244. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vezina P and Stewart J: The effect of
dopamine receptor blockade on the development of sensitization to
the locomotor activating effects of amphetamine and morphine. Brain
Res. 499:108–120. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeziorski M and White FJ: Dopamine
receptor antagonists prevent expression, but not development, of
morphine sensitization. Eur J Pharmacol. 275:235–244. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brady AM, Glick SD and O'Donnell P:
Changes in electrophysiological properties of nucleus accumbens
neurons depend on the extent of behavioral sensitization to chronic
methamphetamine. Ann N Y Acad Sci. 1003:358–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rezayof A, Nazari-Serenjeh F, Zarrindast
MR, Sepehri H and Delphi L: Morphine-induced place preference:
Involvement of cholinergic receptors of the ventral tegmental area.
Eur J Pharmacol. 562:92–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Robinson TE and Berridge KC: Addiction.
Annu Rev Psychol. 54:25–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang XC and Han YF: Pharmacological
profile of uperzine A, a novel acetylcholinesterase inhibitor from
Chinese herb. CNS Drug Reviews. 5:281–300. 1999. View Article : Google Scholar
|
|
52
|
Tang XC, Han YF, Chen XP and Zhu XD:
Effects of huperzine A on learning and the retrieval process of
discrimination performance in rats. Zhongguo Yao Li Xue Bao.
7:507–511. 1986.(In Chinese). PubMed/NCBI
|
|
53
|
Xiong ZQ and Tang XC: Effect of huperzine
A, a novel acetylcholinesterase inhibitor, on radial maze
performance in rats. Pharmacol Biochem Behav. 51:415–419. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Blake MG, Krawczyk MC, Baratti CM and
Boccia MM: Neuropharmacology of memory consolidation and
reconsolidation: Insights on central cholinergic mechanisms. J
Physiol Paris. 108:286–291. 2014. View Article : Google Scholar : PubMed/NCBI
|